Abstract
Bisphosphonates (BPs) slow bone loss by reducing initiation of new basic multicellular units (BMUs). Whether or not BPs simply prevent osteoclasts from initiating new BMUs that resorb bone or also reduce the amount of bone they resorb at the BMU level is not clear. The goal of this study was to determine the effects of BPs on three morphological parameters of individual BMUs, resorption depth (Rs.De), area (Rs.Ar), and width (Rs.Wi). After 1 year of treatment with vehicle (VEH), alendronate (ALN; 0.10, 0.20, or 1.00 mg/kg/day), or risedronate (RIS; 0.05, 0.10, or 0.50 mg/kg/day), resorption cavity morphology was assessed in vertebral trabecular bone of beagle dogs by histology. Animals treated with ALN or RIS at the doses representing those used to treat postmenopausal osteoporosis (0.20 and 0.10 mg/kg/day, respectively) had significantly lower Rs.Ar (−27%) and Rs.Wi (−17%), with no difference in Rs.De, compared to VEH-treated controls. Low doses of ALN and RIS did not affect any parameters, whereas higher doses resulted in similar changes to those of the clinical dose. There were no significant differences in the resorption cavity measures between RIS and ALN at any of the dose equivalents. These results highlight the importance of examining parameters beyond erosion depth for assessment of resorption parameters. Furthermore, these results suggest that in addition to the well-known effects of BPs on reducing the number of active BMUs, these drugs also reduce the activity of osteoclasts at the individual BMU level at doses at and above those used clinically for the treatment of postmenopausal osteoporosis.
Keywords: Basic multicellular unit (BMU), Alendronate, Risedronate, Histomorphometry, Trabecular bone, Antiresorptive
Bisphosphonates (BPs) are commonly used to prevent or treat osteoporosis and other metabolic or oncogenic diseases that result in increased bone remodeling [1]. BPs act by preventing osteoclast activation and causing apoptosis of osteoclasts, resulting in the initiation of fewer active basic multicellular units (BMUs) on bone surfaces [2]. Whether or not BPs affect osteoclast activity at the individual BMU level, i.e., the amount of bone that a team of osteoclasts resorbs, is less clear. Suppressing osteoclast resorption at the BMU level would represent an additional means through which these drugs slow the rate of bone loss.
BMUs occur in vivo as three-dimensional units, yet they are most often assessed in the laboratory as two-dimensional structures via histomorphometry. Numerous techniques have been used to assess morphological properties of the BMU, and although the specific methods can differ, they all focus on reconstructing BMUs in order to estimate various parameters [3–6]. The most common parameter used to determine BMU-level resorption activity is resorption depth (Rs.De), defined as the maximum distance from the cement line of a BMU to the estimated original trabecular surface. In a balanced remodeling system, this would be the same as final wall width, but when resorption and formation are not balanced, wall width could be either greater or less than the Rs.De. Studies examining BP treatment effects on Rs.De have been equivocal, with most showing no difference compared to untreated controls [3, 5, 7–9].
The goal of the current study was to determine the effects of BPs on BMU-level bone resorption measures beyond those of Rs.De. Specifically, we examined how BPs affect resorption space area (Rs.Ar) and resorption space width (Rs.Wi), along with the more commonly assessed Rs.De. We hypothesized that BMU Rs.Ar would be lower in animals treated with BPs compared to controls as a result of lower Rs.De and Rs.Wi.
Methods
Detailed methods concerning this experiment have been previously published [10], and all procedures related to this work were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee before the study initiation. Briefly, 84 skeletally mature female beagles were treated for 1 year with vehicle (VEH), alendronate (ALN; 0.10, 0.20, or 1.00 mg/kg/day), or risedronate (RIS; 0.05, 0.10, or 0.50 mg/kg/day). The middle dose for each drug corresponds, on a mg/kg basis, to the treatment dose for postmenopausal osteoporosis. Before necropsy, most animals were injected with calcein using a 2–12–2–5 labeling schedule; some animals (n = 3 per group) were labeled using a 2–5–2–5 schedule. The shorter interlabel duration was due to a scheduling error. Second lumbar vertebrae were embedded, undecalcified, in plastic, and 8-μm-thick unstained midsagittal sections were prepared [10].
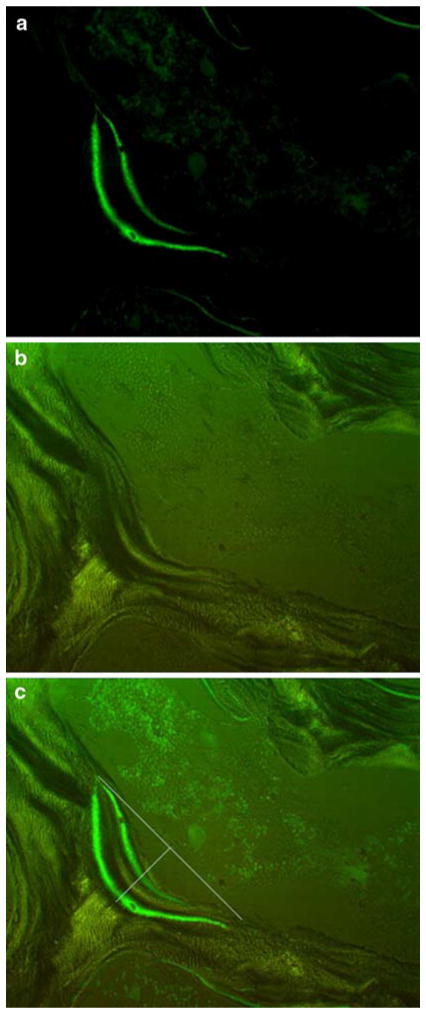
Sections were inspected, blinded to treatment, at ×250 magnification with an Olympus BH-2 microscope under both ultraviolet and polarized light to view the calcein labels and lamellae, respectively. For each animal, resorption cavities were identified for analysis using the following criteria: (1) resorption cavities that resided on a flat, continuous trabecular surface, (2) double calcein labels displayed characteristic bow shaped curves meeting at the ends (Fig. 1a), and (3) lamellae were clear and followed the same contour as the calcein labels (Fig. 1b). Our choice to restrict our analysis to flat surfaces was to eliminate the likely variability associated with estimating the projected bone surface on BMUs located on curves. After identification of BMUs fitting the above criteria, both ultraviolet and polarized light images of the region were taken by a PaxCam camera (MIS Inc.).
Fig. 1.
Photomicrographs of a typical basic multicellular unit (BMU) for which analysis of resorption parameters was conducted. a BMU viewed with epifluorescence to identify calcein labeling which indicates active bone remodeling. b BMU viewed with polarized light to identify the cement line which indicates the limit to erosion of the remodeling unit. c Overlay of the two images used for measurement of BMU morphology along with lines depicting how the surface of the cavity was estimated for assessment of resorption cavity width (Rs.Wi) and depth (Rs.De). Resorption cavity area (Rs.Ar) was defined as the space within the entire cavity, Images are shown at original magnification ×250
Rs.Ar, Rs.Wi, and Rs.De were measured by ImageJ software (NIH) with the calcein and ultraviolet light images overlaid (Fig. 1c). All measures were made by a single observer who was blinded to the treatment. The perimeter of the resorption cavity was outlined by using the endpoints, determined by the lamellar contour and the points where the double calcein labels joined, and the deepest lamellae that followed the contour of the calcein label. Rs.Ar was defined as the space within the perimeter, Rs.Wi as the distance between the endpoints, and Rs.De as the largest distance at a 90-degree angle from the projected bone surface to the cement line of the resorption cavity. This Rs.De constituted the maximum final depth of the individual cavity (Fig. 1c). For each animal, between three and five individual sites were analyzed. Parameters from the multiple resorption cavities were averaged to obtain a single value for each animal.
Variables were compared across groups by a one-way analysis of variance (ANOVA) with significance defined as P < 0.05. Separate one-way ANOVAs were run for VEH + ALN groups and VEH + RIS groups. When ANOVA indicated significant differences, post hoc analysis was performed by Fisher’s protected least significant difference (PLSD) test. Unpaired t-tests were used to assess differences in the dose equivalents of RIS and ALN. All data are presented as mean ± standard error.
Results
Treatment with ALN or RIS at doses consistent with those used for treatment of postmenopausal osteoporosis significantly reduced Rs.Ar by −31% (ALN) and −26% (RIS) compared to VEH-treated animals (Fig. 2a). These doses also significantly reduced Rs.Wi (Fig. 2c) by −25% (ALN) and −14% (RIS), but had no significant effect on resorption cavity depth: −14% (ALN) and −13% (RIS) compared to VEH-treated animals (Fig. 2b). There was no significant difference between dose equivalents of RIS and ALN for any resorption cavity parameters.
Fig. 2.
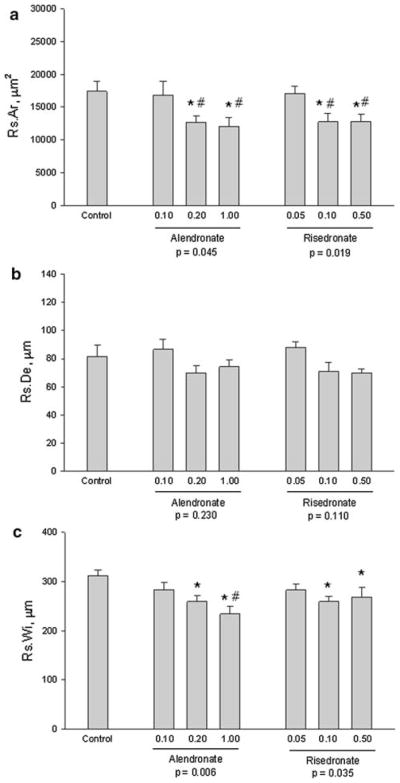
Basic multicellular unit resorption parameters. a Resorption area, b depth, and c width. Differences among groups were assessed separately for control and alendronate groups and for control and risedronate groups with post hoc tests within those groups when appropriate (overall P < 0.05). Data presented as mean ± standard errors from 8–12 animals per group. P < 0.05 vs. control (*) and low dose within treatment (#). No differences were found between RIS and ALN at equivalent doses, nor between the middle and high dose within treatment groups
At lower doses, neither RIS nor ALN significantly altered any parameter of BMU morphology. The highest doses of both RIS and ALN produced changes in BMU morphology similar to those of the osteoporosis-relevant middle doses.
Discussion
BPs are widely used to treat metabolic bone diseases, the most prominent of which is postmenopausal osteoporosis. BPs exert their positive effects on the skeleton by suppressing the amount of bone remodeling [1, 2]. Clinically, this remodeling suppression is assessed by serum/urine biomarkers of collagen breakdown and in some cases through the assessment of bone biopsy samples by histology. In histological analysis, the most prominent effect of BPs is that they reduce the number of active BMUs. In untreated individuals, there tends to be an imbalance between resorption and formation in each BMU, such that each BMU tends to have a net negative bone balance (less bone is formed than is removed) [11, 12]. This, combined with the more transient effect of the greater number of resorption sites produced as a consequence of increased activation frequency, leads to significant bone loss in osteoporosis. By lowering the number of active BMUs with BP treatment, bone loss is slowed.
It has been suggested that in addition to reducing the number of active BMUs, BPs affect the individual activity of osteoclasts within a BMU. Specifically, studies have assessed erosion depth, measured as the distance from the cement line to the bone surface (or estimated bone surface) of an individual BMU, which is indicative of osteoclast activity within a given remodeling site. The results from these analyses are equivocal, showing a tendency for BP treatment to reduce Rs.De, but rarely reaching statistical significance [3, 5, 7–9, 13]. In the current study, we have expanded the morphological assessment of individual BMUs to include both Rs.Ar and Rs.Wi, in addition to the more traditional measure of Rs.De. We show that BPs, at doses equal to and above those used to treat postmenopausal osteoporosis result in smaller Rs.Ar and Rs.Wi of individual BMUs with a nonsignificant trend toward lower Rs.De. These results provide evidence that in addition to the effect of suppressing the number of remodeling units, BPs also reduce the amount of bone resorbed within each BMU. Because Rs.De is not significantly reduced, this is likely caused not by the reduction in individual activity of an osteoclast, but by fewer osteoclasts (perhaps due to increased apoptosis) working within an individual BMU. The implications of these data are significant in that they provide evidence of a second tissue-level mechanism—reduced BMU resorption size—for reduced bone loss with BPs. Of course, this would depend on formation at the individual BMU level being unaffected with BPs. We have previously shown, in these same specimens, that wall width (a measure of osteoblast refilling of remodeling sites) was not altered by BPs [10].
Previous analyses on these same vertebrae have also shown that these BP doses significantly suppressed activation frequency, the rate of new BMU initiation [10], and that the effects were dose-dependent with RIS. In the current work, we show that only the two higher doses of each agent significantly reduced BMU-level resorption activity, with no difference between the two higher doses of RIS. This leads us to speculate that these two effects of BPs, suppression of the number of BMUs and osteoclast activity within the BMU, are controlled independent of one another, at least for RIS.
Several methods have been used to assess resorption cavities morphology [3–6]. These techniques, which were used on human biopsy and animal samples, are similar to the current work in that the BMU is reconstructed by making various assumptions about where the initial bone surface was before resorption. One different aspect of the current work is that we restricted our analysis to those surfaces that were actively forming at the time of sacrifice (defined by the presence of calcein label). This was done in order to assure that the BMUs we measured in the treated animals were in fact active during the period of treatment. If nonlabeled cavities were assessed, it would not be possible to know that the BMU was formed in the presence of BP treatment. As with the current work, most of the previous methods suffer from the limitation of trying to assess a three-dimensional structure (the BMU) in two dimensions. This means that the area, width, and depth we report may not directly correspond to a volumetric measurement. One exception is where the remodeling site was reconstructed in three dimensions. That study found a significant reduction in Rs.De with 3 months of RIS treatment (−25%) although it is not clear whether these measures were restricted to those sites that were remodeled during treatment [3]. Our Rs.De results differ in that we did not show a significant effect of BP treatment, although the magnitude of difference between control and treated animals was comparable (−15%). What the current work shows, however, is that significant effects of BPs exist in other parameters, including Rs.Wi and, most importantly, the resorption cavity area, which previous methods have not assessed. On this basis, we suggest that the assessment of additional parameters such as Rs.Ar can provide important BMU-level information. Ultimately, the development of three-dimensional methods to assess the BMU are essential to advance our ability to study how alterations in its morphology occur with disease and treatment [14].
In conclusion, we show that reductions in resorption at the BMU level likely contribute to the mechanism through which BPs slow bone loss and that assessment of BMU-level resorption is best done through comprehensive measures of Rs.Ar, Rs.Wi, and Rs.De.
Acknowledgments
This work was supported by NIH grants R01 AR51555, R01 AR047838, and T32 AR007581, and a research grant from the Alliance for Better Bone Health (Procter and Gamble Pharmaceuticals and Sanofi-Aventis) and Eli Lilly. Merck and Co. kindly provided the ALN. This investigation utilized an animal facility constructed with support from Research Facilities Improvement Program (grant C06RR10601) from the NIH National Center for Research Resources.
Footnotes
Dr. Allen receives remuneration from, has consultant/advisory role in, and receives funding from The Alliance for Better Bone Health. Dr. Burr receives remuneration from, has consultant/advisory role in, and receives funding from Eli Lilly and The Alliance for Better Bone Health. Dr. Burr has consultant/advisory role in Amgen.
Contributor Information
Matthew R. Allen, Email: matallen@iupui.edu, Department of Anatomy and Cell Biology, Indiana University School of Medicine, 635 Barnhill Drive, MS-5035, Indianapolis, IN 46202, USA
Antonia M. Erickson, University of California Davis Medical Center, Sacramento, CA, USA
Xiang Wang, University of California Davis Medical Center, Sacramento, CA, USA. University of California, Berkeley, CA, USA.
David B. Burr, Department of Anatomy and Cell Biology, Indiana University School of Medicine, 635 Barnhill Drive, MS-5035, Indianapolis, IN 46202, USA. Department of Orthopaedic Surgery, Indiana University School of Medicine, Indianapolis, IN, USA. Department of Biomedical Engineering Program, Indiana University–Purdue University Indianapolis, Indianapolis, IN, USA
R. Bruce Martin, University of California Davis Medical Center, Sacramento, CA, USA.
Scott J. Hazelwood, California Polytechnic State University, San Luis Obispo, CA, USA
References
- 1.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce RW, Paddock CL, Gleason JR, Sletsema WK, Eriksen EF. The effects of risedronate on canine cancellous bone remodeling: three-dimensional kinetic reconstruction of the remodeling site. J Bone Miner Res. 1995;10:211–221. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Solal ME, Shih MS, Lundy MW, Parfitt AM. A new method for measuring cancellous bone erosion depth: application to the cellular mechanisms of bone loss in postmenopausal osteoporosis. J Bone Miner Res. 1991;6:1331–1338. doi: 10.1002/jbmr.5650061210. [DOI] [PubMed] [Google Scholar]
- 5.Roux C, Ravaud P, Cohen-Solal M, de Vernejoul MC, Guillemant S, Cherruau B, Delmas P, Dougados M, Amor B. Biologic, histologic and densitometric effects of oral risedronate on bone in patients with multiple myeloma. Bone. 1994;15:41–49. doi: 10.1016/8756-3282(94)90890-7. [DOI] [PubMed] [Google Scholar]
- 6.Roux JP, Arlot ME, Gineyts E, Meunier PJ, Delmas PD. Automatic-interactive measurement of resorption cavities in transiliac bone biopsies and correlation with deoxypyridinoline. Bone. 1995;17:153–156. doi: 10.1016/s8756-3282(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 7.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- 9.Storm T, Steiniche T, Thamsborg G, Melsen F. Changes in bone histomorphometry after long-term treatment with intermittent, cyclic etidronate for postmenopausal osteoporosis. J Bone Miner Res. 1993;8:199–208. doi: 10.1002/jbmr.5650080211. [DOI] [PubMed] [Google Scholar]
- 10.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen EF, Mosekilde L, Melsen F. Effect of sodium fluoride, calcium, phosphate, and vitamin D2 on trabecular bone balance and remodeling in osteoporotics. Bone. 1985;6:381–389. doi: 10.1016/8756-3282(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen EF, Hodgson SF, Eastell R, Cedel SL, O’Fallon WM, Riggs BL. Cancellous bone remodeling in type I (post-menopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res. 1990;5:311–319. doi: 10.1002/jbmr.5650050402. [DOI] [PubMed] [Google Scholar]
- 14.Tkachenko EV, Slyfield CR, Tomlinson RE, Daggett JR, Wilson DL, Hernandez CJ. Voxel size and measures of individual resorption cavities in three-dimensional images of cancellous bone. Bone. 2009;45:487–492. doi: 10.1016/j.bone.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]