Abstract
Background
Echocardiographic measurements of left ventricular (LV) mass, left atrial (LA) volume, and LV end-systolic volume (ESV) predict heart failure (HF) hospitalization and mortality. Indexing measurements by body size is thought to establish limits of normality among individuals varying in body habitus. The American Society of Echocardiography recommends dividing measurements by body surface area (BSA), but others have advocated alternative indexing methods.
Methods
Echocardiographic measurements were collected in 1024 ambulatory adults with coronary artery disease. LV mass, LA volume, and LV ESV were calculated using truncated ellipse method and biplane method of disk formulae. Comparison between raw measurements and measurements divided by indexing parameters was made by hazard ratios per standard deviation increase in variable and c-statistics for BSA, BSA0.43, BSA1.5, height, height0.25, height2, height2.7, body weight (BW), BW0.26, body mass index (BMI), and BMI0.27.
Results
Mean LV mass was 192 ± 57 g, mean LA volume was 65 ± 24 mL, and mean LV ESV was 41 ± 26 mL. Average height was 171 ± 9 cm, average BSA was 1.94 ± 0.22 m2, and average BMI was 28.4 ± 5.3 kg/m2. At an average follow-up of 5.6 ± 1.8 years, there were 148 HF hospitalizations, 71 cardiovascular (CV) deaths, and 269 all-cause deaths. There was excellent correlation between raw measurements and those indexed by height (r = 0.98–0.99), and moderate correlation between raw measurements and those indexed by BW (r = 0.73–0.94). C-statistics and hazard ratios per standard deviation increase in indexed variables were similar for HF hospitalization, CV mortality, and all-cause mortality. There were no significant differences among indexing methods in ability to predict outcomes.
Conclusion
The choice of indexing method by parameters of BSA, height, BW, and BMI does not affect the clinical usefulness of LV mass, LA volume, and LV ESV in predicting HF hospitalization, CV mortality, or all-cause mortality among ambulatory adults with coronary artery disease. Continued use of BSA to index measurements of LV mass, LA volume, and LV ESV is acceptable.
Keywords: Body surface area, Echocardiography, Heart failure, Mortality, Quantitative
Echocardiographic measurements of left ventricular (LV) hypertrophy, LV dilatation, and left atrial (LA) dilatation are predictive of adverse events. Specifically, LV wall thickness,1 LV mass,2,3 LA diameter,4,5 LA volume,6 and LVend-systolic volume (ESV)7 are predictive of heart failure (HF) hospitalization and mortality. Indexing measurements to body size may provide additional useful and prognostic information, because larger individuals normally have larger heart size. The ideal indexing method should identify abnormal increases in chamber dimensions through diverse body types. The American Society of Echocardiography recognizes debate about the best indexing method but recommends reporting normal measurements indexed, or divided by, body surface area (BSA).8
Indexing measurements to BSA has limitations, because the BSA calculation is complex,9 has different formulae,10–13 and may falsely normalize obese individuals, obscuring pathologic remodeling. In other words, indexing to BSA may underestimate the prevalence of dilated or hypertrophied chambers in overweight individuals.8 The use of BSA has been questioned,14 and alternative indexes have been proposed, including powers of height, BSA1.5, body weight (BW)0.26, and body mass index (BMI)0.27.15–19 Determination of the best indexing method has application in echocardiography and in any imaging modality where limits of normal size are reported. Standardization of indexing method would allow comparison among reference values and echocardiographic laboratories.20 We hypothesized that chamber volumes and LV mass predict HF hospitalization and mortality, and that BSA is as useful as other indexing methods to adjust measurements for body size.
MATERIALS AND METHODS
Study Participants
The Heart and Soul Study is a prospective cohort study with the objective of evaluating psychosocial factors and health outcomes in patients with coronary disease. Methods and objectives have been described.21 We used administrative databases to identify outpatients with documented coronary artery disease (CAD) at 2 Department of Veterans Affairs Medical Centers (San Francisco Veterans Affairs Medical Center and the Veterans Affairs Palo Alto Health Care System, California), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had at least 1 of the following: a history of myocardial infarction, angiographic evidence of at least 50% stenosis in 1 or more coronary vessels, prior evidence of exercise-induced ischemia by treadmill or nuclear testing, a history of coronary revascularization, or a diagnosis of CAD by an internist or cardiologist.
A total of 15,438 eligible patients were mailed an invitation to participate, and 2,495 responded that they would be interested. Of the 2,495 patients whom we attempted to contact by telephone to schedule a study appointment, 505 could not be reached and 596 declined to participate. An additional 370 patients were excluded because they had a history of myocardial infarction in the prior 6 months, deemed themselves unable to walk 1 block, or were planning to move out of the local area within 3 years.
Between September 2000 and December 2002, a total of 1024 participants enrolled, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of CAD that was documented by their physician (based on a positive angiogram or treadmill test in > 98% of cases).
This protocol was approved by the following institutional review boards: the Committee on Human Research at the University of California, San Francisco; the Research and Development Committee at the San Francisco Veterans Affairs Medical Center; the Medical Human Subjects Committee at Stanford University; the Human Subjects Committee at the Veterans Affairs Palo Alto Health Care System; and the Data Governance Board of the Community Health Network of San Francisco. All participants provided written informed consent.
Data Collection
Each participant completed a detailed interview and questionnaire regarding age, gender, race, medical history, current smoking, and level of alcohol consumption. Echocardiographic studies were performed in the standard left lateral recumbent and supine positions with a commercially available ultrasound system with harmonic imaging (Acuson Sequoia, Siemens Corporation, Mountain View, CA). A single cardiologist (NBS), blinded to clinical and laboratory information, evaluated each comprehensive resting echocardiogram. ESV and LA volume were calculated using the biplane method of discs in the apical 4- and 2-chamber views at end systole, as recommended by the American Society of Echocardiography. LV mass was calculated from 2-dimensional wall thickness using the truncated ellipse method, using the semi-major and minor axis radius of the left ventricle.8
Cardiovascular Outcomes
We conducted telephone follow-up interviews and questioned participants or their proxies regarding hospitalizations and mortality. Medical records, death certificates, and coroner’s reports were retrieved. Two blinded adjudicators reviewed each event, and if there was agreement the outcome classification was binding. If there was disagreement, a third blinded adjudicator reviewed the event and determined the outcome classification. All-cause mortality results were complete for at least 1 year of follow-up for 1,018 of 1,024 individuals. Five individuals were completely lost to follow-up, and 1 individual declined to participate further in the study.
Hospitalization for HF was defined as a clinical syndrome requiring a minimum one-night hospital stay and involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, a third heart sound, cardiomegaly on chest radiography, or pulmonary edema on chest radiography. These clinical signs and symptoms must have represented a clear change from the normal clinical state of the patient and must have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes, eg, sepsis or dehydration) or peripheral or pulmonary edema treated with intravenous diuretics, inotropes, or vasodilators.
Mortality adjudications were based on hospital records, death certificates, and autopsy results. Death was considered due to cardiovascular (CV) causes if the death certificate listed acute myocardial infarction, congestive HF, or arrhythmia as the primary cause of death. Sudden death also was considered CV if it was unexpected, otherwise unexplained, and occurred within 1 hour of the onset of terminal symptoms.
Statistical Analysis
Baseline characteristics are reported as the mean plus or minus standard deviation for continuous variables and as percentages for categoric variables. Given the limitation of differentiating among risk factors by comparisons of hazard ratios (HRs) or c-statistic alone,22–24 we made calculations for both HR and c-statistic for each variable indexed to different parameters of body size. We used Cox proportional hazards (survival) models to calculate HR. To further explore our hypothesis that BSA is as useful as other methods of indexing for body size, we directly compared parameters indexed to basic values (BSA, height, BW, and BMI) with raw measurements using Pearson correlation coefficients. If excellent correlation was identified, less variation in predictive ability of indexing methods would be expected.
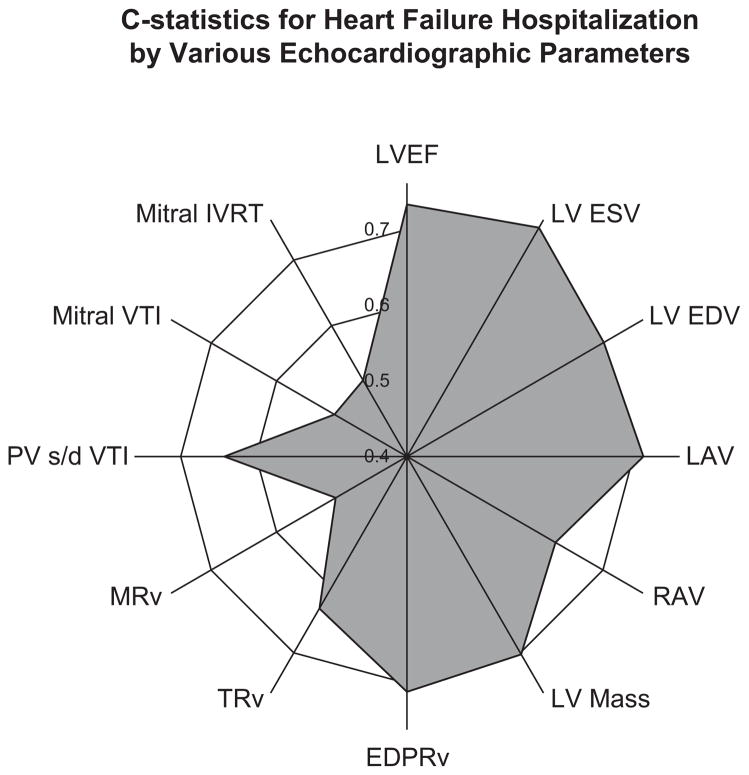
The c-statistics and HR are reported in radial plots, where the further distance from the center indicates higher c-statistics or HR. Figure 1 shows a sample radial plot for various echocardiographic variables measured among our population for the outcome of HF hospitalization. A symmetric circle would indicate equivalence among the different indexing parameters. Asymmetry of the image identifies variables with higher predictive ability as being further from the center. Analyses were performed using software (Statistical Analysis, Version 9.2, SAS Institute Inc, Cary, NC). Outcome events were counted once for each individual (eg, recurrent HF hospitalizations in the same participant were not counted). Predefined end points were HF hospitalization and all-cause mortality.
Figure 1.
Radial plot of c-statistics, or area under the receiver operating characteristic curve, for standard echocardiographic variables in predicting HF hospitalization. Further distance from the center of the plot indicates higher predictive ability. LVEF, Left ventricular ejection fraction (%); LV ESV, left ventricular end-systolic volume (mL); EDV, end-diastolic volume (mL); LAV, left atrial volume (mL); RAV, right atrial volume (mL); EDPRv, end-diastolic pulmonary regurgitation velocity (m/s); TRv, TR velocity (m/s); MRv, mitral regurgitation velocity (m/s); PV s/d VTI, pulmonary vein systolic to diastolic flow ratio by velocity time integral (dimensionless ratio); IVRT, isovolumic relaxation time (ms).
RESULTS
At an average 5.6 ± 1.8 year follow-up, there were 148 HF hospitalizations, 71 CV deaths, and 269 all-cause deaths. Baseline characteristics of study participants are shown in Table 1. The range of BW was 43 to 214 kg. The raw echocardiography measurements correlated most closely with measurements indexed height (r ≥ 0.98) followed by BSA (r ≥ 0.92), BMI (r ≥ 0.81), and BW (r ≥ 0.73), as shown in Table 2. The close correlation of raw measurements to those indexed to BSA and height suggested that these indexing parameters would not significantly reclassify individuals in our study population. However, we explored the predictive ability of all the indexing measurements further by HR, c-statistic, and direct comparison of BSA to height2.7. The standard deviations of each of the calculated variables are shown in Table 3.
Table 1.
Baseline characteristics
| Characteristic | Baseline measurement |
|---|---|
| Age | 67 ± 11 y |
| Weight | 83 ± 18 kg |
| Height | 171 ± 9 cm |
| BSA | 1.94 ± 0.22 m2 |
| BMI | 28.4 ± 5.3 kg/m2 |
| Waist circumference | 101 ± 15 cm |
| Hip circumference | 105 ± 13 cm |
| Systolic blood pressure | 133 ± 21 mm Hg |
| Diastolic blood pressure | 75 ± 11 mm Hg |
| Heart rate | 68 ± 12 beats/min |
| Male | 82% |
| Race | |
| White | 60% |
| Black | 16% |
| Asian | 12% |
| Other race | 12% |
| Diabetes mellitus | 26% |
| History of hypertension | 71% |
| History of MI | 54% |
| History of revascularization | 59% |
| History of stroke | 15% |
| LV end-systolic volume | 41 ± 26 mL |
| LV end-diastolic volume | 101 ± 37 mL |
| LV mass | 192 ± 57 g |
| Left atrial volume | 65 ± 24 mL |
| LV ejection fraction | 62 ± 10 % |
BSA, Body surface area; BMI, body mass index; MI, myocardial infarction; LV, left ventricular. This table summarizes the baseline data of 1024 study participants.
Table 2.
Correlation coefficients
| LV ESV (mL) | LA volume (mL) | LV mass (g) | |
|---|---|---|---|
| No index | 1.0 | 1.0 | 1.0 |
| /BSA, m2 | .98 | .95 | .92 |
| /Height, m | .99 | .99 | .98 |
| /BW, kg | .94 | .83 | .73 |
| /BMI, kg/m2 | .96 | .88 | .81 |
LV ESV, Left ventricular end-systolic volume; LA, left atrial; BSA, body surface area; BW, body weight; BMI, body mass index. The correlation among indexing methods for LV mass, LA volume, and LV ESV using Pearson correlation coefficients is shown. A value near 1 indicates excellent correlation among the different variables.
Table 3.
Standard deviations
| LV ESV (mL) | LA volume (mL) | LV mass (g) | |
|---|---|---|---|
| No index | 32 | 24 | 57 |
| /BSA, m2 | 16 | 12 | 26 |
| /BSA0.43 | 24 | 18 | 40 |
| /BSA1.5 | 12 | 8.9 | 19 |
| /h, m | 0.47 | 0.35 | 0.80 |
| /h0.25 | 11 | 8.2 | 20 |
| /h2 | 0.006 | 0.005 | 0.01 |
| /h2.7 | 0.0004 | 0.0003 | 0.0006 |
| /BW, kg | 0.18 | 0.14 | 0.32 |
| /BW0.26 | 8.2 | 6.0 | 14 |
| /BMI, kg/m2 | 1.2 | .91 | 2.1 |
| /BMI0.27 | 13 | 9.6 | 23 |
LV ESV, Left ventricular end-systolic volume; LA, left atrial; h, height; BW, body weight; BMI, body mass index. These standard deviation measurements were used for calculation of HRs for each indexing method for LV ESV, LA volume, and LV mass.
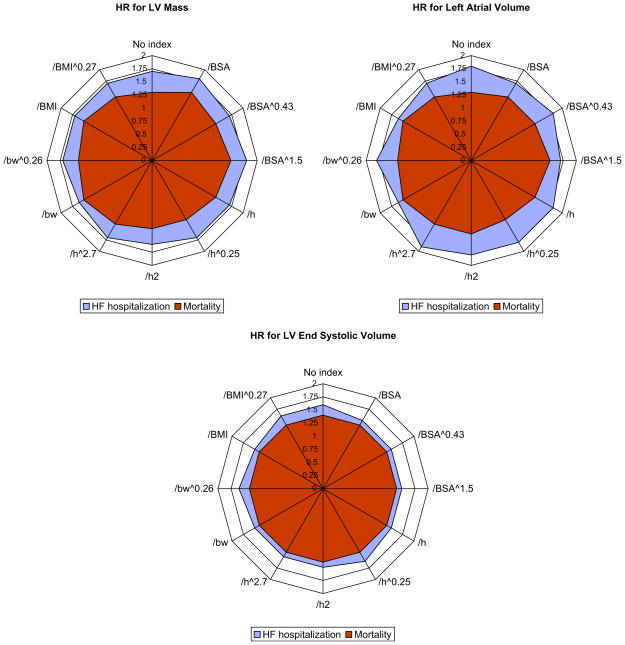
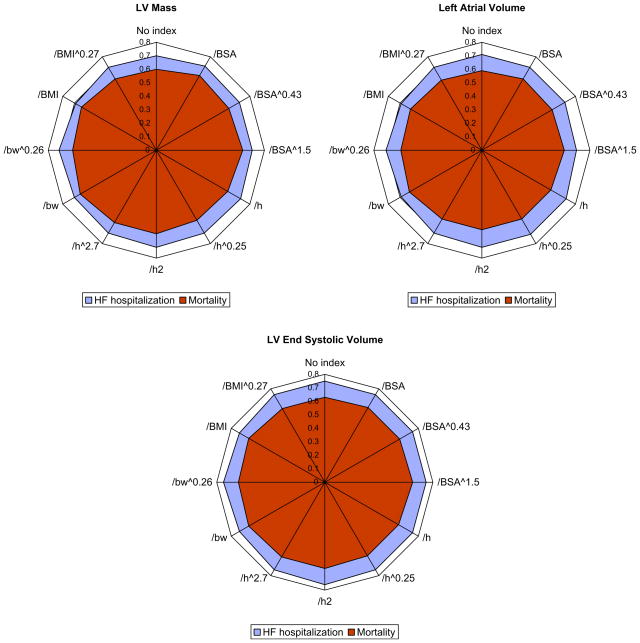
A radial plot of the c-statistics and HRs per standard deviation increase in variables is shown in Figures 2 and 3. The symmetric circular shapes indicate equivalence of the predictive ability for the tested indexing parameters. There were no significant differences among indexing methods in predictive ability by either c-statistic or HRs.
Figure 2.
HRs per standard deviation (as shown in Table 3) increase in variables indexed to different parameters of body size in the entire sample (n = 1,024). A circular configuration of the radial plot indicates equivalence among indexing methods. LV, Left ventricular; BSA, body surface area; h, height; bw, body weight; BMI, body mass index. The numeric values used to create these plots are listed in Table 4.
Figure 3.
C-statistics, or area under the receiver operating characteristic curve, for HF hospitalization and mortality by different methods of indexing for LV mass LA volume, and LV ESV in the entire sample (n = 1024). A circular configuration of the radial plot indicates equivalence among indexing methods. LV, Left ventricular; BSA, body surface area; h, height; bw, body weight; BMI, body mass index. The numeric values used to create these plots are listed in Table 4.
Percentages of events correctly identified by the highest quartile of LV mass indexed to BSA or height2.7 are shown in Figure 4. The green (bottom) bar indicates the percentage of individuals for whom there was agreement in classification. The yellow (top) bar indicates the percentage of individuals identified only by the one indexing method. There was no significant difference among indexing methods in ability to correctly classify individuals who developed HF hospitalization, CV mortality, or all-cause mortality.
Figure 4.
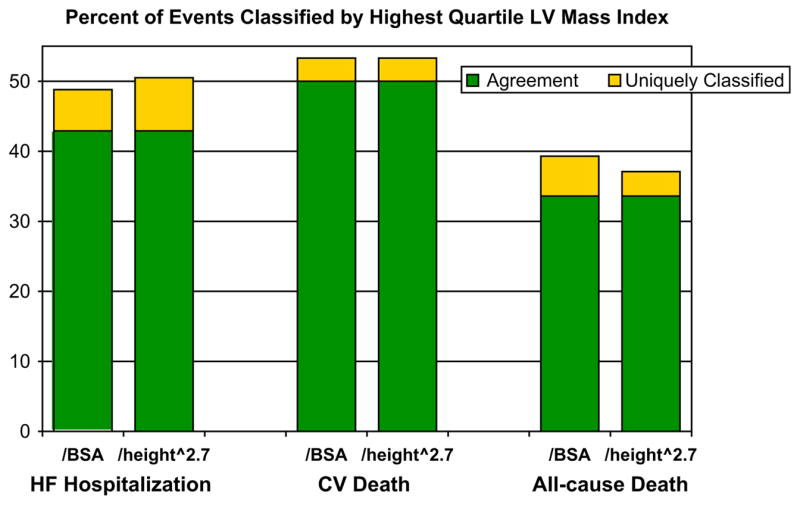
Percentage of HF hospitalizations, CV deaths, or all-cause deaths correctly classified by the highest quartile of LV mass indexed to BSA or height2.7 with results limited to men. The cutoff at the highest quartile indexing was 114 g/m2 for BSA and 52 g/m2.7 for height2.7.
DISCUSSION
We found that indexing parameters including BSA, height, BW, and BMI do not significantly alter the predictive ability of quantitative echocardiographic measurements for HF and mortality among ambulatory adults with CAD. There is at least moderate correlation among indexing parameters, and the c-statistics and HRs do not differ significantly in predictive ability among the sample size of 1024 individuals.
Several indexing parameters have been proposed for LV mass and LA volume among different patient populations. Theoretically, an indexing method that detects increases in chamber size or mass without regard to adiposity is desirable. Height-based parameters would satisfy this criterion. de Simone et al25 showed that LV hypertrophy indexed to height2.7 predicted mortality when inappropriately increased among 294 hypertensive individuals. However, Liao et al26 showed that a minority of individuals are reclassified from a “normal” to an “abnormal” group on the basis of height-based indices, and the predictive value for mortality in this group was not significantly different among height or BSA indexing methods. Our findings extend the results of similarity in indexing method to LV mass, LA volume, and LV ESV with regard to outcomes of both HF hospitalization and mortality.
The predictive value of various indexing methods for HF hospitalization and mortality is complicated by the “U”-shaped relationship between BW and mortality. Although obesity confers a significant risk for development of myocardial infarction,27 HF,28 and mortality,29 improvement in cardiac function after bariatric surgery in morbidly obese individuals has been described.28 Overweight individuals have been shown to have better prognosis when diagnosed with HF or CAD than normal weight or underweight individuals.30–32
Sever al limitations need to be considered in the study findings. Our study population was predominantly men aged more than 50 years, and previous studies have shown at least minor variations in body size relation to cardiac chamber dimensions by age and gender.33–35 We have no data on whether our results apply in childhood or adolescence, where allometric variables may have more impact.36,37 Second, we suspect that the principle of adjusting anatomic measurements to body size should not apply to exclusively individuals with underlying CAD, but we limit our conclusions to individuals enrolled in our study. Others have reported no difference in mortality risk by body size adjusted LV mass among those with or without CAD,26 but our population consists entirely of individuals with CAD. Third, our population consists primarily of overweight and mildly to moderately obese individuals, and our results may not apply to those with morbid obesity or severely underweight individuals.
The scope of this report applies primarily to differences in indexing methods as continuous variables; the possibility of diagnostic advantages using different cutpoints within 1 indexing parameter was addressed only for the highest quartile of LV mass comparing BSA to height2.7. It is possible that separate cutpoints among individual indexing parameters may have better discriminative ability compared with other indexing parameters. Also, the sample size of 1,024 individuals may not have had adequate power to differentiate among advantages in 1 indexing method that may be apparent in a larger population. Finally, we report the prognostic value of echocardiographic measurements divided by body size parameters, and the possibility of a risk score that combines body size parameters, such as waist circumference or BMI, was not addressed.
CONCLUSIONS
Although we considered a theoretic advantage of using different indexing parameters other than BSA to adjust for body size, we found a correlation among indexed variables and similarity of predictive value among ambulatory adults with CAD. Increases in echocardiographic measurements predict outcome when indexed to various powers of height, BW, BMI, and BSA. Given the advantage of standardization among reporting techniques among echocardiography laboratories, it is reasonable to continue using BSA to adjust for LA size, LV mass, and LV ESV in predicting HF hospitalization and mortality among ambulatory adults with CAD.
Table 4.
Data used for construction of radial plots
| C-stats | LV ESV (mL) |
LA volume (mL) |
LV mass (g) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HF | CVD | Death | HF | CVD | Death | HF | CVD | Death | |
| No index | 0.75 | 0.66 | 0.63 | 0.71 | 0.59 | 0.59 | 0.7 | 0.65 | 0.6 |
| /BSA, m2 | 0.75 | 0.68 | 0.64 | 0.71 | 0.62 | 0.61 | 0.72 | 0.69 | 0.64 |
| /BSA^0.43 | 0.75 | 0.67 | 0.64 | 0.71 | 0.61 | 0.6 | 0.71 | 0.67 | 0.62 |
| /BSA^1.5 | 0.75 | 0.68 | 0.65 | 0.7 | 0.63 | 0.61 | 0.71 | 0.70 | 0.64 |
| /h, m | 0.75 | 0.67 | 0.63 | 0.72 | 0.60 | 0.59 | 0.72 | 0.67 | 0.61 |
| /h^0.25 | 0.75 | 0.66 | 0.63 | 0.72 | 0.60 | 0.59 | 0.71 | 0.65 | 0.6 |
| /h2 | 0.76 | 0.68 | 0.64 | 0.72 | 0.61 | 0.59 | 0.72 | 0.68 | 0.62 |
| /h^2.7 | 0.75 | 0.68 | 0.64 | 0.71 | 0.62 | 0.59 | 0.71 | 0.69 | 0.62 |
| /BW, kg | 0.73 | 0.68 | 0.65 | 0.69 | 0.63 | 0.62 | 0.7 | 0.70 | 0.65 |
| /BW^0.26 | 0.75 | 0.67 | 0.64 | 0.71 | 0.61 | 0.6 | 0.72 | 0.67 | 0.62 |
| /BMI, kg/m2 | 0.73 | 0.68 | 0.65 | 0.69 | 0.62 | 0.61 | 0.69 | 0.68 | 0.64 |
| /BMI^0.27 | 0.75 | 0.67 | 0.63 | 0.71 | 0.60 | 0.6 | 0.71 | 0.66 | 0.61 |
| HR | LV ESV (mL) |
LA volume (mL) |
LV mass (g) |
||||||
| HF | CVD | Death | HF | CVD | Death | HF | CVD | Death | |
| No index | 1.6 | 1.2 | 1.4 | 1.8 | 1.5 | 1.3 | 1.7 | 1.5 | 1.3 |
| /BSA, m2 | 1.5 | 1.2 | 1.4 | 1.7 | 1.6 | 1.4 | 1.8 | 1.7 | 1.5 |
| /BSA^0.43 | 1.5 | 1.3 | 1.4 | 1.8 | 1.6 | 1.4 | 1.7 | 1.6 | 1.4 |
| /BSA^1.5 | 1.5 | 1.3 | 1.4 | 1.7 | 1.6 | 1.5 | 1.8 | 1.8 | 1.5 |
| /h, m | 1.5 | 1.3 | 1.4 | 1.8 | 1.6 | 1.4 | 1.7 | 1.8 | 1.4 |
| /h^0.25 | 1.6 | 1.2 | 1.4 | 1.8 | 1.5 | 1.3 | 1.7 | 1.5 | 1.3 |
| /h2 | 1.5 | 1.2 | 1.4 | 1.8 | 1.6 | 1.4 | 1.6 | 1.5 | 1.3 |
| /h^2.7 | 1.5 | 1.2 | 1.4 | 1.9 | 1.7 | 1.4 | 1.7 | 1.6 | 1.4 |
| /BW | 1.5 | 1.3 | 1.4 | 1.6 | 1.6 | 1.5 | 1.6 | 1.6 | 1.5 |
| /BW^0.26 | 1.6 | 1.3 | 1.4 | 1.8 | 1.6 | 1.4 | 1.7 | 1.6 | 1.4 |
| /BMI, kg/m2 | 1.5 | 1.3 | 1.4 | 1.6 | 1.6 | 1.5 | 1.7 | 1.6 | 1.5 |
| /BMI^0.27 | 1.6 | 1.2 | 1.4 | 1.7 | 1.6 | 1.4 | 1.7 | 1.5 | 1.4 |
C-statistics and HRs for events by echocardiographic indexing parameters are shown, with additional results listed for the outcome of CV death. LV, Left ventricular; ESV, end-systolic volume; LA, left atrial; HF, heart failure hospitalization; CVD, cardiovascular death; h, height; BW, body weight; BSA, body surface area; BMI, body mass index.
Acknowledgments
This study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Foundation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Nancy Kirwan Heart Research Fund, and an equipment loan from Siemens Corporation (Mountain View, California).
Footnotes
There are no conflicts of interest to report from any of the authors.
References
- 1.Cooper RS, Simmons BE, Castaner A, Santhanam V, Ghali J, Mar M. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol. 1990;65:441–5. doi: 10.1016/0002-9149(90)90807-d. [DOI] [PubMed] [Google Scholar]
- 2.Turakhai MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2008;102:1131–5. doi: 10.1016/j.amjcard.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol Img. 2009;2:11–20. doi: 10.1016/j.jcmg.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–41. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 5.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–8. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102:70–6. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the heart and soul study. J Am Soc Echocardiogr. 2009;22:190–7. doi: 10.1016/j.echo.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17:863–71. [Google Scholar]
- 10.Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 11.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height weight formula validated in infants, children and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 12.Gehan EA, George SL. Estimation of body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–35. [PubMed] [Google Scholar]
- 13.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55:515–24. doi: 10.1016/j.metabol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Lauer MS, Larson MG, Levy D. Gender-specific reference M-mode values in adults: population-derived values with consideration of the impact of height. J Am Coll Cardiol. 1995;26:1039–46. doi: 10.1016/0735-1097(95)00275-0. [DOI] [PubMed] [Google Scholar]
- 15.Lauer MS, Anderson KM, Larson MG, Levy D. A new method for indexing left ventricular mass for differences in body size. Am J Cardiol. 1994;74:487–91. doi: 10.1016/0002-9149(94)90909-1. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 17.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 18.Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol. 1995;76:699–701. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 19.Neilan TG, Pradhan AD, Weyman AE. Derivation of a size-independent variable for scaling of cardiac dimensions in a normal adult population. J Am Soc Echocardiogr. 2008;21:779–85. doi: 10.1016/j.echo.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Levy D, Larson MG, Benjamin EJ. Interpretation of echocardiographic measurements: a call for standardization. Am Heart J. 2000;139:412–22. doi: 10.1016/s0002-8703(00)90084-x. [DOI] [PubMed] [Google Scholar]
- 21.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JH. The limitation of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–7. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 23.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 25.de Simone G, Palmieri V, Koren MJ, Mensah GA, Roman MJ, Devereux RB. Prognostic implications of the compensatory nature of left ventricular mass in arterial hypertension. J Hypertens. 2001;19:119–25. doi: 10.1097/00004872-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29:641–7. doi: 10.1016/s0735-1097(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 27.Madala MC, Franklin BA, Chen AY, Berman AD, Roe MT, Peterson ED, et al. Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52:979–85. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 28.Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198–202. doi: 10.1016/j.cardfail.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 30.Kang X, Shaw LJ, Hayes SW, Hachamovitch R, Abidov A, Cohen I, et al. Impact of body mass index on cardiac mortality in patients with known or suspected coronary artery disease undergoing myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 2006;47:1418–26. doi: 10.1016/j.jacc.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 31.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 33.Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20–70 years: influence of age, sex and body surface area. J Intern Med. 1989;225:111–5. doi: 10.1111/j.1365-2796.1989.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 34.Spencer KT, Mor-Avi V, Gorcsan J, III, DeMaria AN, Kimball TR, Monaghan MJ, et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multiinstitution acoustic quantification study. Heart. 2001;85:272–7. doi: 10.1136/heart.85.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Simone G, Devereux RB, Roman MJ, Meyer RA, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–6. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 36.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–73. doi: 10.1016/j.jacc.2006.03.004. [DOI] [PubMed] [Google Scholar]