Abstract
Despite decades of research into the effects of exercise on fat metabolism, there is still no clear understanding of how exercise helps to regulate fat mass. Although exercise improves the capacity of muscle to oxidize fat, our studies suggest that moderate duration exercise (≤ 1 hr) has little impact on 24-h fat oxidation.
Keywords: exercise, skeletal muscle, substrate metabolism, oxidative capacity, indirect room calorimetry
INTRODUCTION
Impairments in fat metabolism appear to be important factors in the development of obesity and diabetes. For example, reduced rates of fat oxidation (i.e. increased resting respiratory quotient (RQ)) have been shown to predict weight gain [30], and obese and insulin resistant individuals have a decreased capacity to oxidize fatty acids [11]. Moreover, fatty acid oxidation in isolated skeletal muscle preparations from obese individuals is lower than in muscle from lean individuals [13]. Exercise acutely increases fat oxidation, and endurance training increases the capacity to oxidize fat [10] suggesting that regular exercise could induce loss of fat mass by increasing fat oxidation. Although endurance training increases the capacity of skeletal muscle to oxidize all substrates, including fatty acids, carbohydrate is still the predominant energy source in exercising skeletal muscle. It has been suggested that the major effect of exercise on fat metabolism may occur after exercise, although this has never clearly been demonstrated. Thus, how exercise leads to a reduction in fat mass is still not completely understood. To reduce fat mass, a state of negative fat balance must be achieved. To achieve negative fat balance, one must alter intake or expenditure such that fat oxidation exceeds fat intake. Since fat oxidation is increased during exercise, and since endurance exercise training increases the capacity to oxidize fat, by extension, it is assumed that 1) more fat is oxidized on a day that exercise is performed; and 2) trained individuals will oxidize more fat over 24 compared to sedentary individuals. We have conducted several studies in humans of varying adiposity and training status to determine effects of exercise on 24-h fat oxidation. Our studies have been conducted using whole-room indirect calorimetry. An important element of these studies is that the 24-h measurement period encompasses both exercise and meals, thus reflecting a typical 24-h pattern. This differs from the majority of the studies in the literature on the effects of exercise and exercise training on fat metabolism which have been conducted in laboratory conditions in the fasted state. We have also measured 24-h substrate oxidation under sedentary and exercise conditions in order to provide a more complete picture of the responses to exercise. Before commencing these studies, our hypothesis was that fat oxidation would be elevated on days when exercise was performed, and that fat oxidation would be higher in endurance-trained individuals. To our surprise, we have found that exercise has little, if any, effect on 24-h fat oxidation. Thus, there is an apparent paradox between the effects of acute and chronic exercise on fat metabolism in skeletal muscle versus the effects on 24-h fat oxidation. The purpose of this review is to 1) summarize the effects of exercise on fat metabolism; and 2) discuss the effects of exercise on 24-h fat oxidation.
EFFECTS OF EXERCISE ON FAT METABOLSIM
Before considering the effects of exercise on 24-h fat oxidation and fat balance, it is worthy to briefly review the effects of acute exercise and chronic exercise training on fat metabolism. An extensive review of this topic is beyond the scope of this manuscript, but the reader is referred to excellent recent reviews by Kiens [12], Jeukendrup [10], and Achten [1].
Effects of exercise on fat metabolism in skeletal muscle
The sources of lipid available to skeletal muscle during exercise are circulating very-low-density lipoprotein-triglycerides (VLDL-TG), intramuscular triglyceride (IMTG), and circulating albumin-bound long chain fatty acids (LCFA) derived from lipolysis of subcutaneous and visceral adipocytes. The effects of exercise on oxidization of the different sources of fat have been extensively studied, and the evidence clearly indicates that the primary source of fatty acids (FA) in exercising skeletal muscle is LCFA, especially during prolonged or low-intensity exercise. The contribution of VLDL-TG to total fat oxidation during exercise is low (~5% of total energy expenditure). VLDL-TG hydrolysis is regulated by the activity of lipoprotein lipase (LPL), which is unchanged during moderate exercise, but is markedly elevated following exhaustive or glycogen depleting exercise. Some evidence suggests VLDL-TG may increased after exercise or with an increased intake of dietary fat, but the overall contribution to whole body fat oxidation is still substantially less than that of LCFA. The available evidence also suggests that IMTG may provide up to 10% of total energy expenditure during prolonged exercise (≥ 1 hr), although the contribution may be slightly higher in females, and that oxidation of IMTG may be increased in the post-exercise period, if subjects remain fasted.
The uptake of free fatty acids (FFA) by skeletal muscle during exercise is achieved via the combined processes of diffusion and protein-mediated uptake. Although initial research in this area suggested that uptake and oxidation of FFA was primarily determined by the rate of lipolysis in adipose tissue, more contemporary studies have demonstrated that several transport proteins play an important role in facilitating FFA uptake across the sarcolemma, including fatty acid translocase (FAT/CD36), fatty acid transport protein (FATP), and a plasma membrane bound fatty acid binding protein (FABPPM). LCFA transport within the muscle is also mediated via a cytoplasmic fatty acid binding protein (FABPc).
Transmitochondiral FA transport is dependent on a transport system involving carnitine as a carrier and two carnitine palmitoyl transferases (CPTI/CPTII). CPTI is inhibited by malonyl-CoA, the product of acetyl-CoA carboxylase and substrate of malonyl-CoA decarboxylase. Phosphorylation of these enzymes by 5′-AMP-activated protein kinase (AMPK), sensing conditions of energy deficit (e.g. exercise), results in a decline in malonyl CoA concentrations, relieving malonyl-CoA dependent inhibition, and thereby increasing mitochondrial uptake and oxidation of FA. While this regulation of CPTI appears to contribute to the regulation of fat oxidation during exercise, it is clear that it is not the only factor involved. Mitochondrial uptake of glycolytic products and the availability of free carnitine for the CPTI may also have a significant impact on the uptake and oxidation of FA during exercise.
Endurance exercise training increases the capacity skeletal muscle fat oxidation by increasing mitochondrial density, the activity of enzymes involved in β-oxidation, and oxygen delivery to muscle. Recent evidence also suggests that endurance training increases the gene expression and protein content of several FA transporters, which may aid in the uptake and delivery of FA to mitochondria.
Whether training alters the source of fatty acids oxidized during exercise is not clear. Training increases LPL activity, and LPL activity is higher in trained individuals, which suggest that VLDL-TG oxidation may be increased by training. These adaptations may be augmented by the increased capillarization within the trained muscle, which increases the surface of the endothelium, thereby increasing the binding sites for LPL, but also decreasing perfusion distance from endothelium to plasma membrane. IMTG content has been shown to increase with training, but it appears that this adaptation requires that adequate amounts of fat are included in the diet. Whether IMTG utilization increases with training is controversial.
At the same absolute workload, whole-body lipolytic rates are similar in sedentary and endurance-trained individuals and are not affected by endurance training; thus, delivery of LCFA to skeletal muscle is the same. However, during exercise performed at the same relative intensity, lipolytic rate is greater in trained individuals and is increased with training. Although the mechanism is not known, the increase may be related to the greater absolute work being performed.
Exercise and fat oxidation
It is well-established that whole body fat oxidation increases with exercise intensity up to ~55-65% of VO2max, but decreases at higher exercise intensity [1, 22]. Why fat oxidation decreases at high exercise intensities is not completely understood, but evidence suggests a decrease in FFA availability due to a decrease in blood flow to adipose tissue, a limited capacity per unit time to generate ATP from oxidation of plasma FFA, or a decrease in the activity of CPT1 [1]. The exercise intensity at which maximal fat oxidation rates occur varies according to training status, sex, and mode of exercise. Maximal fat oxidation rates (~05.-0.6 vs. ~0.4-0.5 mg · kg−1 · min) and the intensities at which this occurs (59% - 64% vs. 47-52% of maximal oxygen consumption (VO2max)) are higher in highly trained vs. moderately trained individuals. Maximal fat oxidation rates appear to be higher in women compared to men; fat oxidation rates are higher at a given exercise intensity in females, and occurs at a higher exercise intensity. Although the mechanism is not clear, the ability of females to utilize more fat during exercise may be due to differences in levels of circulating hormones and catecholamines, a more oxidative muscle fiber type distribution, an increased sensitivity to catechomlamine stimulated lipolysis, or increased activity of hormone-sensitive lipase [1]. Maximal fat oxidation rates also appear to be higher during walking and running compared to cycling, which may reflect recruitment of a smaller muscle mess and a lower catecholamine response during cycling [1].
It is also well-established that endurance training increases fat oxidation during submaximal exercise [1]. These adaptations have commonly been observed in response to moderate intensity (60-75% of VO2max) exercise training programs lasting 6-12 weeks. A few studies have shown that daily moderate intensity training sessions (2 hr/day) induce an increase in fat oxidation during exercise within 7-10 days. In addition, sprint interval training at very high power outputs (150-300%) produce similar increases in oxidative capacity as does moderate-intensity, longer duration training programs, and high-intensity interval training (90%) has been shown to elicit similar increases in as little as two weeks [5].
The effect of resistance training of fat oxidation has not been as well-studied as the effects of endurance training. Most studies in this area have measured the acute effects of resistance exercise on fat oxidation in the period after exercise. These studies have reported that RQ is significantly decreased immediately following or the day after a bout of resistance exercise relative to a non-exercise control day, findings which have been interpreted to suggest that fat oxidation is enhanced in the post-exercise period [1]. The effects of regular resistance training on fat oxidation are unclear with some studies reporting an increase, and others showing no change [1].
EFFECTS OF EXERCISE ON 24-H FAT OXIDATION
To examine the independent effects of exercise on 24-h substrate oxidation requires proper attention to the state of energy balance and macronutrient intake. If subjects are in positive energy balance (intake>expenditure), carbohydrate oxidation increases and fat oxidation decreases [9]; conversely, if subjects are in negative energy balance (intake<expenditure) carbohydrate oxidation decreases and fat oxidation increases. Macronutrient intake will also affect fat oxidation. Carbohydrate overfeeding yields an immediate and profound shift in fuel utilization, suppressing the oxidation of fat and increasing carbohydrate oxidation and energy expenditure. In contrast, fat is not effective in promoting its oxidation during overfeeding and also does not stimulate an increase in energy expenditure [8].
Pre-study diet and exercise can also affect fat oxidation. For example, increasing the intake of dietary fat from 20 to 50% causes a substantial reduction in 24-h RQ within 24-hs [24]. With regards to exercise, Schrauwen et al. [25] have demonstrated that performance of glycogen-depleting exercise performed the day before measurement in a room calorimeter increases fat oxidation in the subsequent 24-h period. Thus, studies aimed at determining the independent effects of exercise on 24-h fat oxidation should employ procedures to control pre-study exercise as well as energy and macronutrient intake.
Early studies
Relatively few studies have considered the effects of exercise on 24-h fat oxidation. It is likely that the small size of some of the earliest room calorimeters prohibited the use of exercise equipment. In addition, early calorimeters had a slower response time and could not accurately measure energy expenditure and substrate oxidation during short intervals. One of the earliest studies utilizing indirect room calorimetry to study the effects of exercise on 24-h substrate oxidation was performed in the calorimeter located in Lausanne, Switzerland [2]. Ten male subjects were studied for 42 hrs, and the primary aim was to determine the effects of exercise on energy expenditure. The subjects entered the room on day 1, and performed 3 hrs of exercise at 50% of VO2max on day 2. RQ was lower in the period after exercise compared to the same period on the non-exercise day, leading the authors to conclude that fat oxidation was increased by exercise. However, it is likely that subjects were in a state of negative energy balance on the exercise day, which would have artificially elevated fat oxidation. Indeed, it was estimated that the EE during the 3 hr exercise trial was 2100 kcal, but intake was only ~800 kcal higher on the exercise day. There was also no indication of pre-study dietary or exercise control.
In a subsequent study performed at the University of Alabama, Treuth et al. [28] compared the effects of low- versus high-intensity exercise on 24-h energy expenditure and substrate oxidation. Eight women were studied over 24-h periods that involved either low-intensity (LI) exercise (60 minutes of stationary cycling at 50% of VO2max) or high-intensity (HI) exercise with an identical work output (interval cycling with 2 minutes exercise at a workload corresponding to 100% VO2max with 2 minutes recovery). Pre-study dietary control was achieved using food records to record intake for three days and instructing subjects to consume the same diet prior to each testing day. Energy intake during the calorimeter stay was 1.3 times the resting metabolic rate, with a macronutrient composition of 65% carbohydrate, 20% fat, and 15% protein. As a group, subjects were in energy balance during the LI trial, but were in negative energy balance during the HI trial; this is because EE was significantly higher (~2045 vs. 1885 kcal) during the HI trial. Despite the fact that RQ was significantly lower during LI exercise (0.85 ± 0.03 vs. 0.93 ± 0.05), there were no differences in RQ over 24-hs (0.86 ± 0.03 vs. 0.87 ± 0.02, LI and HI, respectively) or during sleep (0.84 ± 0.03 vs. 0.84 ± 0.02, LI and HI, respectively). 24-h fat oxidation was 79 ± 21 vs. 79 ± 24 grams for the LI vs. HI protocol. It was concluded that 24-h fat oxidation was similar because although more lipid was used during exercise in the LI trial, more lipid was used after the HI exercise. In a similar study, Saris and Schrauwen at Maastricht University [23] studied eight obese men (body mass index (BMI) = 31± 1 kg/m2) in a room calorimeter over a period of 2 days. On alternate days, subjects performed an equivalent amount of either HI or LI exercise. In the LI protocol, subjects cycled for 60 min at 38% of the maximal work capacity (maximal watts, Wmax). In the HI protocol subjects cycled for 30 min alternating between 80% and 50% Wmax for 2.5 min intervals. Pre-study dietary control was achieved by providing subjects fixed amounts of food that were based on a food intake diary completed prior to the study. During the calorimeter measurements, subjects were fed a diet that achieved individual energy balance, and the macronutrient composition of the diet was 37% fat, 48% carbohydrate, and 15% protein. Although RQ during the LI exercise was significantly lower than during the HI exercise (0.91 ± 0.00 vs. 0.93 ± 0.01), there were no differences in 24-h RQ (0.89 ± 0.01 for both conditions). Interestingly, in the post-exercise period (2 hrs after each exercise bout), RQ tended to be higher after LI compared to HI exercise (0.90 ± 0.01 vs. 0.87 ± 0.02, P=0.06). The authors concluded that differences in substrate oxidation during HI and LI exercise are compensated for during post-exercise period, leading to similar substrate oxidation patterns of 24-h.
Studies at the University of Colorado
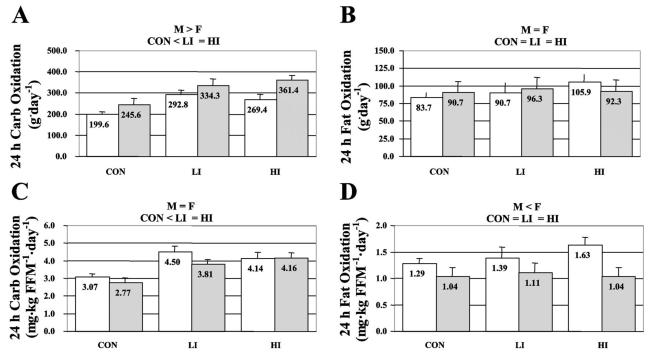
We have performed several studies in different subject groups to examine the effects of exercise on 24-h fat oxidation. In our first study [15], 16 moderately active (3-5 hrs/wk of exercise), non-obese adults (8 men and 8 women) were studied on three occasions; a sedentary day (Con), a LI exercise day, and a HI exercise day. LI and HI exercise consisted of 400 kcal of continuous stationary cycling at 40% of 70% of VO2max. Strengths of our study design were 1) the inclusion of a sedentary control day, which permitted a comparison between days with and without exercise; 2) precisely matching the EE during exercise under the LI and HI conditions, rather than total work, with workloads determined prior to the calorimeter testing using a metabolic cart; 3) achieving energy balance for each individual subject by setting energy intake to energy expenditure measured during a preliminary stay in the calorimeter (mean energy balance across conditions was 12.5 ± 29.2 kcal for Con, −6.8 ± 42.7 kcal for LI, and −54.5 ± 24.5 kcal for HI (NS); and 4) imposing strict dietary controls prior to each calorimeter stay; for 3 days before each calorimeter stay, subjects were provided a diet (30% fat, 15% protein, 55% carbohydrate) estimated to meet free-living energy requirements (RMR × 1.5-1.8, depending on self-reported physical activity level). Although RQ during the exercise differed significantly according to exercise intensity (0.88 ± 0.01, 0.91 ± 0.01 for LI and HI, respectively), 24-h RQ was not affected by exercise intensity (0.87 ± 0.01, 0.87 ± 0.01 for LI, and HI, respectively), and did not significantly differ from non-exercise control condition (0.86 ± 0.01). Both 24-h EE and carbohydrate oxidation were significantly elevated on the exercise days (Con < LI = HI), but 24-h fat oxidation was not significantly different across conditions (Figure 1). Thus, in agreement with previous studies, we found no effect of exercise intensity on 24-h fat oxidation, but our study extended the results of the previous studies by also demonstrating that fat oxidation on days with exercise is not different from a sedentary control day.
Figure. 1.
Absolute (A and B) and relative (C and D) 24-h carbohydrate (Carb) and fat oxidation in men (M; gray bars) and women (F; open bars). Values are means ± SE; nos. within bars are means. Con, no-exercise day; LI, low-intensity aerobic exercise day; HI, high-intensity aerobic exercise day; FFM, fat-free mass. (P > 0.05).
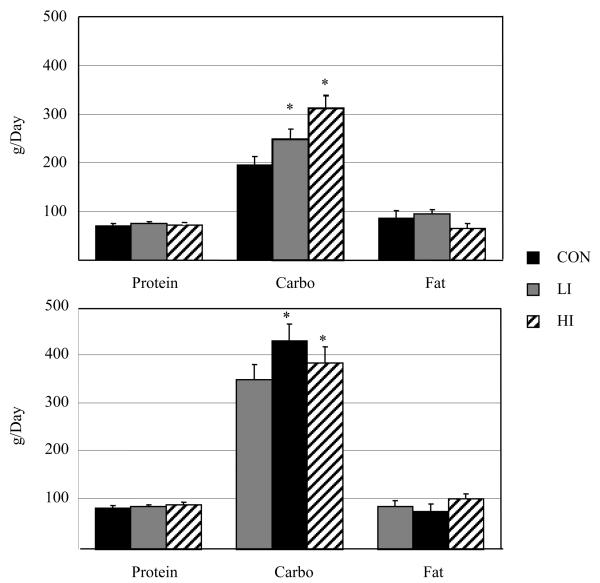
To determine the effects of obesity on 24-h fat oxidation, we have performed a similar study in 15 sedentary obese (BMI ≥ 30 kg/m2) and 11 sedentary lean (BMI ≤ 25 kg/m2) individuals [17]. Subjects were studied on a non-exercise control day (CON), on a LI exercise day (60 minutes of stationary cycling at 40% of VO2max), and a HI exercise day (30 minutes at 70% of their VO2max). Individual energy balance was tightly controlled (~ ± 200 kcal) by setting energy intake to EE measured during a preliminary calorimeter stay and adjusting for the expected exercise EE, and pre-study dietary control was employed as in our previous study. In obese subjects, 24-h RQ did not significantly differ under CON (0.88 ± 0.01), LI (0.90 ± 0.01), or HI (0.88 ± 0.01) conditions. In lean subjects, 24-h RQ was significantly higher during HI (0.89 ± 0.01) compared with the LI (0.86 ± 0.01) and CON (0.86 ± 0.02). 24-h fat oxidation did not differ across conditions and was 90 ± 13, 79 ± 16, 86 ± 15 g/day in obese individuals (CON, LI, and HI, respectively), and 92 ± 13, 92 ± 16, 73 ± 15 g/day in lean individuals (Figure 2). Thus, consistent with our previous studies, there was no evidence that fat oxidation was increased with exercise. Eleven of the obese individuals were restudied after achieving a diet-induced weight loss (9.8% ± 3.0%), and 24-h RQ was still not significantly different across conditions (0.87 ± 0.02, 0.87 ± 0.02, 0.84 ± 0.01 for CON, LI, and HI, respectively) [19], suggesting that weight loss does not increase daily fat oxidation on days when exercise is performed.
Figure 2.
24-h substrate oxidation in obese (TOP) and lean (bottom) subjects. Con, no-exercise day; LI, low-intensity aerobic exercise day; HI, high-intensity aerobic exercise day
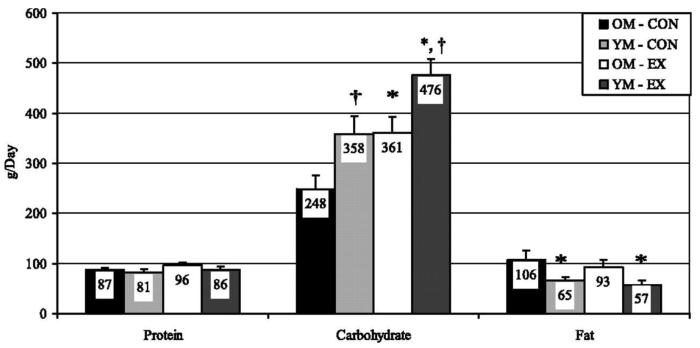
We also compared the effects of exercise on 24-h substrate oxidation in older (60-75 yr, n = 7) and younger (20-30 yr, n = 7) men [18]. The men were studied on a day without exercise (CON) and a day with exercise (EX, 300 kcal of stationary cycling performed at 60% of VO2max). We used the same methods to achieve energy balance as in our previous studies, and imposed the same level of pre-study dietary control. In agreement with our other studies, exercise did not affect 24-h RQ in either the older (0.85 ± 0.05 vs. 0.87 ± 0.03 on the CON and EX days, respectively) or younger men (0.90 ± 0.03 vs. 0.92 ± 0.03 on the CON and EX days, respectively). Fat oxidation (Figure 3) was also not different on the CON and EX day in either the older (106 ± 21 vs. 98 ± 14 vs. g/day, respectively) or younger men (66 ± 8 vs. 57 ± 9 g/day).
Figure 3.
2. 24-h Substrate oxidation in older (OM) and younger men (YM) on the Con and Ex days. Error bars are SE. *YM vs. OM, P < 0.05. †Con vs. Ex, P < 0.05.
Effects of endurance training
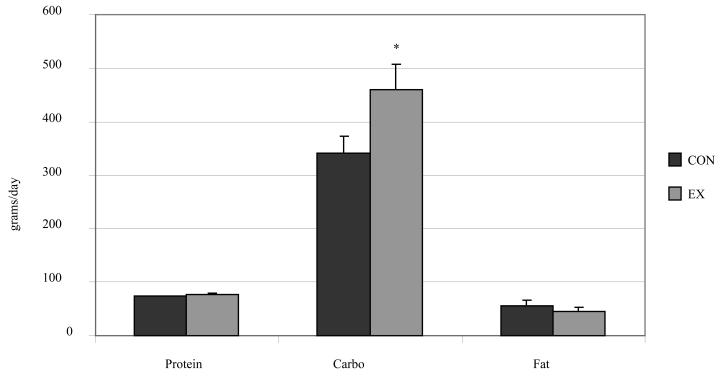
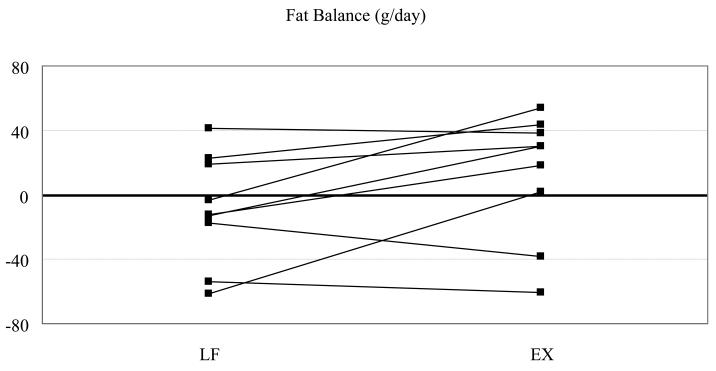
To our knowledge, no studies have considered the effects of endurance training on 24-h fat oxidation. Since training increases fat oxidation during exercise, it is widely assumed that 24-h fat oxidation will be higher in trained individuals on a day when exercise is performed. To address this, we have recently studied n a group of endurance-trained individuals under non-exercise and exercise conditions [20]. During the exercise condition, subjects performed 60 minutes of stationary cycling at 55% of VO2max, an intensity which should be close to the maximal fat oxidation rates [1]. Pre-study dietary control and energy balance was achieved as in our previous studies. We have studied nine competitive runners and triathletes (5 females, VO2max = 46.1 ± 1.5 ml/kg/min; 4 males, VO2max = 54.2 ± 3.6 ml/kg/min, with VO2max measured using stationary cycling ergometry. All subjects reported a minimum of 5 hours per week of endurance training (average = 7 ± 1 hrs). 24-h RQ was 0.90 ± 0.04 (mean ± SD) on the non-exercise day (CON), and 0.92 ± 0.04 on the exercise day (EX). 24 h carbohydrate oxidation was significantly higher in the EX compared to the CON day (459 ± 45 vs. 342 ± 31 g, mean ± SE), but 24 h fat oxidation was unchanged (55 ± 9 vs. 44 ± 8 g on the CON and EX day, respectively) (Figure 4). Fat balance was also not affected by exercise (−4 ± 10 vs. 26 ± 10 g). Strikingly, 5 of the 9 athletes achieved negative fat balance on the CON day, but only 2 of these subjects achieved negative fat balance on the exercise day (Figure 5). Thus, even in endurance-trained individuals, we see no evidence of an increase in 24 h fat oxidation on the day when exercise is performed. It is possible that 24 h oxidation would be higher in endurance-trained individuals compared to untrained individuals. We are currently completing studies in sedentary individuals using the same exercise protocol (55% of VO2max for 1 h) which will address this question.
Figure 4.
24 h substrate oxidation in endurance-trained individuals (N=9) studied under sedentary (CON) and exercise (EX) conditions. *EX vs. CON, P<0.05
Figure 5.
Individual fat balance values in endurance-trained individuals (N=9) studied under sedentary (CON) and exercise (EX) conditions.
Resistance exercise and resistance exercise training
We have also studied the effects of acute resistance exercise on 24-h fat oxidation [14, 16], employing the same dietary controls as in our other studies. For obvious reasons, performing resistance exercise in a room calorimeter presents some unique challenges. Thus, resistance exercises were performed using an exercise machine that could be easily used in the calorimeter (see description in [14]). In these studies, subjects performed a 60-minute circuit consisting of 4 sets of 10 exercises at 70% of exercise-specific 1 RM. During sets 1-3, subjects performed 10 repetitions, but on the 4th set, subjects exercised to failure. Exercises were performed in pairs (bench press and standing mid-row; bi-lateral leg extension and unilateral leg curls; triceps extension and biceps curl; abdominal crunch and military press) and were repeated every 3 minutes. After completion of each exercise pair, subjects were permitted to rest until the next 3-minute interval. We found that 24-h fat oxidation on the day that resistance exercise was performed (68 ± 13 g/day) was not different to that measured on the non-exercise control day (80 ± 17 g/day) [16], or a day where stationary cycling was performed (~1 hr at 70% VO2max) (75± 12 vs. 95 ± 18 g/day, respectively) [14].
We are aware of only 1 study that determined the effects of strength training on 24-h fat oxidation [27]. Thirteen older women (67 ± 1yr) were studied before and after a 16 wk resistance exercise training program. Diet for the three days prior to and during the calorimeter stays was identical before and after training. Body weight, body fat, and fat free mass did not change. 24-h EE was unchanged, but 24-h RQ (0.90 ± 0.01 vs. 0.82 ± 0.01) and 24-h carbohydrate oxidation (180 ± 4 vs. 113 ± 10 g/day) significantly decreased, and 24-h fat oxidation increased (42 ± 6 vs. 81 ± 7 g/day). Energy balance was not reported, but it appears that subjects were in positive energy balance during both stays, which would have the tendency of increasing carbohydrate oxidation. Whether the older age of these individuals contributed to this result is not clear. However, it is worth noting the baseline 24-h fat oxidation in this group (42 ± 6 g/day) is much lower than we have observed in our studies of either younger men and women (~60-90 g/day) or older men (~90-100 g/day).
WHY DOESN'T 24-H FAT OXIDATION INCREASE WHEN EXERCISE IS PERFORMED?
If acute exercise and chronic exercise training induce changes in skeletal muscle that favor an increase in fat oxidation, why doesn't 24-h fat oxidation increase when exercise is performed? The most likely factor is the effect carbohydrate intake on lipolysis and fat oxidation. It is important to note that most studies of the effects of exercise on fat metabolism have been performed with subjects in the fasted state. In room calorimeter studies, the length of the measurement period (≥ 24 hrs) necessitates that subjects be fed meals. Thus, in the room calorimeter studies performed to date, exercise has been performed in the post-absorptive or post-prandial state. We would argue that this approach represents a more typical daily pattern for most individuals. However, ingesting as little as 60 g (240 kcals) of carbohydrate during the hour before exercise can reduce lipolysis and fat oxidation during subsequent exercise [3], and the increase in fat oxidation during exercise can be blunted for up to six hours following consumption of a meal [21]. This is largely caused by an insulin-induced suppression of lipolysis [7, 21], but a decrease in CPT activity has also been suggested [4]. Consequently, the exercising muscle will increase the oxidation of glycogen to compensate for the decrease in fat oxidation [7]. Moreover, the endurance training-induced increase in fat oxidation is prevented when a carbohydrate rich diet is consumed [6].
Another possible explanation for the lack of evidence supporting an effect of exercise on 24 hr oxidation is possible that the magnitude of increase in fat oxidation (e.g. <10 grams/day) is below the sensitivity of most room calorimeters. The trends we see would tend to argue against this possibility. In our studies, we have consistently observed a slightly higher RQ and higher fat oxidation in most individual subjects on the days that exercise is performed, even in endurance trained individuals (Figure 4) Another factor to consider in the room calorimeter studies is that total EE is lower than would be observed in free-living individuals. However, in our studies, even though 24-h EE is higher in the endurance-trained individuals compared to untrained individuals, 24-h RQ is increased on days when exercise is performed.
It is important to note that our studies have been performed with subjects in energy balance. Although studying subjects in energy balance may be perceived as artificial situation, the aims of our studies to date has been to determine the independent effects of exercise on fat oxidation, which can only be done if subjects are in energy balance. This may not be the ideal metabolic context in which the effect of exercise would emerge. Exercise may prime peripheral tissues to respond differently to a metabolic challenge, like overfeeding or calorie restriction. For example, Smith and colleagues have eloquently demonstrated that adaptation to an increase in dietary fat is more complete when high levels of physical activity are performed [26]. Thus, the impact of exercise may become apparent in the free-living environment, under certain metabolic challenges or feeding patterns that we have yet to recapitulate in the laboratory.
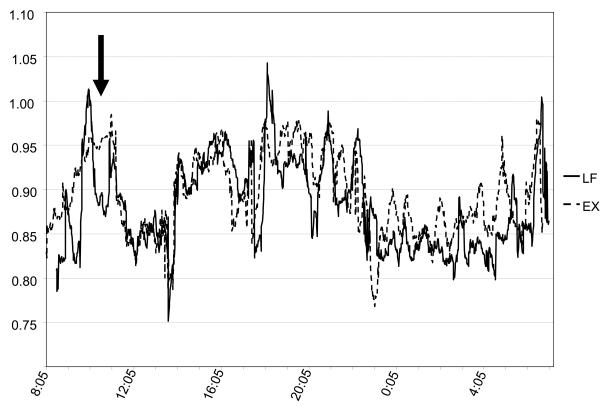
It could be argued, even though exercise does not impact 24 h RQ or fat oxidation, exercise may improve the ability to modulate fuel utilization. This could thereortically lead to a compensation in fat oxidation in the post-exercise period, perhaps during sleep. For example, Schrauwen and Saris reported that differences in substrate oxidation during low- and high-intensity exercise were compensated in the post-exercise period. In that study, RQ was lower during exercise on the LI day compared to the HI day, but tended (P=0.06) to be higher (on average) during the 2 hrs immediately after exercise. However, sleeping RQ was not affected, and fat balance did not differ between conditions. Importantly, subjects were in positive fat balance under both conditions. Thus, in the context of 24 hr fat balance, small differences in fat oxidation in the post-exercise period appear to have no affect on fat balance. We routinely compare 24 h RQ profiles within subjects, and have not found evidence that a meaningful compensation occurs, even in endurance-trained individuals (Figure 6). Finally, in most of the subjects we have studied, 24 h RQ is slightly higher and fat oxidation is slightly lower on the exercise day compared to the non-exercise day, although on a group level, these differences are not significant. This suggests that the effects of diet in modulating 24 h substrate oxidation are much more potent than the effects of exercise, assuming energy balance.
Figure 6.
Representative 24 h RQ profile in an endurance-individual under sedentary (CON) and exercise (EX) conditions. The bolded arrow indicates the exercise period.
The finding that exercise does not increase 24-h fat oxidation when individuals are studied in energy balance provokes two questions. First, how do endurance-trained individuals maintain a low fat mass? Fat mass will not decrease unless fat oxidation exceeds fat intake. Thus, it is possible that endurance-trained individuals maintain a low fat mass because their habitual fat intake is lower than their total fat oxidation. It is also possible that individuals participating in regular endurance training are in a state of negative energy balance on most of their training days. It seems reasonable that an individual expending high levels of energy (e.g. 800 or more kcals per day) may not completely replace the calories expended during training. Even if this difference were small (e.g. <100 kcal), it would create a state of negative energy balance and increase 24 h fat oxidation, and the repetitive effect over sequential days could have a substantial effect on fat mass. However, our ability to detect such small differences, especially in free-living individuals, is limited. Another factor to consider is the effect of consecutive days of high-intensity training. As mentioned previously, glycogen-depleting exercise increases fat oxidation in the subsequent 24-h period [25]. Thus, if glycogen stores are not completely restored prior to the subsequent exercise training session, fat oxidation may be increased. Additionally, it should be mentioned that endurance-trained individuals are likely to perform some training sessions for longer durations than we have studied so far. Since LCFA becomes a much more important fuel source with increasing exercise duration, and also because this type of training is likely to deplete muscle glycogen stores, it is possible that 24 fat oxidation is increased in response to long-duration exercise bouts (e.g. > 1 hr). Finally, we have only studied responses to cycling exercise, which as discussed above, may have lower maximal fat oxidation rates compared to running and walking.
The second question is how can exercise be used to increase fat oxidation in those individuals who desire to use exercise to lose fat mass? For example, since the increases in fat oxidation are most pronounced in the fasted state, would exercising in the fasting state promote an increase in 24-h fat oxidation? This is an intriguing possibility, but it cannot be ruled out that there would be compensatory decrease in fat oxidation in the post-exercise period once a meal was consumed. Moreover, since fat oxidation is suppressed for up to 6 hours following consumption of carbohydrate, it is not likely that most individuals would find this to be an appealing option. Another possibility is that 24-h fat oxidation may be increased by altering the type of fat consumed. For example, Votruba et al. [29] have demonstrated that prior exercise increases the oxidation of dietary oleate but not dietary palmitate, suggesting that exercise increases monounsaturated fat oxidation more than saturated fat oxidation. Thus, recommendations for exercise for the purposes of reducing fat mass may need to be specific for dietary fat composition.
CONCLUSIONS
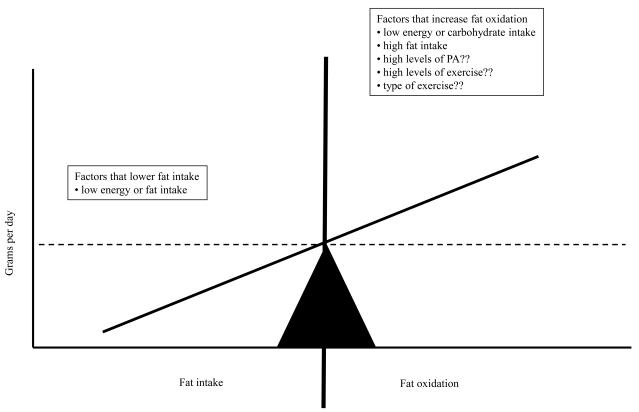
Implicit in our review is the notion that in order to achieve a decrease in fat mass, a state of negative fat balance must be achieved. A model of how negative fat balance may be achieved is presented in Figure 7. To achieve negative fat balance, one must alter intake or expenditure such that fat oxidation exceeds fat intake. Energy and macronutrient intake are powerful modulators of fat balance. If energy intake is less than expenditure, fat oxidation will increase, independent of macronutrient intake, inducing a state of negative fat balance. Negative fat balance can also be achieved even when in energy balance, provided that fat intake is less than oxidation. Ironically, high fat diets will increase fat oxidation, but in this instance, negative fat balance would only be achieved if the oxidation rate exceeds the intake. In our model, the effects of high levels of physical activity and exercise remain unclear. It is widely assumed by both the scientific community and the general public that high levels of physical activity and exercise would induce an increase in fat oxidation and therefore negative energy balance. Our studies would suggest that energy and macronutrient intake is a more important modulator of daily fat balance, and therefore, that exercise recommendations made for the sake of regulating fat mass cannot be made without also considering energy and macronutrient intake. Unfortunately, we cannot at this time we cannot make recommendations of how energy and macronutrient intake can be modulated to induce negative fat balance. We can state with a degree of confidence that if energy intake is maintained below expenditure, than exercise will induce a state of negative fat balance. However, whether manipulations of macronutrient intake will be as effective remains to be demonstrated. Finally, our studies have only considered acute exercise, i.e. exercise performed on a single day in the calorimeter. The effects of consecutive days of exercise on fat balance remains to be studied.
Figure 7.
Proposed model to explain the effects of dietary intake and fat oxidation on fat balance. Negative fat balance is achieved when energy expenditure exceeds energy intake or when fat oxidation exceeds fat intake. Although the effects of dietary manipulation on fat balance is well understood, the effects of exercise and physical activity on fat balance are not as well characterized.
The evidence that level of exercise affects body weight is strong but our incomplete understanding of why could limit our ability to develop optimum strategies for using exercise to address the current epidemic of obesity. On one hand, we can just be content with a recommendation to the population to increase exercise. However a more complete understanding of the impact of exercise on energy balance may help develop more specific and better ways to optimize the impact of exercise on body weight. This will require studies that go beyond assessing the amount and type of energy intake in response to exercise and the impact of exercise on macronutrient oxidation rates. This will also require studies where exercise and energy intake are combined in ways that reflect how exercise is done in daily life. While these studies are difficult, we believe it we must understand the interaction of exercise and food intake on energy and macronutrient balance that may provide the most useful information.
Although we do not discount the numerous benefits of regular endurance exercise on various aspects of health (e.g. improving lipid profiles and insulin sensitivity), we do think it is time to reconsider how we promote exercise as a means of inducing fat loss. Indeed, the plethora of studies that demonstrate that exercise produces less than an expected weight loss appears consistent with our conclusion that in non-fasted individuals, exercise has little effect on daily fat oxidation. We offer that in individuals performing moderate amounts of exercise (e.g. < 1 hr/day), the primary benefit of endurance exercise on body weight may be in preventing additional weight gain or in preventing weight regain after weight loss, but understanding exactly how to prescribe exercise to achieve fat mass loss requires further investigation. Finally, we suggest that it is time to put the myth that low-intensity exercise promotes a greater “fat burn” to rest. Clearly, exercise intensity does not have an effect on daily fat balance, if intake is unchanged. We must educate the public that participation in moderate doses of exercise will not burn more fat unless changes are made to energy or fat intake.
SUMMARY.
Exercise increases the capacity of muscle to oxidize fat, but moderate duration exercise (≤ 1 hr) does not impact 24-h fat oxidation or fat balance.
ACKNOWLEDGEMENTS
These studies could not have been performed without the assistance of the Nursing, Clinical Lab, and Dietary Staffs of the University of Colorado Denver Clinical Translational Research Center (CTRC). We are grateful for their support.
Funding: The work described within has been supported by the following grants
Institutional National Research Service Award (NIH AG00279) and Mentored Scientist Award (NIH K01DK061348) (Dr. Melanson)
NIH R01 DK42549, HL 049331 (Dr. Hill)
The University of Colorado GCRC (NIH M01 RR00051)
References
- 1.Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20(7-8):716–27. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bielinski R, Schutz Y, Jequier E. Energy metabolism during the postexercise recovery in man. Am J Clin Nutr. 1985;42(1):69–82. doi: 10.1093/ajcn/42.1.69. [DOI] [PubMed] [Google Scholar]
- 3.Coyle EF, Coggan AR, Hemmert MK, Lowe RC, Walters TJ. Substrate usage during prolonged exercise following a preexercise meal. J Appl Physiol. 1985;59(2):429–33. doi: 10.1152/jappl.1985.59.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Coyle EF, Jeukendrup AE, Wagenmakers AJ, Saris WH. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am J Physiol. 1997;273(2 Pt 1):E268–75. doi: 10.1152/ajpendo.1997.273.2.E268. [DOI] [PubMed] [Google Scholar]
- 5.Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 6.Helge JW, Richter EA, Kiens B. Interaction of training and diet on metabolism and endurance during exercise in man. J Physiol. 1996;492(Pt 1):293–306. doi: 10.1113/jphysiol.1996.sp021309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol. 1997;273(4 Pt 1):E768–75. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
- 8.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62(1):19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr. 1983;38(6):989–98. doi: 10.1093/ajcn/38.6.989. [DOI] [PubMed] [Google Scholar]
- 10.Jeukendrup AE. Regulation of fat metabolism in skeletal muscle. Ann N Y Acad Sci. 2002;967:217–35. doi: 10.1111/j.1749-6632.2002.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 11.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24(5):933–41. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 12.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–43. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279(5):E1039–44. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 14.Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, Hamilton JT, Hill JO. Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Med Sci Sports Exerc. 2002;34(11):1793–800. doi: 10.1097/00005768-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Melanson EL, Sharp TA, Seagle HM, Horton TJ, Donahoo WT, Grunwald GK, Hamilton JT, Hill JO. Effect of exercise intensity on 24-h energy expenditure and nutrient oxidation. J Appl Physiol. 2002;92(3):1045–52. doi: 10.1152/japplphysiol.00706.2001. [DOI] [PubMed] [Google Scholar]
- 16.Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, Hamilton JT, Hill JO. Twenty-four-hour metabolic responses to resistance exercise in women. J Strength Cond Res. 2005;19(1):61–6. doi: 10.1519/14293.1. [DOI] [PubMed] [Google Scholar]
- 17.Melanson EL, Cornier MA, Bessesen DH, Grunwald GK, MacLean PS, Hill JO. 24 H Metabolic Responses to Low- and High-Intensity Exercise in Lean and Obese Humans. Obesity Research. 2006;14:p180–182. [Google Scholar]
- 18.Melanson EL, Donahoo WT, Grunwald GK, Schwartz R. Changes in 24-h substrate oxidation in older and younger men in response to exercise. J Appl Physiol. 2007;103(5):1576–82. doi: 10.1152/japplphysiol.01455.2006. [DOI] [PubMed] [Google Scholar]
- 19.Melanson EL, Bessesen DH, Cornier MA, MacLean PS, Grunwald GK, Hill JO. 24 hr substrate oxidation following diet-induced weight loss. Obesity. 2008;16(S1):S53. [Google Scholar]
- 20.Melanson EL, Gozansky WS, Barry D, MacLean PS, Hill JO. Changes in 24-h substrate oxidation in response to exercise in endurance-trained individuals. Phsyiologist. 2008;51(6):56. [Google Scholar]
- 21.Montain SJ, Hopper MK, Coggan AR, Coyle EF. Exercise metabolism at different time intervals after a meal. J Appl Physiol. 1991;70(2):882–8. doi: 10.1152/jappl.1991.70.2.882. [DOI] [PubMed] [Google Scholar]
- 22.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(3 Pt 1):E380–91. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 23.Saris WH, Schrauwen P. Substrate oxidation differences between high- and low-intensity exercise are compensated over 24 hours in obese men. Int J Obes Relat Metab Disord. 2004;28(6):759–65. doi: 10.1038/sj.ijo.0802631. [DOI] [PubMed] [Google Scholar]
- 24.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66(2):276–82. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- 25.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am J Physiol. 1997;273(3 Pt 1):E623–9. doi: 10.1152/ajpendo.1997.273.3.E623. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood J, Windhauser M, Volaufova J, Bray GA. Concurrent physical activity increases fat oxidation during the shift to a high-fat diet. Am J Clin Nutr. 2000;72(1):131–8. doi: 10.1093/ajcn/72.1.131. [DOI] [PubMed] [Google Scholar]
- 27.Treuth MS, Hunter GR, Weinsier RL, Kell SH. Energy expenditure and substrate utilization in older women after strength training: 24-h calorimeter results. J Appl Physiol. 1995;78(6):2140–6. doi: 10.1152/jappl.1995.78.6.2140. [DOI] [PubMed] [Google Scholar]
- 28.Treuth MS, Hunter GR, Williams M. Effects of exercise intensity on 24-h energy expenditure and substrate oxidation. Med Sci Sports Exerc. 1996;28(9):1138–43. doi: 10.1097/00005768-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Votruba SB, Atkinson RL, Schoeller DA. Prior exercise increases dietary oleate, but not palmitate oxidation. Obes Res. 2003;11(12):1509–18. doi: 10.1038/oby.2003.202. [DOI] [PubMed] [Google Scholar]
- 30.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5 Pt 1):E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]