Abstract
Summary
While nitrogen-containing bisphosphonates have been shown to reduce fracture risk in postmenopausal women and men, their safety in the period after a fracture is unclear. In fully adjusted multivariable regression models, bisphosphonate use in the post-fracture period was associated with an increased probability of non-union [odds ratio (OR) 2.37, 95% confidence interval (CI) 1.13–4.96]. Clinicians might consider waiting for several months before introduction of a bisphosphonate after a fracture.
Introduction
While nitrogen-containing bisphosphonates have been shown to reduce fracture risk in postmenopausal women and men, their safety in the period after a fracture is unclear. We examined the risk of non-union associated with post-fracture bisphosphonate use among a group of older adults who had experienced a humerus fracture.
Methods
We conducted a nested case–control study among subjects who had experienced a humerus fracture. From this cohort, cases of non-union were defined as those with an orthopedic procedure related to non-union 91–365 days after the initial humerus fracture. Bisphosphonate exposure was assessed during the 365 days prior to the non-union among cases or the matched date for controls. Multivariable logistic regression models were examined to calculate the OR and 95% CI for the association of post-fracture bisphosphonate use with non-union.
Results
From the cohort of 19,731 patients with humerus fractures, 81 (0.4%) experienced a non-union. Among the 81 cases, 13 (16.0%) were exposed to bisphosphonates post-fracture, while 69 of the 810 controls (8.5%) were exposed in the post-fracture interval. In fully adjusted multivariable regression models, bisphosphonate use in the post-fracture period was associated with an increased odds of non-union (OR 2.37, 95% CI 1.13–4.96). Albeit limited by small sample sizes, the increased risk associated with bisphosphonate use persisted in the subgroup of patients without a history of osteoporosis or prior fractures (OR 1.91, 95% CI 0.75–4.83).
Conclusions
In this study of older adults, non-union after a humerus fracture was rare. Bisphosphonate use after the fracture was associated with an approximate doubling of the risk of non-union.
Keywords: Bisphosphonate, Epidemiology, Fracture, Non-union
Introduction
Randomized controlled trials clearly demonstrate the benefit of nitrogen-containing bisphosphonates such as alendronate, risedronate, and ibandronate in reducing fractures in select populations [1]. However, it has never been clear when they should be initiated in the post-fracture period. Based on their inhibition of both bone formation and resorption, there are theoretical concerns that their use too close to the fracture date may retard callus formation leading to non-union [2, 3]. Some, but not all animal models, support this possibility [4–7]. A recent study demonstrated that the precise timing of bisphosphonate use was very important [8].
To date, there are only sparse human data on this issue [9]. The study of non-union risk among bisphosphonate users is difficult. Typical randomized controlled trials (RCTs) are inadequate to answer this question, because non-unions are relatively rare events, and RCTs generally do not follow up patients long enough to determine the resolution of the fracture event. Large observational studies may be more appropriate, but most prospective cohort studies have inadequate data regarding the precise initiation dates for medications. The use of pharmacy and health care utilization databases offer a possible setting for studying this issue. These data sets are limited, however, by their lack of information on bone mineral density (BMD), often used for the diagnosis of osteoporosis, but they do provide precise information about diagnoses, surgical procedures, and prescription filling patterns.
Humerus fractures provide a useful example for studying the potential relation between bisphosphonate use and non-union. Humerus fractures are common osteoporotic fractures, with an incidence of 2.3 per 1,000 patient years in those over 60 years of age [10]. Approximately 70% of such fractures are associated with low BMD, and the risk of hip fracture is doubled in the year after a humerus fracture [11]. These observations highlight the need for osteoporosis treatment, such as a bisphosphonate, in most patients after a humerus fracture.
Thus, we studied the potential association between bisphosponate use and humeral non-union using health care utilization data comprising over 1 million elderly adults. We hypothesized an association between bisphosphonate use after a humeral fracture and subsequent non-union.
Materials and methods
Design
We conducted a nested case–control study within a large cohort of older adults who sustained a fracture of the humerus. Cases were defined as those patients who underwent a procedure for a non-union between 91 and 365 days following the date of humerus fracture. Ten controls who had experienced a humerus fracture but had not experienced non-union were matched with each case based on calendar time. The risk of non-union associated with bisphosphonate exposure before and after the initial fracture was calculated through multivariate adjusted logistic regression models controlling for relevant covariates. We also assessed the risk of exposure to other osteoporosis medications such as raloxifene and calcitonin.
Study population
The study population comprised Medicare beneficiaries from two large US states during the period 1996–2004. All patients were also enrolled in a state run pharmacy benefits program that provides medications at minimal expense to low-income elderly. The co-payments for medications were $4–8 dollars per prescription during the study period, and no restrictions were in place regarding osteoporosis medication prescribing.
Patients were eligible for inclusion in the study cohort if they sustained a humerus fracture defined by ICD-9-CM diagnosis codes 812.00–812.3x. This algorithm has been found to have a positive predictive value over 95% [12]. Eligible patients were excluded if they did not demonstrate use of the health care system by requiring at least one Medicare and one drug claim during the two 6-month periods preceding the humerus fracture. Since Medicare reimburses for medications but does not provide information on such claims for patients during their first 100 days in a nursing home, those admitted to a nursing home after their humerus fracture were also censored.
This study received approval from the Partners Health-care Institutional Review Board. As well, Data Use Agreements are in place with the relevant bodies.
Study endpoint (non-union)
Humeral non-union was defined based on the presence of a surgical procedure for non-union (see Table 1) occurring 91–365 days after the initial humerus fracture. This definition is consistent with diagnostic criteria used by previous articles and textbooks [13–15]. Ten controls who had experienced the initial humerus fracture but did not undergo a procedure for non-union at the time of matching were matched with each case based on calendar time.
Table 1.
Procedures used to define non-union of a humerus fracture
| Common procedural terminology code | Description |
|---|---|
| 24430 | Repair of non-union or mal-union |
| 24435 | Repair of non-union or mal-union |
| 24515 | Open treatment of humeral shaft fracture with plate/screws |
| 24516 | Treatment of humeral shaft fracture |
Exposures of interest
The primary exposure of interest was bisphosphonate use, including oral preparations such as alendronate, etidronate, and risedronate, and intravenous zoledronic acid. Since the study period only ran through 2005, there was no use of ibandronate in the study population. As well, there was no use of zoledronic acid in the nested case–control population. Therefore, this study only considered the use of oral bisphosphonates.
Drug utilization was assessed through examining pharmacy claims for medication dispensing. These electronic claims are automatically generated at each filling of a medication at pharmacies and are not necessarily what was prescribed (and not filled) nor what patients actually used. However, pharmacy claims data are generally considered the most complete and accurate sources of drug utilization information available [16]. The pharmacy data comprise the name of the drug, the strength, the number of pills, and the date of dispensing. From this information, we built a longitudinal record of drug utilization for each patient. Gaps between prescriptions were considered days without medication, based on pharmacy dispensing data.
Drug utilization was examined during the 365 days prior to cohort-defining humerus fracture (pre-fracture exposure). In addition, drug utilization was assessed during the period after the humerus fracture but before the case-defining humeral non-union (post-fracture exposure). The primary exposure of interest was bisphosphonate use post-fracture. To separate the post-fracture from the pre-fracture period, we created mutually exclusive exposure categories (i.e., post-fracture and not pre-fracture). Sensitivity analyses assessed bisphosphonate use pre-fracture and not post-fracture. Similar exposure categories were created for other osteoporosis medications, including calcitonin and raloxifene, to assess their effect on non-union. We assessed the use of these drugs as a comparison with the effect of bisphosphonates on non-union. No information on calcium and vitamin D use or blood levels was available for analysis.
Covariates
All covariate information was assessed during 365 days before the case-defining event or its matched control date. Sociodemographic factors included age, gender, and race. Osteoporosis-related factors included prior fracture, a diagnosis of osteoporosis, and use of glucocorticoids or hormone therapy. Other comorbid conditions included a prior diagnosis of diabetes or rheumatoid arthritis. Health services information included prior hospitalization, number of physician visits, and number of different medications used [17]. In addition, a validated claims-based adaptation of the Charlson comorbidity index was assessed [18]. In addition, we included an indicator of whether there was instrumentation at the time of the humeral fracture.
Statistical analyses
We first assessed the cumulative incidence of humeral non-union among the total cohort of patients who sustained a humerus fracture. The adjusted odds ratio of non-union associated with bisphosphonate use after a humeral fracture was estimated from multivariate conditional logistic regression, including all covariates listed above. In case–control studies with risk-set sampling, odds ratios are interpreted as hazard rate ratios and approximate relative risks for rare events [19]. We first examined age- and sex-adjusted models and then fully adjusted models. Finally, to better determine whether pre-existing osteoporosis or severity of humeral fracture introduced confounding bias, we examined logistic regression models among relevant subgroups of patients, i.e., those without a fracture in the 365 days prior to the humerus fracture and those without instrumentation at the time of the humeral fracture.
All analyses were run in SAS (version 9.0).
Results
From the eligible study population of Medicare beneficiaries, we were able to identify 19,731 patients who were diagnosed with fracture of the humerus. Eighty-one of these patients (0.41%) underwent a non-union procedure 91–365 days after the initial humeral fracture. The median time between the humeral fracture and the procedure for non-union was 187 days (interquartile range 124–274 days). These 81 patients comprised the cases, and 810 controls (10:1) were matched with them on calendar data for the nested case–control analyses.
Characteristics of cases and controls are shown in Table 2. The mean age of the case–control population was nearly 80 years, with a slightly higher mean among controls. The source population is over 80% female, and 95% of cases in this study were women versus 91% of controls. The vast majority of cases and controls were Caucasian, again reflecting the composition of the source population. Cases were more likely to have been hospitalized in the prior year than controls. Their comorbidity index scores were more likely to be in the upper third of the distribution, and they were more likely to have been diagnosed with diabetes.
Table 2.
Baseline characteristics of study population from the 12 months prior to the index fracture of the humerus
| Non-union (cases) N (%) or mean (±SD) |
No non-union (controls) | P value | |
|---|---|---|---|
| N | 81 | 810 | |
| Age, mean | 77 (±9) | 80 (±8) | 0.03 |
| Age, by category | 0.01 | ||
| 65–74 | 31 (38) | 189 (23) | |
| 75–84 | 35 (43) | 374 (46) | |
| 85+ | 15 (19) | 247 (30) | |
| Female gender | 77 (95) | 756 (91) | 0.5 |
| Race, by category | 0.9 | ||
| Caucasian | 77 (95) | 768 (95) | |
| African American | 2 (2) | 26 (3) | |
| Other | 2 (2) | 16 (2) | |
| Hospitalization, yes | 31 (38) | 223 (28) | 0.04 |
| Number of physician visits, mean | 9 (±6) | 8 (±6) | 0.2 |
| Number of different medications, mean | 9 (±6) | 8 (±5) | 0.6 |
| Comorbidity score | 0.01 | ||
| Zero to one | 14 (17) | 265 (33) | |
| Two to four | 25 (31) | 239 (30) | |
| Five or more | 42 (52) | 306 (38) | |
| History of a fracture | 22 (27) | 165 (20) | 0.15 |
| History of osteoporosis | 11 (14) | 111 (14) | 0.9 |
| History of oral glucocorticoid use | 8 (10) | 66 (8) | 0.6 |
| Surgery at time of index fracture | 8 (10) | 19 (2) | <0.001 |
| Prior diabetes | 33 (41) | 238 (29) | 0.03 |
| Prior hormone therapy | 4 (5) | 23 (3) | 0.3 |
| Prior rheumatoid arthritis | 6 (7) | 36 (4) | 0.2 |
P values were calculated using the Student’s t test for continuous normally distributed variables and a chi-square test for categorical variables
Bisphosphonate exposures for cases and controls are shown in Table 3. Few patients were receiving bisphosphonates before or after the humeral fracture. The mean exposure durations were approximately 1 month.
Table 3.
Bisphosphonate exposure for cases and controls
| Bisphosphonate exposure | Cases |
Controls |
||
|---|---|---|---|---|
| Pre-fracture | Post-fracture | Pre-fracture | Post-fracture | |
| Any exposure, n (%) | 3 (3.7) | 13 (16.0) | 28 (3.5) | 69 (8.5) |
| Days of exposure, mean | 36 (±11) | 26 (±23) | 37 (±24) | 26 (±20) |
There was no intravenous bisphosphonate use observed in this cohort
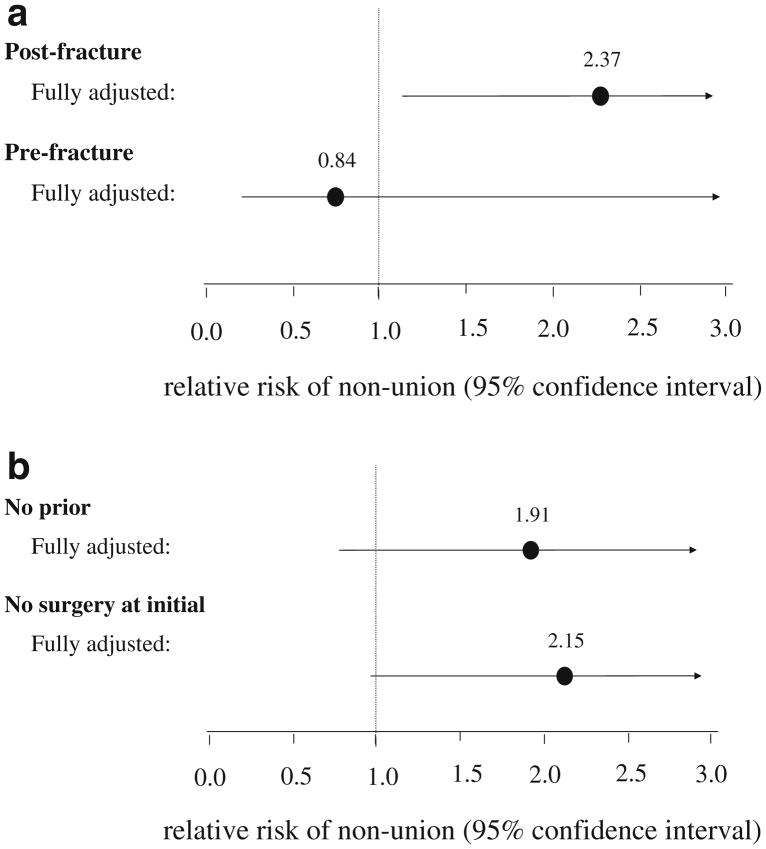
We next examined the relative risk of non-union associated with post-fracture bisphosphonate use (see Fig. 1a). Age- and gender-adjusted models (RR=1.84, 95% confidence interval, CI, 0.94–3.60) as well as fully adjusted models (RR=2.37, 95% CI 1.13–4.96) gave similar relative risk estimates. There was no elevation in risk associated with pre-fracture bisphosphonate use (RR=0.84, 95% CI 0.19–3.74). Similar models were examined for calcitonin and raloxifene use. There was no elevation in relative risk of non-union for use of these agents post-fracture (RR=0.57, 95% CI 0.13–2.50). There did appear to be an elevated risk of pre-fracture use, although the number of cases was small and the confidence intervals were wide (RR=2.12, 95% CI 0.52–8.63).
Fig. 1.
The adjusted relative risk and 95% confidence intervals based on models that included all variables listed in Table 1. Since the events are rare, the calculated odds ratios approximate the relative risk. a The results for post-fracture and pre-fracture bisphosphonate use. b The results for post-fracture bisphosphonate use among patients without a prior fracture nor a diagnosis of osteoporosis and without surgery at the time of the humerus fracture
Finally, to limit the effect of residual confounding by fracture severity or prior osteoporosis, fully adjusted models were re-run in two subgroups: (1) patients without prior fracture or osteoporosis and (2) patients without instrumentation at the time of the initial humeral fracture. These subgroup analyses showed relative risk estimates similar to the overall study population (see Fig. 1b).
Discussion
Fractures of the humerus in adults are associated with low bone mineral density and are considered to be osteoporotic fractures [10]. Most major guidelines recommend osteoporosis treatment, including the use of nitrogen-containing bisphosphonates, for patients with fractures such as those of the humerus. However, prior literature has raised concerns about a possible theoretical association between bisphosphonate use and fracture non-union. Since non-union is a relative rare event, study of this association is challenging in most clinical datasets. We examined this relationship using a very large health care utilization database comprised of older adult Medicare beneficiaries who are also enrollees of state run drug benefit programs for older adults. In this nested case–control study, bisphosphonate use after a fracture of the humerus was associated with an approximate doubling of the risk of non-union. This was not the case for bisphosphonate use before the humeral fracture and also not the case for use of post-fracture calcitonin or raloxifene. This relationship of post-fracture bisphosphonate use and non-union persisted in subgroup analyses of patients without prior osteoporosis or fractures as well as in patients who had not had instrumentation at the time of their humerus fracture.
While this study is the largest to date of the potential relationship between post-fracture bisphosphonate use and non-union, there are a number of methodologic limitations. First, the severity of osteoporosis as measured by bone mineral density may confound the relationship between non-union and post-fracture bisphosphonate use. If low bone mineral density is a risk factor for non-union and is associated with a higher likelihood of post-fracture bisphosphonate use, then this unmeasured factor could induce a relationship that is not causal. It is also possible that bisphosphonates could have been prescribed to patients in response to possible non-union, a form of protopathic bias [20]. The fact that the relationship between post-fracture bisphosphonate use and humeral non-union persisted among patients without a prior fracture nor a diagnosis of osteoporosis suggests that this relationship is not due to low bone mineral density. Moreover, the fact that the relationship was not observed among patients using calcitonin or raloxifene post-fracture also argues against confounding.
Second, we did not have information about calcium or vitamin D use or serum levels of calcium and 25-hydroxyvitamin D. In addition, several other risk factors for non-union were also missing from the study database, including obesity, osteoporosis, alcoholism, smoking, poor bone quality, and scar tissue. Third, pharmacy claims information do not equate to what a patient actually took. Patients can fill prescriptions and not take the medications. As well, some patients obtain drugs without filling prescriptions through use of samples. Fourth, the definition of non-union of a humeral fracture was based on procedure data and not actual clinical records with radiographs. Misclassification of exposures and endpoints typically biases relative risks toward the null rather than away from it. Finally, the patients we studied were predominantly older white adults with low incomes. It is possible that our results would not generalize to other populations.
Several strengths of our analyses are also worth noting. Since non-union is a relatively rare event, large health care utilization databases likely will be the best source for large cohorts to study this phenomenon. Moreover, precise information about drug utilization will be important. Health care utilization databases, while not perfect, may give the best information on the timing between bisphosphonate use and non-union. We have demonstrated the value of this approach in prior work [21]. It is important to note that the cohort we used in our prior work has been updated in the current work.
While prior literature suggests the possibility that bisphosphonates are associated with non-union, there are no clear data supporting this from clinical trials. As we note above, RCTs of bisphosphonates for fracture prevention did not start bisphosphonates in the immediate post-fracture period, and many did not follow up patients who sustained fractures long enough to determine whether a fracture non-union occurred. One interesting exception is the HORIZON Recurrent Fracture Trial that enrolled 2,127 patients who had sustained hip fractures and followed them up for a median of 1.9 years [9]. In this trial, patients received an intravenous injection of zoledronic acid within 90 days after a hip fracture. Delayed healing or non-union was reported in slightly more patients in the zoledronic acid group, 3.2% versus 2.7% in the placebo group (risk ratio 1.17, 95% confidence interval 0.72–1.90), but this difference was not statistically significant. While this trial suggests that the use of a bisphosphonate in the post-fracture period is not associated with a large increase in the risk of non-union, it is not clear from the report exactly when during the 90-day period the bisphosphonate was given. As well, all of the hip fractures in the HORIZON trial would have been treated initially with instrumentation, and all of the patients were vitamin D replete prior to receiving the bisphosphonate, and all received calcium and vitamin D supplementation during the trial. Thus, the effect of a bisphosphonate on hip non-union may not be comparable to our example of humerus fracture. There have been data from a rodent fracture model that zoledronic acid does not delay endochondral fracture repair, but weekly dosing did delay hard callus remodeling [22]. Thus, the relationship between bisphosphonates and non-union may be agent and dose specific. It is also possible that cortical healing, such as that required in humeral fractures, is more vulnerable than metaphyseal trabecular healing. We cannot find any support for this in the literature, but it is a possible explanation for our findings.
In conclusion, we observed an approximate two-fold increased risk of non-union among older adult patients who used a bisphosphonate after a fracture of the humerus. While this risk was statistically significant, its clinical significance is unclear. We found that non-union occurred in approximately one in 250 patients. If bisphosphonate use causes a doubling of the risk of non-union, then the risk goes from one in 250 to one in 125, corresponding to a number needed to harm of approximately 125. Since bisphosphonate use among high-risk patients is associated with an approximate 50% reduction in fracture risk and the morbidity and mortality associated with a new fracture are often much greater than a non-union, it would appear unwise to suggest not using a bisphosphonate after a fracture. Thus, delaying bisphosphonate use for the first few months after a fracture might be wise, as long as the delay does not interfere with the ultimate initiation of such agents.
Footnotes
Conflicts of interest There is no specific support for this project. Dr. Solomon receives salary support from the NIH (AR 047782, AR 055989, AG 027066, DA 022600) and AHRQ. He has served as an unpaid member of an Advisory Board to Amgen for a non-osteoporosis-related product. He provides epidemiologic consulting to CORRONA. Dr. Hochberg receives salary support from the NIH and the Department of Veterans Affairs and is a consultant to the following companies that have products in the field of osteoporosis: Amgen, Merck & Co., Inc., Novartis Pharma A.G., Proctor & Gamble Pharmaceutical Co., and Roche Pharmaceutical Co. Dr. Schneeweiss is Principal Investigator of the Brigham and Women’s Hospital DEcIDE Research Center on comparative effectiveness research funded by the Agency of Healthcare Research and Quality.
Contributor Information
D. H. Solomon, Email: dsolomon@partners.org, Division of Pharmacoepidemiology, Harvard Medical School, Boston, MA, USA. Division of Rheumatology, Immunology, and Allergy, Harvard Medical School, Boston, MA, USA. Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Division of Rheumatology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA
M. C. Hochberg, Division of Rheumatology and Clinical Immunology, University of Maryland School of Medicine, Baltimore, MD, USA. Department of Medicine and Division of Gerontology, University of Maryland School of Medicine, Baltimore, MD, USA. Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
H. Mogun, Division of Pharmacoepidemiology, Harvard Medical School, Boston, MA, USA. Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
S. Schneeweiss, Division of Pharmacoepidemiology, Harvard Medical School, Boston, MA, USA. Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23(4):570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 2.Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Jt Surg, Br Vol. 2007;89 (4):425–433. doi: 10.1302/0301-620X.89B4.18301. [DOI] [PubMed] [Google Scholar]
- 3.Fleisch H. Can bisphosphonates be given to patients with fractures? J Bone Miner Res. 2001;16(3):437–440. doi: 10.1359/jbmr.2001.16.3.437. [comment] [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Mori S, Mashiba T, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17(12):2237–2246. doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- 5.Peter CP, Cook WO, Nunamaker DM, Provost MT, Seedor JG, Rodan GA. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res. 1996;14(1):74–79. doi: 10.1002/jor.1100140113. [DOI] [PubMed] [Google Scholar]
- 6.Boyce RW, Paddock CL, Gleason JR, Sletsema WK, Eriksen EF. The effects of risedronate on canine cancellous bone remodeling: three-dimensional kinetic reconstruction of the remodeling site. J Bone Miner Res. 1995;10(2):211–221. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Mori S, Kaji Y, Kawanishi J, Akiyama T, Norimatsu H. Concentration of bisphosphonate (incadronate) in callus area and its effects on fracture healing in rats. J Bone Miner Res. 2000;15(10):2042–2051. doi: 10.1359/jbmr.2000.15.10.2042. [DOI] [PubMed] [Google Scholar]
- 8.Amanat N, McDonald M, Godfrey C, Bilston L, Little D. Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res. 2007;22(6):867–876. doi: 10.1359/jbmr.070318. [DOI] [PubMed] [Google Scholar]
- 9.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TV, Center JR, Sambrook PN, Eisman JA. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153(6):587–595. doi: 10.1093/aje/153.6.587. [DOI] [PubMed] [Google Scholar]
- 11.Lauritzen JB, Schwarz P, McNair P, Lund B, Transbol I. Radial and humeral fractures as predictors of subsequent hip, radial or humeral fractures in women, and their seasonal variation. Osteoporos Int. 1993;3(3):133–137. doi: 10.1007/BF01623274. [DOI] [PubMed] [Google Scholar]
- 12.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45 (7):703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 13.Healy WL, Jupiter JB, Kristiansen TK, White RR. Nonunion of the proximal humerus. A review of 25 cases. J Orthop Trauma. 1990;4(4):424–431. [PubMed] [Google Scholar]
- 14.Foster RJ, Dixon GL, Jr, Bach AW, Appleyard RW, Green TM. Internal fixation of fractures and non-unions of the humeral shaft. Indications and results in a multi-center study. J Bone Jt Surg, Am Vol. 1985;67(6):857–864. [PubMed] [Google Scholar]
- 15.Wheeless CR. Wheeless’ textbook of orthopaedics. Data Trace; Towson: 2007. [Google Scholar]
- 16.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ, Greenland S. Modern epidemiology. Lippincott Williams and Wilkins; Philadelphia: 1998. [Google Scholar]
- 20.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya T, Levin R, Vrahas MS, Solomon DH. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53(3):364–367. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 22.McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008;43(4):653–662. doi: 10.1016/j.bone.2008.05.019. [DOI] [PubMed] [Google Scholar]