Abstract
Knee ligament injuries frequently happen when the joint transitions from non-weight bearing (NWB) to weight bearing (WB). To gain insight into the mechanism that produces these injuries, physically active females (N = 41) and males (N = 39) underwent measurement of coupled joint displacements [anterior tibial translation (ATT) and varus–valgus and internal–external rotations] and neuromuscular responses as the knee transitioned from NWB to WB in response to a 40% body weight load applied under the control of gravity. The transition from NWB to WB produced no difference in ATT between males and females; however, significant sex-based differences were noted for both transverse and frontal plane knee motions. With the knee NWB, females were in a greater absolute valgus compared to males (6.6 vs. 5.0°), and moved through greater varus motion than males during the transition from NW to WB (2.3 vs. 1.4°), resulting in similar valgus alignment for both sexes at peak WB (4.3 vs. 3.6°). In the transverse plane, the knees of females were positioned in more external rotation compared to males when NWB (1.4 vs. –0.3°), then females externally rotated their knees while males internally rotated their knees during the transition from NWB to WB. This resulted in a 3.4° difference in transverse plane knee position at peak WB (2.3 vs. –1.1°). Our findings suggest that the coupled knee motions produced during the transition from NWB to WB are sex dependent, and may provide insight into the knee motion patterns that place females at increased risk of knee ligament injury.
Keywords: knee biomechanics, tibiofemoral joint, joint laxity, axial compression
Knee motion is influenced by the passive stabilizing structures, loads applied to the joint, active muscle forces, and articular geometry.1 Once a compressive force is applied to the tibiofemoral joint, there is a dramatic increase in joint stiffness and a subsequent reduction in the magnitude of tibiofemoral displacement in response to applied loads.11,2 However, as the knee transitions from non-weight bearing (NWB) to weight bearing (WB), anterior translation of the tibia relative to the femur (ATT) has been observed, 1,3,4 and this is restrained by the anterior cruciate ligament (ACL) in healthy knees. 3 This ATT is likely produced by the anterior directed forces produced by the patellofemoral mechanism (with the knee near extension) 1 and tibial geometry (e.g., posterior directed slope of the tibial plateau), 1,5 which act in combination to produce an anterior directed shear force on the proximal tibia that strains the ACL.3 This is supported by Cerulli et al. 6 who examined the strain behavior of the ACL during a single leg hop. They noted an increase in ACL strain values prior to foot contact (i.e., during the flight phase), a further increase in magnitude of ACL strain during the landing phase until peak ground reactionforce was achieved, and elevated strain values until the limb was unloaded. At an upper limit, excessive axial compressive load applied to cadaver tibiofemoral joints (simulating the reaction forces produced when landing from a jump) can produce ACL ruptures. 7
Fewer studies have examined ACL strain biomechanics during WB in combination with internal–external or varus–valgus torques applied to the knee. Fleming et al. 3 demonstrated the ACL comes under strain in WB, and when combined with applied internal–external and varus–valgus torques, ACL strain values increased across a range of internal torques and remained constant across a range of external and varus–valgus torques. In other work, ACL strain values were 30% greater when a loading impulse creating an external flexion moment about the knee was applied in combination with a valgus moment compared to when the flexion load was applied alone.8 For these reasons, strain on the ACL during the transition from NWB to WB may ultimately depend on the combined (or coupled) motions that occur about the knee at the time of foot strike. This is consistent with a recent systematic review of ACL injury mechanisms which indicated that the ACL is more likely to be loaded when anterior directed loads are applied to the tibia in combination with frontal and/or transverse-plane knee loadings, particularly when the knee is at or near full extension.9 However, the coupled motions that occur in combination with ATT during the transition from NWB to WB are not well understood.
Patient-based questionnaires and video analysis of athletes suffering non-contact ACL trauma haveoffered insight into ACL injury biomechanics.10-12These studies indicate that a large proportion of these injuries appear to occur when landing from a jump or during plant-and-cut movements when the knee is near extension at or near the time the foot contacts the ground. One report11observed an abrupt increase in knee valgus angle within 30–40 ms of foot contact. Hence, under-standing the coupled knee motions that occur during weight acceptance may provide insight into the positioning of the joint at the time the ACL is strained as a result of full WB. Because females are more prone to these non-contact ACL injury mechanisms, it is possible that these coupled knee motionsare different for males and females. In this context, we are not aware of any controlled, laboratorystudies that have examined the 3D kinematics of the knee as it transitions from NWB to WB conditions. Therefore, our purpose was to compare males and females on coupled tibiofemoral joint motions (ATT, varus–valgus, and internal–external rotation motions) and muscle reflex activation (onset time, reflex amplitude) produced when the knee transitioned from NWB to WB.
METHODS
Participants were 41 females (21.9±2.8 years, 58.1±6.0 kg, 162.9±6.7 cm) and 39 males (22.6±2.6 years 81.7±14.0 kg, 177.8±10.1 cm) with similar distributions of anterior knee laxity (mean = 6.6±1.8 vs.6.8±2.3 mm, median=6.3 vs. 6.6 mm) and genu recurvatum (mean 3.8±3.8° vs. 3.3±3.9°, median 3.0 vs. 2.3°) values, and who were physically active (2–10 h/week) with no previous knee injury or metabolic or neurological disorder. Subjects were excluded if they had a body mass index >30 (BMI wt/ht2); if they smoked or could not abstain from alcohol for 24h prior to any testing; if they had a history of knee injury involving the osteochondral surface, ligament, tendon, capsule, or menisci; if they had any medical conditions affecting the connective tissue; if they had a vestibular or balance disorder; or if they were physically active less than 2 or more than 10 h/week. The rationale for recruiting males and females with similar knee laxity values is that there is a direct relationship between anterior knee laxity and ATT during the transition from NWB to WB (i.e., a 1-mm increase of anterior knee laxity is associated with a 0.5-mm increase of ATT).4 Healthy knees were confirmed using items 1–3 of the 2000 International Knee Documentation Committee subjective knee form (IKDC-9).13 All females were tested on a day during the first 6 days of their menstrual cycle to control for hormone effects on joint laxity.14 As part of a larger study,participants attended a session where it was first determined if they met the entry criteria for the study, then written consent was obtained using a form approved by the University Institutional Review Board, and they were measured for height, weight, and pre-screened for anterior knee laxity. Of a total of 80 people screened, 80 were studied. They then completed a familiarization session, approximately 2 weeks before testing, where they were instructed on and experienced all testing procedures by completing the exact tests they would perform on the day of testing. On the day of testing, subjects were measured for anterior knee laxity and genu recurvatum, maximal voluntary isometric (MVIC) contractions of the thigh muscles, and joint kinematics and neuromuscular responses during the transition from NWB to WB. All testing was performed on the dominant limb, defined as the stance leg when kicking a ball.
Anterior knee laxity (AKL) was measured as the anterior displacement of the tibia relative to thefemur at 133 N of an applied load using the KT2000™ Knee Arthrometer (MED-metric Corp; San Diego, CA). Genu recurvatum (GR) was measured with a hand-held goniometer as the amount of maximal active knee extension with the subject supine and the distal thigh elevated on a bolster. The same investigator who had previously established excellent test–retest measurement reliability [ICC(2,3) (SEM) = 0.96 (0.3 mm) for AKL, 0.97 (0.5°) for GR] measured all laxity values. To prepare for surface electromyography (sEMG) measurements, all skin areas were cleaned, then 10-mm bipolar Ag–AgCl surface electrodes (Medicotest Blue Sensor Model #N-00-S; Ambu Products, Germany) were attached over the vastus medialis (VM) and lateralis (VL), and the medial hamstring(MH) and biceps femoris (BF), with a 2.5-cm center-to-center distance. The reference electrode was placed on the contralateral anterior tibial shaft. Manual muscle testing confirmed signal fidelity and absence of cross talk. To normalize muscle responses obtained during testing, participants were positioned in a dynamometer (Biodex Medical Systems Inc., Shirley, NY) at 20° of knee flexion and asked to complete three, 5-s maximal effort isometric knee extension and knee flexion contractions. sEMG signals were recorded using a 16-channel Myopac telemetric system (Run Technologies, Mission Viejo, CA), with an amplification of 1 mV/V, frequency bandwidth of 10–1,000 Hz, CMRR of 90 dB min at 60 Hz, input resistance of 1MΩ, and internal sampling rate of 8 KHz.
Neuromuscular response characteristics and knee kine-matics were then measured during the transition from NWB to WB with the subject positioned in the Vermont Knee Laxity Device (VKLD; University of Vermont, Burlington, VT) using previously established methods4 (Fig. 1). The VKLD measures displacement of the tibia relative to the femur as the knee transitions from non-weight bearing to weight bearing, and characterizes the anterior–posterior load-displacement behavior of the knee.15 Features of the VKLD include the capability to apply quantifiable loads to the tibiofemoral joint under the control of gravity, by first creating an absolute zero shear load condition across the tibiofemoral joint while it is un-weighted to establish a reproducible neutral initial position of the tibia relative to the femur, and then to apply standardized compressive loads through the ankle and hip axes of rotation of the limb to simulate weight-bearing. Subjects were positioned supine in the VKLD with their dominant foot strapped to the foot plate, and the anatomical ankle and hip flexion axes were aligned with the mechanical axes of rotation of the VKLD counterweight system (Fig. 1).With the sEMG electrodes still attached, six-degree-of-freedom position sensors (Mini Birds Ascension Technologies, Burlington, VT) were securely attached to the lateral thigh, patella, and the anteromedial aspect of the proximal tibia of the dominant limb. Hip, knee, and ankle joint centers were estimated using the centroid method as previously described.4 The initial anatomical position was established in the VKLD with the knee fully extended and the 2nd metatarsal aligned with the vertical axis of the VKLD.
Figure 1.
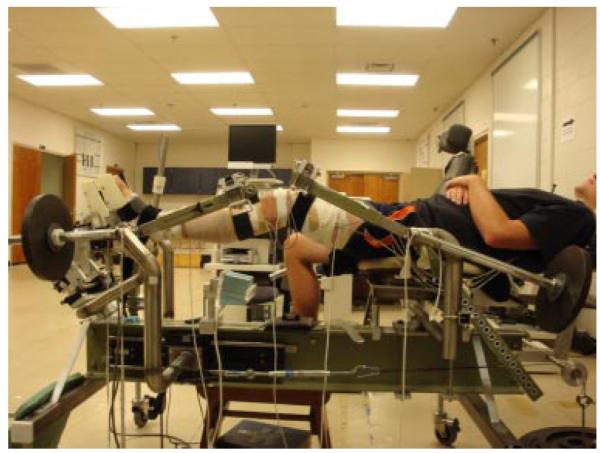
Subject positioned in VKLD with counterweights applied.
Following digitization and initial knee alignment, a counter-weight system was applied to the leg to offset gravity loads acting on the lower extremity.15 The ankle and knee were flexed to 90° and 20°, respectively, and the subjects were instructed torelax their leg muscles. The 20° knee flexion angle is consistent with previous reports of knee flexion at ground contact during landing16 and cutting17 maneuvers, and is also consistent with the knee flexion angle when ACL injury occurs as reported in video analysis-based studies.12,18 Prior to each test trial, three anterior-to-posterior directed forces were applied to the proximal tibia to identify a reproducible reference position of the tibia relative to the femur, and knee flexion angle was reconfirmed within ±5° using a hand-held goniometer and real-time data from the position sensors. These procedures established a resultant zero shear and compressive load across the tibiofemoral joint (confirmed by the load transducer located at the subjects’ foot), and created the same standardized initial conditions for kinematic measurements across all subjects and trials. Data collection began with the subjects’ knee NWB and continued as the foot cradle was unlocked and the compressive force equal to 40% of the subject’s bodyweight was applied longitudinally through the ankle and hip axes of rotation under the control of gravity to simulate WB.4 The subjects were asked to maintain the same 20° knee position upon joint loading. Following two to three practice trials, three trials transitioning from NWB to WB were completed while kinematic data were collected. Three additional trials were completed to collect sEMG data. These data were collected separately because the electromagnetic system caused significant interference with the sEMG signal. Pilot data confirmed strong measurement consistency of the sEMG signal from the thigh muscles between the first and last three trials (all ICC(2,3) >0.98).
Kinematic data were collected (100 Hz) and analyzed using the Motion Monitor (Innovative Sports Training, Chicago, IL) electromagnetic tracking system and software. A six-degree-of-freedom load transducer (Model MC3A, Advanced Medical Technology, Inc; Watertown, MA) located at the foot measured compressive loads at 500 Hz. Force data were offline low-pass filtered at 60 Hz using a 4th order, zero-lag Butterworth filter, and kinematic data were low-pass filtered at 10 Hz using a 4th order zero lag Butterworth filter. The initiation of weight acceptance was defined as the time when the compressive force exceeded 10 N. A segmental reference system quantified the three-dimensional kinematicsof the knee during the transition from NWB to WB. ATT was calculated as the displacement of the sensor located on the proximal shank relative to the sensor on the distal thigh in the A–P plane between NWB (just prior to the initiation of weight acceptance) and peak axial compressive loading (WB). During this same time interval, initial (NWB) and peak (WB) varus–valgus and internal–external rotation positions of the knee were recorded. sEMG data sampled at 1,000 Hz were synchronized with the software’s trigger sweep acquisition mode, using the same foot contact threshold of 10 N. SEMG signals were band pass filtered from 10 to 350 Hz, using a 4th-order, zero-lag Butterworth filter then processed with a centered RMS algorithm for MVIC trials (100 ms time constant) and during weight acceptance(5 ms time constant). Muscle onset (ms) was defined as the time when muscle activation exceeded 5 SD of the baseline sEMG signal for ≥ 10 ms. Muscle amplitude was defined as the mean normalized RMS amplitude (% MVIC) over the time from muscle onset to peak WB load. The average of three trials was analyzed for each measure. T-tests compared males and females on anterior knee laxity, genu recurvatum, and ATT. Separate repeated measures ANOVAs compared males and females on varus–valgus and internal–external rotation positions of the tibia relative to the femur at initial (NWB) and peak WB, and on muscle onset timing and amplitude for the VM, VL, MH, and BF muscles during the transition from NWB to WB. All analyses were evaluated at p ≤ 0.05. Post hoc testing consisted of main effects testing.
RESULTS
Per our screening criteria, females and males had similar anterior knee laxity (6.6±1.8 mm vs. 6.8±2.3 mm, p = 0.711) and genu recurvatum (3.8±3.8° vs.3.3±3.9°, p = 0.605) values, and these values are within the normal ranges previously reported in the literature for physically active males and females.19-22 During the transition from NWB to WB, females and males went through the same amount of knee flexion (8.2 ±3.9° vs. 8.9±3.9, p = 0.442), achieved peak axial loads within a similar time frame (296.6±58.8 ms vs. 316.0±69.0 ms, p 0.181), and produced the same magnitude of ATT(7.6±2.0 mm vs. 7.5±3.1 mm, p = 0.842). Figure 2 displays the typical timing sequence of the dependent variables measured. However, significant sex by weight bearing status interactions were noted for the coupled frontal (p = 0.033) and transverse plane (p = 0.001) knee motions. In the frontal plane, female knees compared to male knees started in more valgus with the joint NWB (Mean = 6.6±2.5° vs. 5.0±3.0°; p = 0.011), moved through greater varus during the transition from NWB to WB (2.3±1.9° vs.1.4±2.0°; p = 0.033; Fig. 3), and ended in similar valgus alignment at the peak WB force (4.3±3.1° vs. 3.6±3.5°; p = 0.333). In the transverse plane, female knees were initially positioned (NWB) in external rotation (1.4±2.2°) while male knees were positioned more neutral (–0.3±2.7°) (p = 0.002). During the transition from NWB to WB, female knees underwent external rotation while male knees internally rotated (0.9±2.2° vs. –0.8±2.0°, p = 0.001) (Fig. 3). This resulted in a 3.4° difference in transverse plane knee position at peak WB for females versus males (2.3±3.3° vs. –1.1°±3.7°, p < 0.001). For muscle reflex amplitudes, a sex by side by muscle interaction was observed (p = 0.014), with females activating their vastus medialis (15.5±7.8% vs. 12.6%±6.5% MVIC) and biceps femoris (7.4%±4.3% vs. 4.9%±3.4% MVIC) muscles to a greater level than males (Fig. 4). There were no sex differences in muscle onset times by sex (p = 0.402), sex by muscle (p = 0.541), or sex by muscle by side (p = 0.722) (Fig. 5). In all subjects, the quadriceps responded before the hamstrings (92.6±19.8 ms vs. 120.9±29.3 ms, p < 0.001) and with higher amplitudes (12.7%±5.6% vs.4.7±3.4%, p < 0.001) in response to the WB load.
Figure 2.
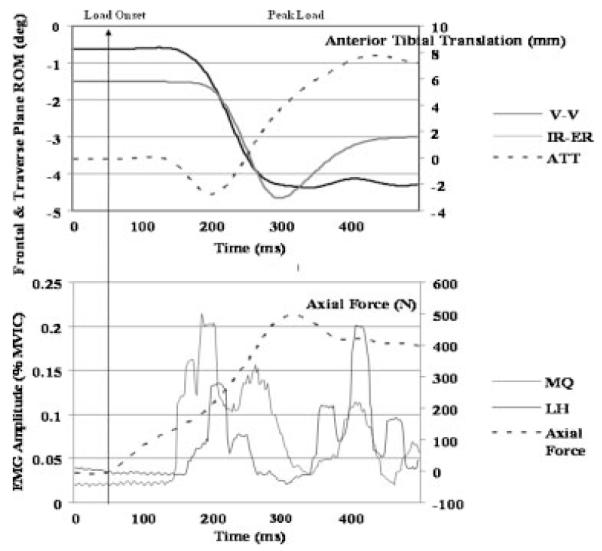
Representative trial showing temporal sequence of axial load, anterior tibial translation, frontal and traverse plane kinematics, and surface EMG amplitude.
Figure 3.
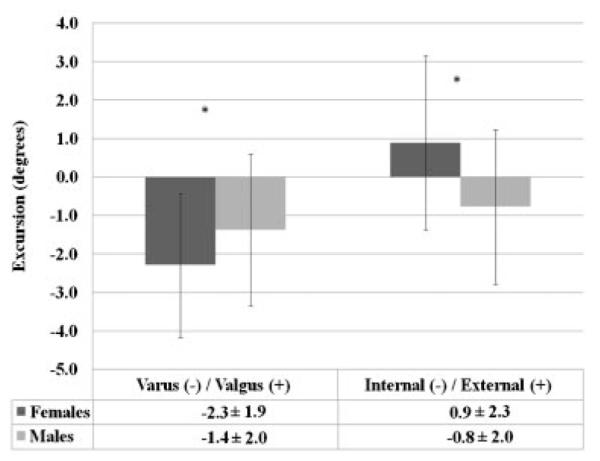
Sex differences in coupled transverse and frontal plane knee motions during the transition from NWB to WB load. *Females ≠ males (p < 0.05).
Figure 4.
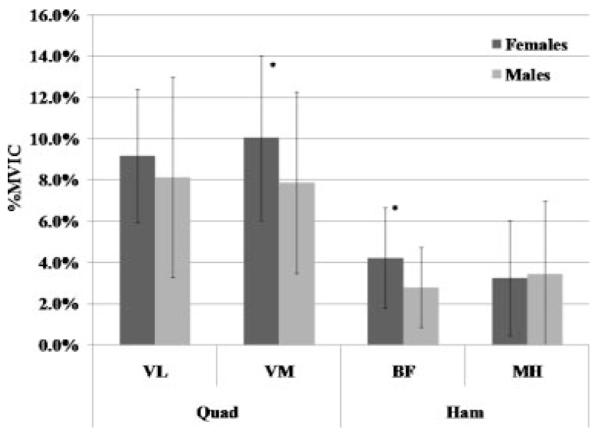
Reflex amplitudes (% MVIC) of the medial and lateral quadriceps (VM, VL) and hamstring (MH, BF) muscles during the transition from NWB to WB. *Females ≠ males (p < 0.05).
Figure 5.
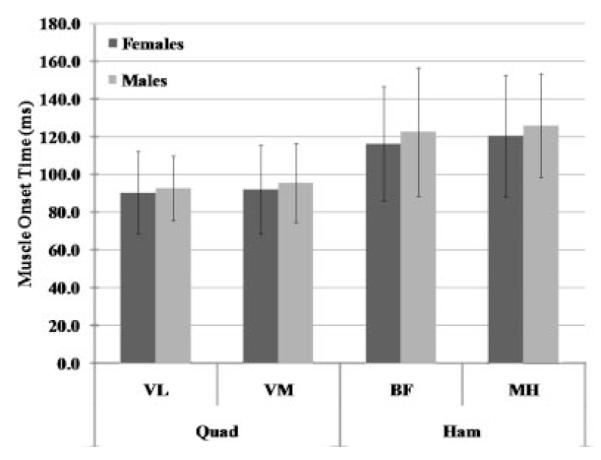
Muscle onset times (ms) of the medial and lateral quadriceps (VM, VL) and hamstring (MH, BF) muscles during the transition from NWB to WB.
DISCUSSION
Prior video-based studies of non-contact ACL injuries revealed that a large proportion of these injuries occur during sports when landing and cutting as the foot contacts the ground with the knee near extension during the transition from NWB to WB.10-12,23 For example, Krosshaug et al.18 estimated that ACL rupture occurred between 25–50 ms after initial contact of the foot with the playing surface with the knee between 18–24° of flexion. To gain insight into the neuro-mechanical behavior of the knee during events that lead up to what many consider a common non-contact injury mechanism, single leg landing, our investigation focused on the transition of the knee from NWB to WB when a gravity controlled load of 40% body weight was applied to the foot with the knee flexed to 20°, and compared males and females on knee kinematics and neuromuscular behavior. To facilitate these sex-based comparisons, two aspects of the study were carefully controlled. First, we selected males and females with similar A–P laxity and hyperextension of the knee in an effort to control for the known effects of knee laxity on knee biomechanics.4 Second, we used the same approach to establish the initial NWB position of the lower extremity for all subjects so that the loads applied to the knee during NWB were the same between subjects. Using this approach, the transition from NWB to WB occurred over an average of 300 ms, with the thigh muscles responding to the application of the WB load with muscle onset times of 90 ms (quadriceps) to 120 ms (hamstrings). Thus, this research model allowed us to capture 90 – 120 ms of kinematic data that was largely controlled by the noncontractile tissues.
Our primary findings were that males and females produced similar magnitudes of ATT during the transition from NWB to WB (anticipated by the a priori matching of A–P laxity values between groups), but this was accompanied by different patterns of coupled rotations of the tibia relative to the femur. When NWB, females were initially positioned in greater knee valgus and external rotation compared to males. During the transition from NWB to WB, varus knee motion accompanied ATT for both sexes, however, females moved into more varus than males, and female knees externally rotated while male knees internally rotated. These coupled motions resulted in similar valgus alignment at peak ATT, but a 3.4° difference in internal–external knee position (females 2.3° external rotation, males – 1.1 internal rotation). Sex differences in neuromuscular control strategies were also observed, with females activating their VM and BF to a greater proportion of their MVIC than males. Based on the known moment arms and independent actions of these muscles,24,25 these activation patterns are consistent with findings of greater varus displacement (greater VM activation) and external tibial rotation motion (greater BF activation) in females compared to males.
The observed sex differences were produced by an externally applied compressive load that acted through the centers of the ankle and hip joints, and occurred in the absence of externally applied torques about the long axis of the lower extremity. Hence, the differences observed were likely produced by sex differences in anatomy (e.g., lower extremity alignment, internal geometry of the knee joint articular surfaces) and/or the neuromuscular control strategies used. For example, females have greater internal femoral torsion, quadriceps and tibiofemoral angles, and genu recurvatum compared to males.21 As we only controlled for sex differences in genu recurvatum, these other alignments may explain, at least in part, the greater initial valgus and external tibial positions in females compared to males when the joint is NWB, and the greater magnitude of tibial external rotation infemales during the transition from NWB to WB. With regard to internal knee joint geometry, recent studies indicate that females have a greater posterior directed slope of the tibial plateau, tend to have smaller lateral-to-medial coronal tibial slopes,26 and have smaller tibial articular surface areas that result in smaller moment arms for the extensor and flexor muscle groups.24 An increase in the posterior directed slope of the tibia, either in the medial or lateral compartments, is associated with an increase in the magnitude of the anterior directed force component of the compressive force that acts on the corresponding articular surfaces of these compartments.26 Our observation that females undergo external rotation of the tibia during the transition of the lower extremity from NWB to WB while males undergo internal rotation may be explained, at least in part, by differences in the posterior directed slopes of the medial and lateral portions of the tibial plateau. Further work is needed to determine the extent to which these known sex differences in anatomy may influence and explain the sex differences in coupled motions and neuromuscular responses that were observed in the current study and are associated with ACL injury.
Although the thigh muscles were activated before peak joint displacements, when considering their relatively low level of activation and the added time required to generate force in the muscle (i.e., electromechanical delay), the observed neuromuscular responses were more likely a result of thetibiofemoral joint displacements, rather than a controlling factor in the joint displacements observed. As such, the early positioning of the tibiofemoral joint during weight acceptance (and before substantial muscle forces can be generated) may have an important effect on the force distribution about the knee and ACL which can be further modified by other external loads applied to the lower extremity during landing and cutting maneuvers. For example, in work by Fleming et al.,3 only anterior shear forces and internal torques were found to strain the ACL when the knee was NWB, and muscle contraction was not present. However, when the knee was WB, external torques and varus–valgus moments also strained the ACL. Therefore, in the normal knee, when ATT is coupled with internal–external or varus–valgus displacements during weight acceptance, ACL strain values may be greater than when the knee remains more neutral in the frontal and transverse planes. Whether the observed sex differences in transverse plane knee motion results in different ACL strain biomechanics when coupled with similar anterior–posterior and varus–valgus knee positions requires further study.
Relating our findings from this laboratory study of single leg transfer from NWB to WB to prior video-based ACL injury research is challenging. While video-based studies of ACL injuries capture thetrauma, they are limited with regard to quantifying the neuromechanical response of the subject’s lower extremity. Our study did not examine ACL trauma; however, we did quantify knee neuromechanics during the transition from NWB to WB such as occurs during a single leg landing. Moreover, every injury involves different combinations of loads applied to the knee. Appreciating these limitations, qualitative comparisons can begin to link our laboratory-based findings to ACL injury events that occur during more complex landing and cutting activities. For example, Olsen et al.12 performed video analysis of ACL injuries produced during landing and reported that the knee was near extension, in a valgus position, and that a certain amount of internal or external rotation of the tibia occurred at the time of injury. In our investigation, the knee was also near extension, in a valgus position, and either underwent external rotation (females) or internal rotation (males) in response to WBWhen considering the coupled motions of anterior translation and rotation of the tibia relative to the femur during the transition from NWB to WB, both external27 and internal torques of the tibia28 have been implicated in the ACL injury mechanism when combined with an anterior directed load to the proximal tibia that is produced by contraction of the quadriceps muscles acting through the patellofemoral mechanism. Further, Krosshaug et al.18 introduced the hypothesis that knee-loading patterns in non-contact ACL injuries may be sex dependent, and cite evidence from prior laboratory-based motion analysis studies that females land with their knee moving into valgus–external rotation while males land with varus–internal rotation.29,30 Although these studies focused on complex landing and cutting activities in comparison to our controlled study, we also found that on average, females underwent external tibial rotation while males internally rotated during the transition from NWB to WB with the knee near full extension.
In summary, coupled knee motions are produced during the transition from NWB to WB, and this occurs during the time frame when ACL injuries are thought to occur. These coupled motions occurred during the application of joint compressive loads and prior to the initiation of muscle forces in response to the applied load. This suggests that the displacements were initially controlled by joint geometry and the ligaments and then, subsequently, by the forces produced by the muscles. Hence, the sex differences in coupled knee motions that we observed during the transition from NWB to WB are more likely produced by sex differences in anatomy than neuromuscular responses, and may shed light into the sex-dependent coupled knee motions patterns reported by Krosshaug et al. during non-contact ACL injuries. However, our findings are limited to a controlled laboratory study of knee biomechanics in response to a relatively low (40% body weight applied under the control of gravity) load that results in peak axial loads of 56%–59% BW with a time to peak load of ~300 ms. While this load was applied under the control of gravity (as occurs during a drop landing), the laboratory conditions used in the current investigation differ in comparison to what occurs during landing froma jump when the axial loads are reported to be higher (e.g., 3–4 × BW) and occur over a shorter period of time (40 – 80 ms). This may be attributed to the preactivation that occursin anticipation of landing. Future work should determine if these same differences occur during common load acceptance activities, and the ultimate impact these differences have on the load distribution of the knee joint and the strain biomechanics of the ACL.
ACKNOWLEDGMENTS
The project described was supported by Grant #R01 AR053172-01A1 from NIH-NIAMS.
REFERENCES
- 1.Torzilli PA, Deng X, Warren RF. The effect of joint-compressive load and quadriceps muscle force on knee motion in the intact and anterior cruciate ligament-sectioned knee. Am J Sports Med. 1994;22:105–112. doi: 10.1177/036354659402200117. [DOI] [PubMed] [Google Scholar]
- 2.Markolf KL, Bargar WL, Shoemaker SC, et al. The role of joint load in knee stability. J Bone Joint Surg. 1981;63-A:570–585. [PubMed] [Google Scholar]
- 3.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34:163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 4.Shultz SJ, Shimokochi Y, Nguyen A, et al. Non-weight bearing anterior knee laxity is related to anterior tibial translation during transition from non-weight bearing to weight bearing. J Orthop Res. 2006;24:516–523. doi: 10.1002/jor.20040. [DOI] [PubMed] [Google Scholar]
- 5.Dejour H, Bonnin M. Tibial translation after anterior cruciate ligament rupture. J Bone Joint Surg. 1994;76-B:745–749. [PubMed] [Google Scholar]
- 6.Cerulli G, Benoit DL, Lamontagne M, et al. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: case report. Knee Surg Sports Traumatol Arthrosc. 2003;11:307–311. doi: 10.1007/s00167-003-0403-6. [DOI] [PubMed] [Google Scholar]
- 7.Meyer EG, Haut RC. Excessive compression of the human tibiofemoral joint causes ACL rupture. J Biomech. 2005;38:2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Withrow TJ, Huston LJ, Wojtys EM, et al. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. 2006;21:977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Shimokochi Y, Shultz SJ. Mechanisms of non contact anterior cruciate ligament injuries. J Athl Train. 2008;43:396–408. doi: 10.4085/1062-6050-43.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boden BP, Dean GS, Feagin JA, et al. Mechanisms of anterior cruciate ligament injury. Orthop. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 11.Krosshaug T, Slauterbeck JR, Engebretsen L, et al. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scan J Med Sci Sports. 2007;17:508–519. doi: 10.1111/j.1600-0838.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 12.Olsen O, Myklebust G, Engebretsen L, et al. Injury mechanisms for anterior cruciate ligament injuries in team handball. Am J Sports Med. 2004;32:1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 13.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 14.Shultz SJ, Sander TC, Kirk SE, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uh BS, Beynnon BD, Churchill DL, et al. A new device to measure knee laxity during weightbearing and non-weight bearing conditions. J Orthop Res. 2001;19:1185–1191. doi: 10.1016/S0736-0266(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 16.Decker MJ, Torry MR, Wyland DJ, et al. Gender differences in lower extremity kinematics, kinetics, and energy absorption during landing. Clin Biomech. 2003;18:662–669. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 17.Sigward SM, Powers CM. The influence of gender on knee kinematics, kinetics and muscle activation patterns during side-step cutting. Clin Biomech. 2006;21:41–48. doi: 10.1016/j.clinbiomech.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: Video analysis of 39 cases. Am J Sports Med. 2007;35:359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 19.Beynnon BD, Bernstein I, Belisle A, et al. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33:1298–1304. doi: 10.1177/0363546505275149. [DOI] [PubMed] [Google Scholar]
- 20.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen AD, Shultz SJ. Sex differences in lower extremity posture. J Orthop Sports Phys Ther. 2007;37:389–398. doi: 10.2519/jospt.2007.2487. [DOI] [PubMed] [Google Scholar]
- 22.Trimble MH, Bishop MD, Buckley BD, et al. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech. 2002;17:286–290. doi: 10.1016/s0268-0033(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Teitz CC. Video analysis of ACL injuries. In: Griffin LY, editor. Prevention of non contact ACL injuries. American Association of Orthopaedic Surgeons; Rosemont, IL: 2001. pp. 87–92. [Google Scholar]
- 24.Buchanan TS, Kim AW, Lloyd DG. Selective muscle activation following rapid varus/valgus perturbations at the knee. Med Sci Sports Exerc. 1996;28:870–876. doi: 10.1097/00005768-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Hirokawa S, Solomonow M, Luo Z, et al. Muscular co-contraction and control of knee stability. J Electromyogr Kinesiol. 1991;1:199–208. doi: 10.1016/1050-6411(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi J, Chandrashekar N, Gill B, et al. The tibial plateau and its influence on the biomechanics of the tibiofemoral joint: a sex-based comparison in a blinded study. J Athl Train. 2008;43:556. [Google Scholar]
- 27.Ebstrup JF, Bojsen-Moller F. Anterior cruciate ligament injury in indoor ball games. Scan J Med Sci Sports. 2000;10:114–116. doi: 10.1034/j.1600-0838.2000.010002114.x. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JA, Coker TP, Heaton LM, et al. Natural history of anterior cruciate tears. Am J Sports Med. 1979;7:305–313. doi: 10.1177/036354657900700601. [DOI] [PubMed] [Google Scholar]
- 29.Chappell JD, Herman DC, Knight BS, et al. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am J Sports Med. 2005;33:1022–1029. doi: 10.1177/0363546504273047. [DOI] [PubMed] [Google Scholar]
- 30.McLean SG, Lipfert SW, Van den Bogert AJ. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004;36:1008–1016. doi: 10.1249/01.mss.0000128180.51443.83. [DOI] [PubMed] [Google Scholar]


