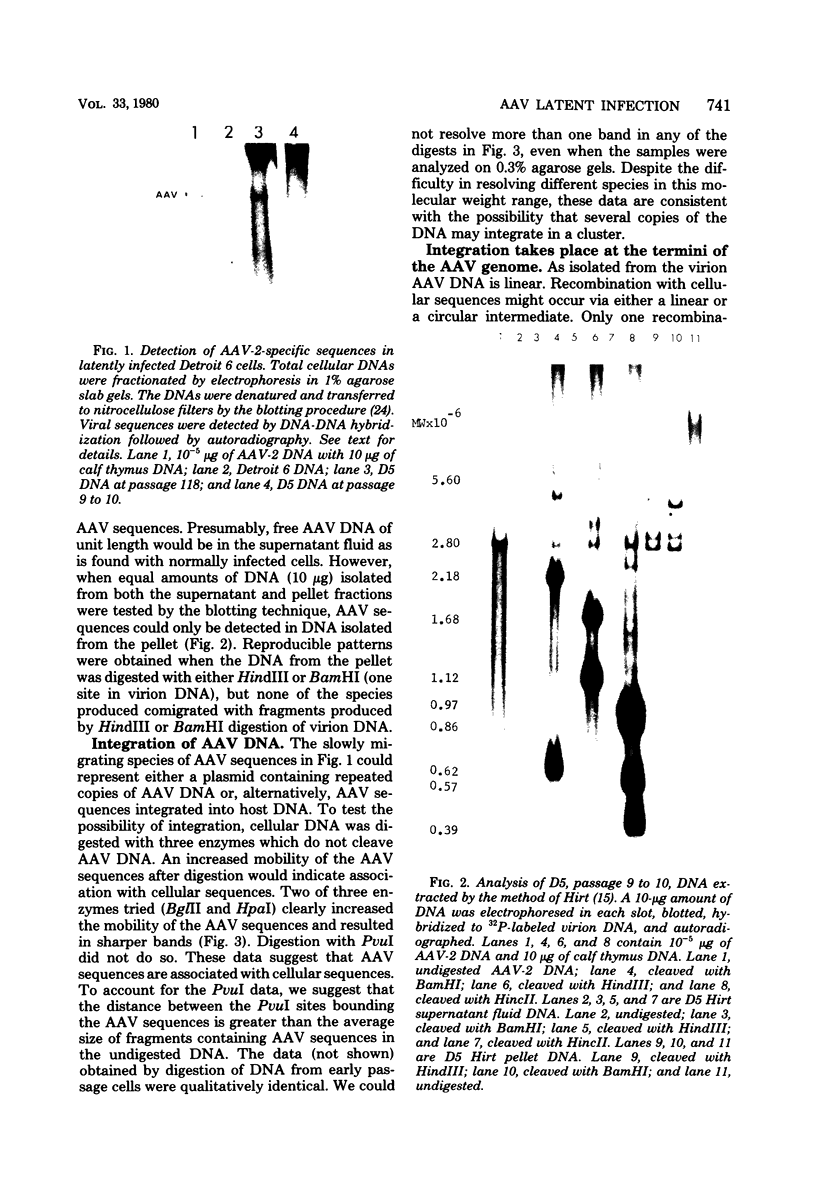
Abstract
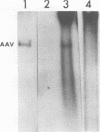
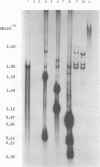
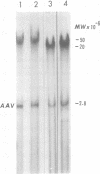
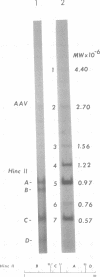
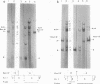
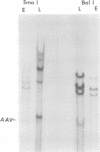
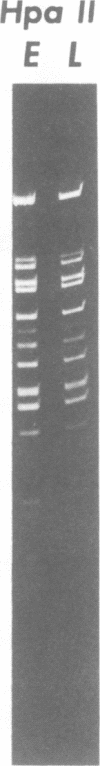
A clone of human cells (Detroit 6) latently infected by adeno-associated virus (AAV) has been characterized with regard to the status of the viral DNA. In both early (9 to 10) and late (118) passages of the clone, AAV-DNA was recombined with host DNA, at least in some cases as a head-to-tail tandem repeat, via the terminal sequences of the viral genome. However, it was not possible to distinguish between integration into chromosomal DNA and very large plasmids (< 20 x 10(6) molecular weight) which contain both viral and cellular DNA sequences. Although evidence for some modifications of the viral sequence was obtained, most of the integrated sequences appeared to be intact. In some cases sequences of undetermined origin separated adjacent copies of the viral genome. Free copies of the AAV genome were detectable in late passage cells, but not in early passage cells. The orientation of nucleotide sequences present in the free AAV DNA from late passage cells was indistinguishable from that of virion DNA. With the notable exception, the organization of the integrated AAV sequences as determined by restriction enzyme digestion remained constant with continued passage. Digestion with SmaI, which cleaves within the palindromic region of the terminal repetition in AAV DNA, produced reproducibly different patterns when early and late passage DNAs were compared. Several models for rescue of free copies of the genome from the integrated DNA are possible, all of which involve the terminal repetition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- BERMAN L., STULBERG C. S., RUDDLE F. H. Long-term tissue culture of human bone marrow. I. Report of isolation of a strain of cells resembling epithelial cells from bone marrow of a patient with carcinoma of the lung. Blood. 1955 Sep;10(9):896–911. [PubMed] [Google Scholar]
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W., Fife K. H., Lusby E. Adeno-associated virus DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):781–787. doi: 10.1101/sqb.1979.043.01.085. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Pinkerton T. C., Thomas G. F., Hoggan M. D. Detection of adeno-associated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology. 1975 Dec;68(2):556–560. doi: 10.1016/0042-6822(75)90298-6. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Laughlin C. A., de la Maza L. M., Myers M. Adeno-associated virus autointerference. Virology. 1979 Jan 30;92(2):449–462. doi: 10.1016/0042-6822(79)90149-1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Establishment and characterization of KB cell lines latently infected with adeno-associated virus type 1. Virology. 1977 Oct 1;82(1):84–92. doi: 10.1016/0042-6822(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Adeno-associated virus DNA replication: nonunit-length molecules. Virology. 1979 Feb;93(1):57–68. doi: 10.1016/0042-6822(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kirschstein R. L., Smith K. O., Peters E. A. Inhibition of adenovirus 12 oncogenicity by adeno-associated virus. Proc Soc Exp Biol Med. 1968 Jul;128(3):670–673. doi: 10.3181/00379727-128-33095. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Houlditch G. S., Mumford D. M. Influence of adeno-associated satellite virus on adenovirus-induced tumours in hamsters. Nat New Biol. 1973 Jan 10;241(106):44–46. doi: 10.1038/newbio241044b0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]