Abstract
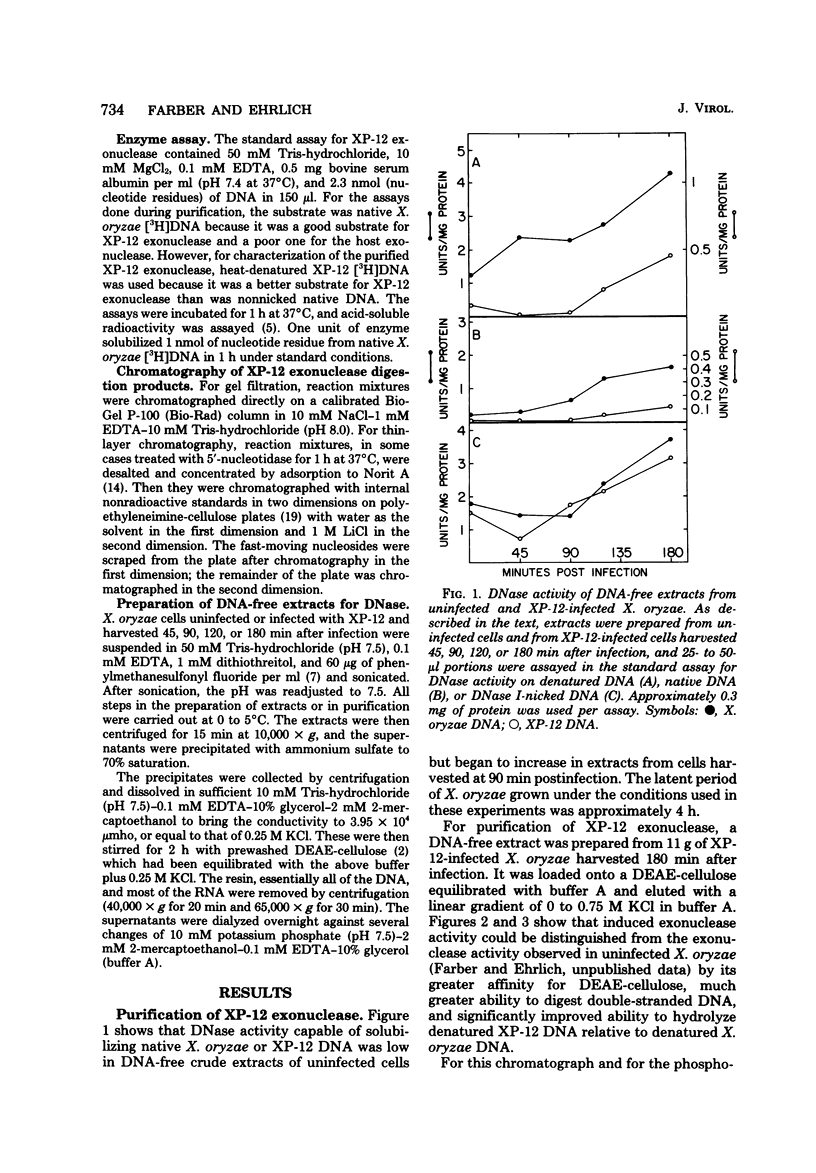
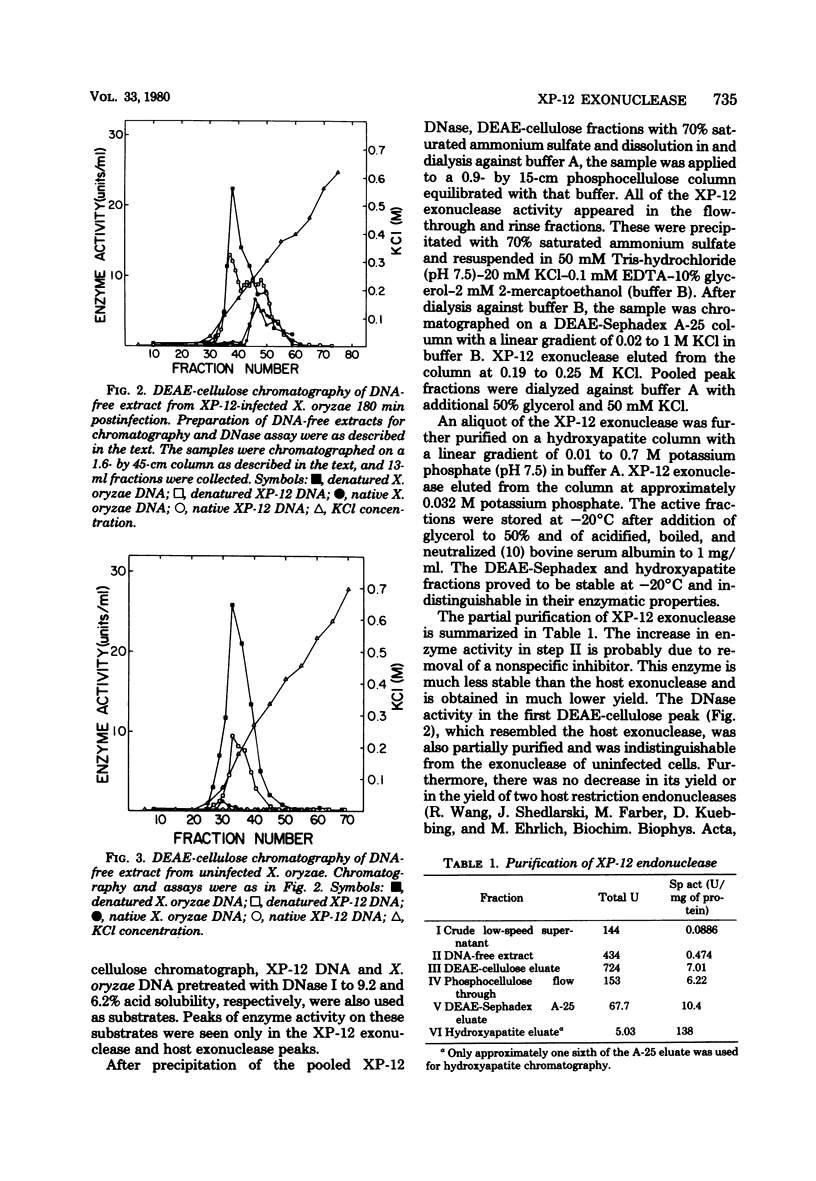
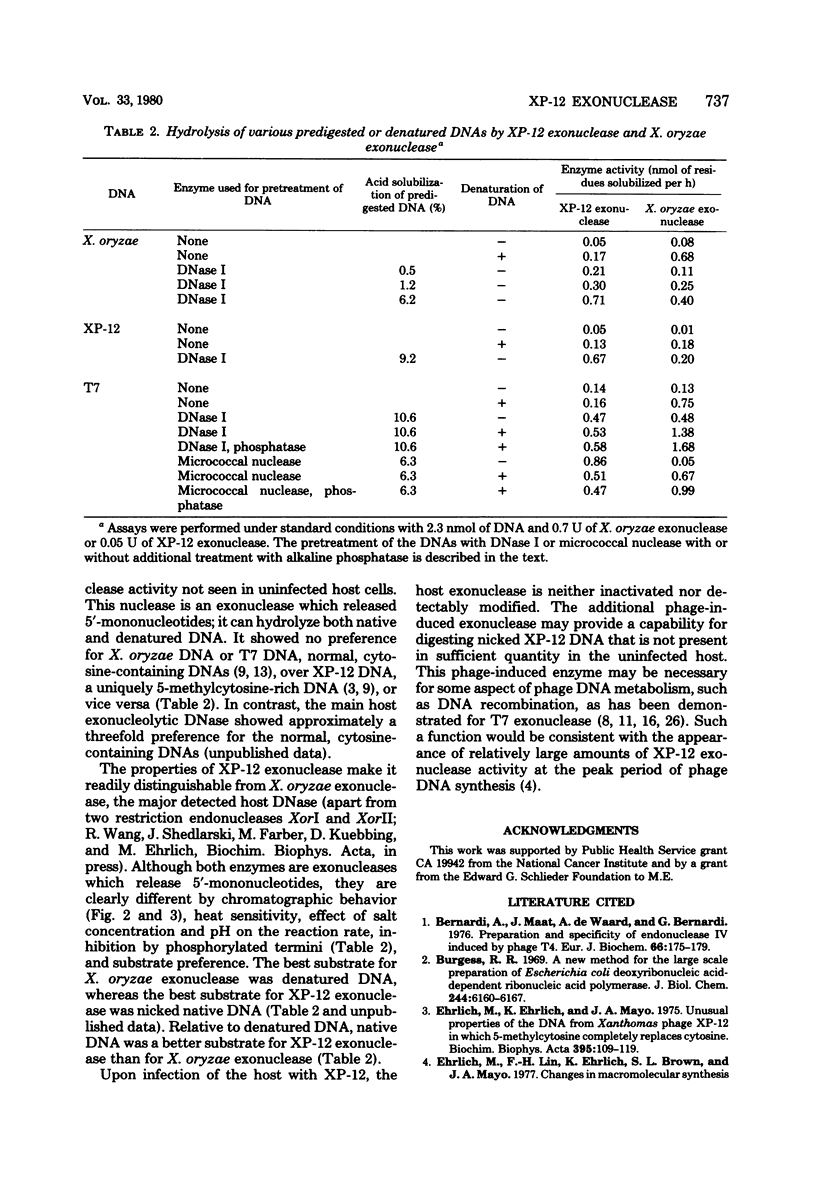
An exonuclease has been partially purified from XP-12-infected Xanthomonas oryzae which is not found in uninfected X. oryzae. Although both the phage-induced exonuclease and the major host exonucleolytic DNase released 5'-mononucleotides, these enzymes differed in their chromatographic behavior, pH optimum, salt inhibition, and heat sensitivity. These two exonucleases preferred different substrates. Nicked native DNA was the best substrate for the phage-induced enzyme, whereas denatured DNA was the best substrate for the host enzyme. Also, the host enzyme had a significant preference for denatured or nicked, normal cytosine-containing DNA (e.g., X. oryzae or T7 DNA) over similarly denatured or nicked 5-methylcytosine-rich DNA (namely, XP-12DNA), whereas the phage-induced enzyme hydrolyzed both types of DNA equally well.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi A., Maat J., de Waard A., Bernardi G. Preparation and specificity of endonuclease IV induced by bacteriophage T4. Eur J Biochem. 1976 Jun 15;66(1):175–179. doi: 10.1111/j.1432-1033.1976.tb10437.x. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Lin F. H., Ehrlich K., Brown S. L., Mayo J. A. Changes in macromolecular synthesis in Xanthomonas oryzae infected with bacteriophage XP-12. J Virol. 1977 Sep;23(3):517–523. doi: 10.1128/jvi.23.3.517-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Sarafyan L. P., Myers D. J. Interaction of microbial DNA with cultured mammalian cells. Binding of the donor DNA to the cell surface. Biochim Biophys Acta. 1976 Dec 13;454(3):397–409. doi: 10.1016/0005-2787(76)90266-5. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574–579. doi: 10.1016/0003-2697(78)90784-4. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. The involvement of genes 3,4,5 and 6 in genetic recombination in bacteriophage T7. Virology. 1975 May;65(1):281–285. doi: 10.1016/0042-6822(75)90031-8. [DOI] [PubMed] [Google Scholar]
- Kuo T. T., Huang T. C., Teng M. H. 5-Methylcytosine replacing cytosine in the deoxyribonucleic acid of a bacteriophage for Xanthomonas oryzae. J Mol Biol. 1968 Jul 14;34(2):373–375. doi: 10.1016/0022-2836(68)90263-5. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., PRATT E. A. On the structure of the glucosylated hydroxymethylcytosine nucleotides of coliphages T2, T4, and T6. J Biol Chem. 1960 Nov;235:3254–3259. [PubMed] [Google Scholar]
- LUNAN K. D., SINSHEIMER R. L. A study of the nucleic acid of bacteriophage T7. Virology. 1956 Aug;2(4):455–462. doi: 10.1016/0042-6822(56)90003-4. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Deoxyribonucleases of Pneumococcus. J Biol Chem. 1967 Jul 10;242(13):3108–3120. [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol. 1974 Nov;14(5):1040–1048. doi: 10.1128/jvi.14.5.1040-1048.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeles S., Kammen H. O. Use of activated charcoal for adsorption and elution of ribooligonucleotides. Anal Biochem. 1966 Dec;17(3):540–544. doi: 10.1016/0003-2697(66)90189-8. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- OHSAKA A., MUKAI J. I., LASKOWSKI M., Sr THE USE OF PURIFIED MICROCOCCAL NUCLEASE IN IDENTIFYING THE NUCLEOTIDE TERMINUS BEARING A FREE 5'-MONOPHOSPHATE. J Biol Chem. 1964 Oct;239:3498–3504. [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Hurwitz J. Enzymatic breakage of deoxyribonucleic acid. I. Purification and properties of endonuclease II from T4 phage-infected Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6182–6191. [PubMed] [Google Scholar]
- Sadowski P. D., Hurwitz J. Enzymatic breakage of deoxyribonucleic acid. II. Purification and properties of endonuclease IV from T4 phage-infected Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6192–6198. [PubMed] [Google Scholar]
- Shimizu K., Sekiguchi M. 5' leads to 3'-Exonucleases of bacteriophage T4. J Biol Chem. 1976 May 10;251(9):2613–2619. [PubMed] [Google Scholar]
- Short E. C., Jr, Koerner J. F. Separation and characterization of deoxyribonucleases of Escherichia coli B. II. Further purification and properties of an exonuclease induced by infection with bacteriophage T2. J Biol Chem. 1969 Mar 25;244(6):1487–1496. [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. Intermediates in genetic recombination of bacteriophage T7 DNA. J Mol Biol. 1977 Jan 25;109(3):423–426. doi: 10.1016/s0022-2836(77)80021-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]


