Abstract
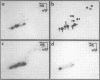
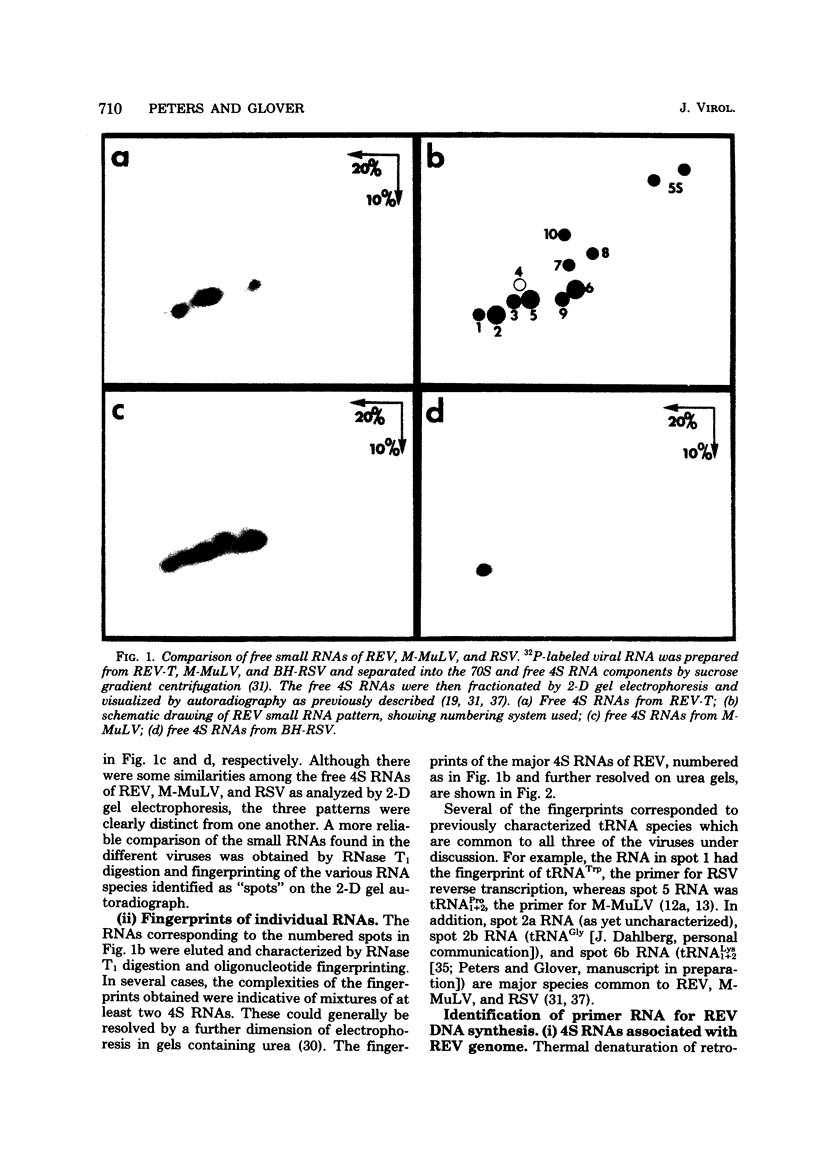
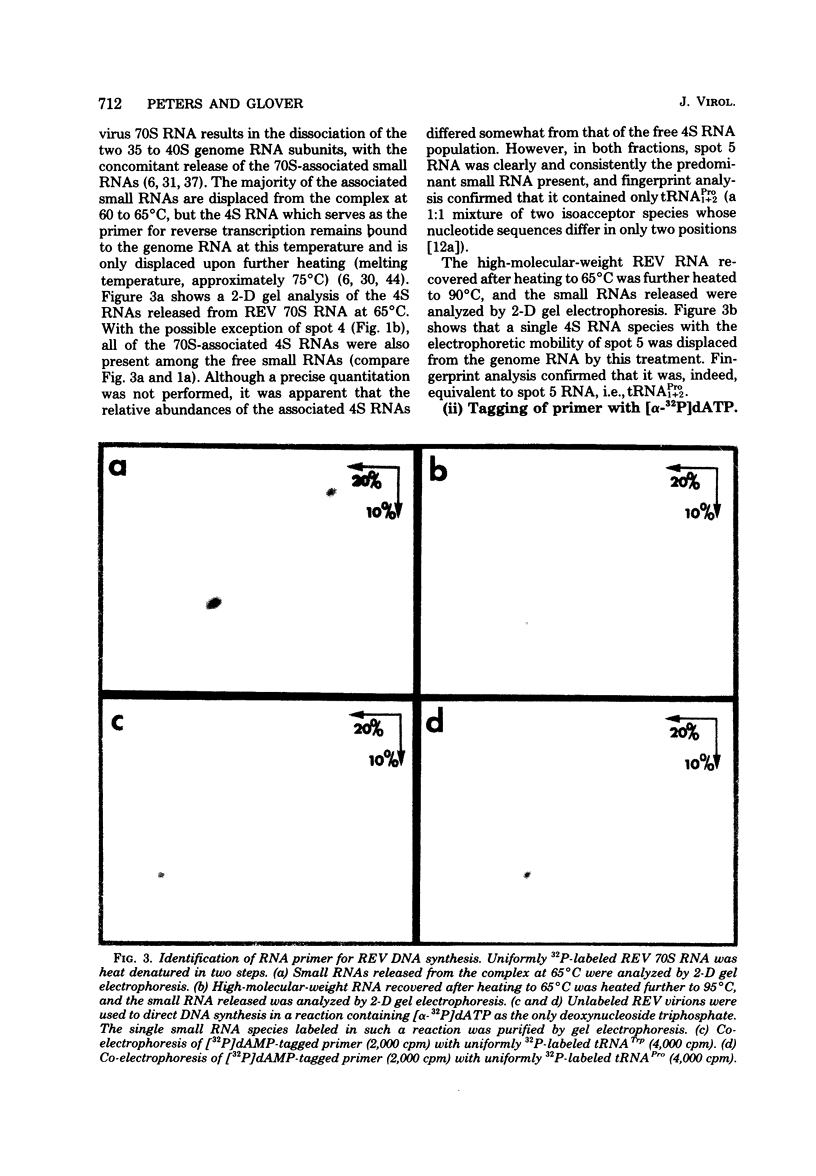
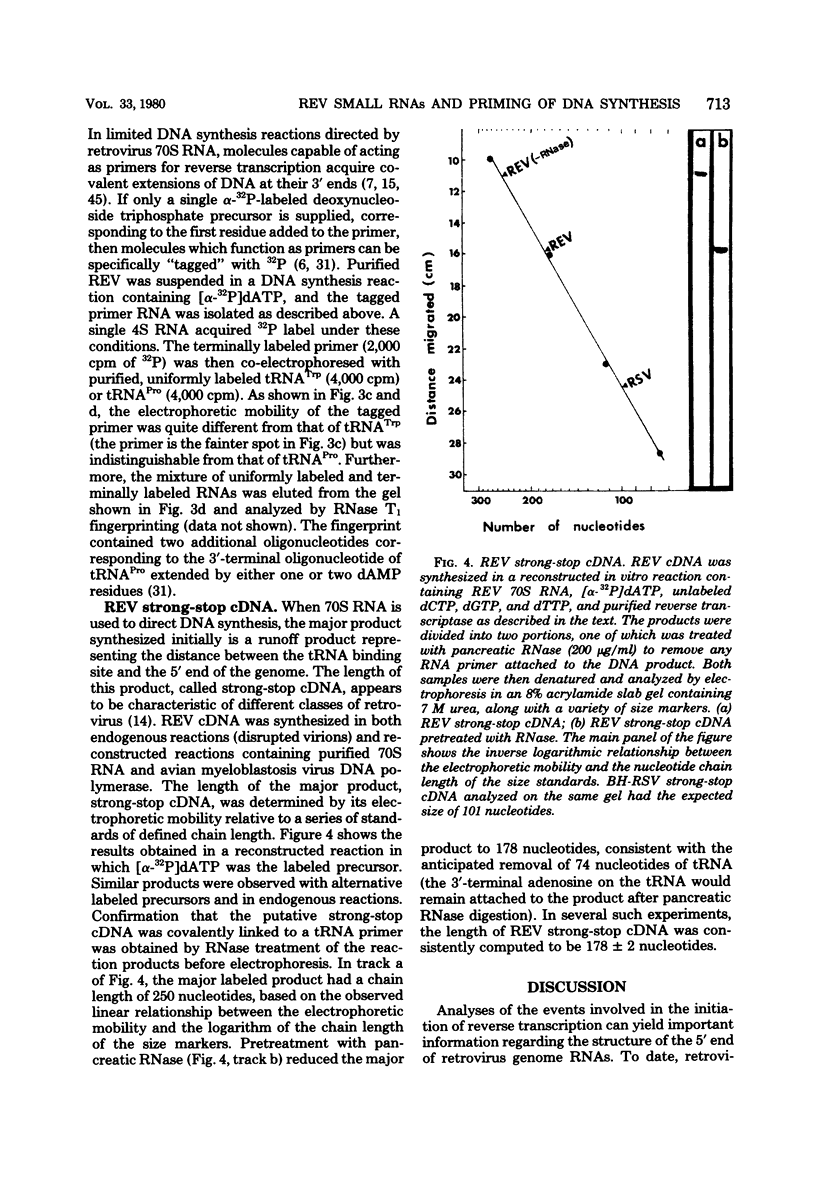
The small RNAs of avian reticuloendotheliosis virus (REV) were analyzed by two-dimensional polyacrylamide gel electrophoresis and compared with those of murine leukemia virus and avian sarcoma virus. Although there were some similarities among the three virus types, the patterns of small RNAs were distinct. By characterizing the small RNA which is most tightly associated with REV genome RNA and which can be labeled in limited DNA synthesis reactions, the primer for REV reverse transcription was identified as tRNAPro. This is consistent with previous reports that REV is more closely related to retroviruses of mammalian origin than to other avian viruses. In contrast, REV strong-stop complementary DNA is longer than any previously characterized strong-stop products of avian or mammalian retroviruses. The REV group may, therefore, have been derived from an as yet unidentified mammalian type C virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Hunter E., Aaronson S. A. Avian reticuloendotheliosis viruses: evolutionary linkage with mammalian type C retroviruses. J Virol. 1979 May;30(2):508–514. doi: 10.1128/jvi.30.2.508-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman H. P., Gilden R. V., Oroszlan S. Reticuloendotheliosis virus: detection of immunological relationship to mammalian type C retroviruses. J Virol. 1979 Mar;29(3):1221–1225. doi: 10.1128/jvi.29.3.1221-1225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Bolognesi D. P., Langlois A. J., Nichols J. L. The initial nucleotide sequence of DNA transcribed from avian myeloblastosis virus 70 S RNA by RNA-dependent DNA polymerase. Virology. 1975 May;65(1):163–172. doi: 10.1016/0042-6822(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Eiden J. J., Quade K., Nichols J. L. Interaction of tryptophan transfer RNA with Rous sarcoma virus 35S RNA. Nature. 1976 Jan 22;259(5540):245–247. doi: 10.1038/259245a0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dahlberg J. E., Sawyer R. C., Harada F., Taylor J. M., Levinson W. E., Bishop J. M., Goodman H. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. II. Structure of a 4S RNA primer. J Virol. 1974 May;13(5):1134–1142. doi: 10.1128/jvi.13.5.1134-1142.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Harada F., Peters G. G., Dahlberg J. E. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979 Nov 10;254(21):10979–10985. [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G. A method for classification of 5' termini of retroviruses. Nature. 1978 Jun 1;273(5661):358–364. doi: 10.1038/273358a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hunter E., Bhown A. S., Bennett J. C. Amino-terminal amino acid sequence of the major structural polypeptides of avian retroviruses: sequence homology between reticuloendotheliosis virus p30 and p30s of mammalian retroviruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2708–2712. doi: 10.1073/pnas.75.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R. Relationship of reticuloendotheliosis virus to the avian tumor viruses: nucleic acid and polypeptide composition. J Virol. 1973 May;11(5):741–747. doi: 10.1128/jvi.11.5.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A., Temin H. M. Purification and properties of spleen necrosis virus DNA polymerase. J Virol. 1975 Oct;16(4):797–806. doi: 10.1128/jvi.16.4.797-806.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Lack of serological relationship among DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J Virol. 1973 Sep;12(3):440–448. doi: 10.1128/jvi.12.3.440-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Gelderblom H., Pauli G., Friis R. A comparative study of the avian reticuloendotheliosis virus: relationship to murine leukemia virus and viruses of the avian sarcoma-leukosis complex. Virology. 1975 Jun;65(2):546–557. doi: 10.1016/0042-6822(75)90059-8. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Mosser A. G. Polypeptide composition of spleen necrosis virus, a reticuloendotheliosis virus. J Virol. 1975 May;15(5):1088–1095. doi: 10.1128/jvi.15.5.1088-1095.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Dahlberg J. E. RNA-directed DNA synthesis in Moloney murine leukemia virus: interaction between the primer tRNA and the genome RNA. J Virol. 1979 Aug;31(2):398–407. doi: 10.1128/jvi.31.2.398-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. A., Baxter-Gabbard K. L., Levine A. S. Avian reticuloendotheliosis virus (strain T): V. DNA polymerase. Virology. 1972 Jan;47(1):251–254. doi: 10.1016/0042-6822(72)90259-0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Purchase H. G., Witter R. L. The reticuloendotheliosis viruses. Curr Top Microbiol Immunol. 1975;71:103–124. doi: 10.1007/978-3-642-66193-8_3. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Formation of reticuloendotheliosis virus pseudotypes of Rous sarcoma virus. J Virol. 1977 Jun;22(3):634–639. doi: 10.1128/jvi.22.3.634-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cordell-Stewart B., Rohde W., Goodman H. M., Bishop J. M. Reassociation of 4 S and 5 S RNA's with the genome of avian sarcoma virus. Virology. 1975 May;65(1):248–259. doi: 10.1016/0042-6822(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Garfin D. E., Levinson W. E., Bishop J. M., Goodman H. M. Tumor virus ribonucleic acid directed deoxyribonucleic acid synthesis: nucleotide sequence at the 5' terminus of nascent deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3159–3163. doi: 10.1021/bi00712a024. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Spencer J. L., Okazaki W., Witter R. L., Crittenden L. B. Phenotypic mixing between reticuloendotheliosis virus and avian sarcoma viruses. Virology. 1977 Jul 1;80(1):127–135. doi: 10.1016/0042-6822(77)90385-3. [DOI] [PubMed] [Google Scholar]
- Waters L. C. Lysine tRNA is the predominant tRNA in murine mammary tumor virus. Biochem Biophys Res Commun. 1978 Apr 14;81(3):822–827. doi: 10.1016/0006-291x(78)91425-0. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- Zeigel R. F., Theilen G. H., Twiehaus M. J. Electron microscopic observations on RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):709–729. [PubMed] [Google Scholar]