Abstract
The goal of the study was to determine how the changed balance of host naïve and regulatory T cells observed after conditioning with total lymphoid irradiation (TLI) and anti-thymocyte serum (ATS) promotes tolerance to combined organ and bone marrow transplants. Although previous studies showed that tolerance was dependent on host natural killer T (NKT) cells, the current study shows that there is an additional dependence on host CD4+CD25+ Treg cells. Depletion of the latter cells before conditioning resulted in rapid rejection of bone marrow and organ allografts. The balance of T cell subsets changed after TLI and ATS with TLI favoring mainly NK T cells and ATS favoring mainly Treg cells. Combined modalities reduced the conventional naïve CD4+ T cells 2800 fold. The host type Treg cells that persisted in the stable chimeras had the capacity to suppress alloreactivity to both donor and third party cells in the MLR. In conclusion, tolerance induction after conditioning in this model depends upon the ability of naturally occurring regulatory NKT and Treg cells to suppress the residual alloreactive T cells that are capable of rejecting grafts.
Keywords: Allograft tolerance, Antithymocyte globulin, Bone marrow transplantation, CD4+ CD25+T cells, T regulatory cells, Radiation
Introduction
The induction of tolerance to allografts by establishing stable mixed chimerism after non-myeloablative conditioning was first reported more than 30 years ago (1-3). Since then several laboratories have studied the cellular basis of tolerance in the mixed chimera model in a variety of inbred and outbred laboratory animals (4, 5, 6). Recently, the approach of combined organ and hematopoietic cell transplantation has been successfully applied to tolerance induction in humans (7, 8, 9). Conditioning regimens used to achieve mixed chimerism and tolerance include lethal and sublethal total body irradiation (TBI) with or without thymic irradiation and anti-T cell antibodies (4, 5, 6), TLI with and without anti-T cell antibodies (1, 2, 3, 8, 10, 11, 12), co-stimulatory blockade with or without rapamycin therapy or cytoreduction (13, 14, 15, 16), injection of naturally occurring CD4+CD25+ Treg (nTreg) cells combined with radiation cytoreduction (17, 18), and chemical cytoreduction combined with thymic irradiation, and anti-T cell antibodies (7, 9). Although central and peripheral clonal deletion in chimeras can explain the lack of reactivity of host immune cells to donor alloantigens (6, 19), host regulatory T cells that remain after cytoreduction or that are injected after cytoreduction can also play an important role in the engraftment of the donor organ and hematopoietic cells (12, 17, 18).
In the mixed chimera and tolerance induction model with TLI, anti-thymocyte serum (ATS), and bone marrow transplantation, tolerance is dependent on the residual host natural killer (NK) T cells (12). Since nTregs and Tregs induced from CD4+CD25- T cell precursors (iTregs) have been shown to play an important role in promoting tolerance to allografts in both chimeric and non-chimeric mouse models (15, 20, 21, 22, 23), we determined the role of residual host Tregs in the mixed chimera model using TLI and ATS conditioning in the current study. The requirement for host Tregs was determined by selectively depleting these cells pretransplant with a single injection of anti-CD25 mAb, a strategy that has been reported to interfere with tolerance induction (24). The results show that deficiency in either NK T cell or Tregs prevents chimerism and tolerance in the TLI and ATS model. This lymphodepletive-conditioning regimen facilitated tolerance by altering the balance of host T cell subsets to markedly favor the NKT cells and Tregs over alloreactive host naïve (CD62LhiCD44lo) T cells.
Materials and Methods
Mice
Adult 8- to 10-week-old, wild type BALB/c (H-2d), wild-type C57BL/6 (H-2b), p53-/- C57BL/6 and wild type C3H (H-2k) mice were obtained from The Jackson Laboratory and the Department of Comparative Medicine, Stanford University (Stanford, CA) as per existing protocols. CD1d-/- BALB/c and wild type mice were bred and/or maintained in the Department of Comparative Medicine, Stanford University (Stanford, CA).
Cardiac Transplantation and Monitoring for Graft Survival
Neonatal C57BL/6 heart grafts were transplanted into a pouch in the ear pinna of BALB/c hosts on day 0 according to the procedure described by Trager et al. (25). Heart grafts were monitored daily for visible contractions and survival was based on the time interval until contractions stopped.
Bone Marrow Transplantation and TLI
The procedure for bone marrow transplantation was described in details previously (12). TLI was delivered to the abdomen, lymph nodes, thymus, and spleen with shielding of the skull, lungs, limbs, pelvis and tail (5, 6). Irradiation was started on day 14 before transplantation and 10 doses of 240 cGy each were administered. The last dose of TLI was administered to BALB/c mice 24 hr before the allogeneic bone marrow cell infusions.
Rabbit Antithymocyte Serum and Anti-CD25 mAb Treatment
Rabbit ATS was purchased from Accurate Chemical and Scientific Inc. (Westbury, NY). BALB/c recipients were injected i.p. with 0.05 ml of ATS in 0.5 ml of saline on days 0, 2, 6, 8, and 10 after heart transplantation. Depletion of Tregs was accomplished by a single intraperitoneal injection of anti-CD25 mAb purchased from eBioscience, Inc., as described previously (24).
Immunofluorescent Staining Reagents and Monoclonal Antibodies
Anti-CD4-PE, anti-CD8-PE, anti-H-2Kb-FITC, anti-TCRαβ-PE, anti-TCRαβ-APC, and anti-CD16/32 monoclonal antibodies (mAb) to block FcR-γ II/III were purchased from BD Pharmingen (San Diego, CA). Staining for surface expression of CD25 and/or intracellular Foxp3 used anti-CD25 and anti-Foxp3 mAbs from eBioscience, Inc. (San Diego, CA). CD1d-tetramers conjugated to PE were obtained from the National Institutes of Health Tetramer Facility, Rockville, MD.
Immunofluorescent Staining and Chimerism Analysis
Single cell suspensions of blood or spleen cells were stained for surface markers with appropriate mAbs at 4°C in staining buffer containing propidium iodide and Fc receptor blocking antibodies as described previously (1), 7, 9, 10). Cell staining was analyzed on a FACS Vantage (Becton Dickinson, Mountain View, CA) (1, 7. Analysis of chimerism in the blood was performed by one color staining of total white blood cells with the anti-H-2Kb mAb. Staining procedures for CD4+CD25+Foxp3+ T cells were a modification of those reported previously using the eBioscience regulatory T cell staining kit (26).
Statistical Analysis
Statistical analyses of differences in heart graft survival between different groups of mice were performed using the log-rank test of Kaplan-Meier plots or Chi-square analysis at day 100. Comparisons of the fraction of mice in different groups that were chimeras were performed by chi-square analysis. Comparisons of mean percentages of absolute numbers of T cells and subsets in the spleen of different groups of mice were made using the two tail student t test of independent means. Comparisons of the mean stimulation index in the MLR were performed using the student t test as well.
Results
Tolerance and Chimerism require host regulatory T cells despite lymphodepletion
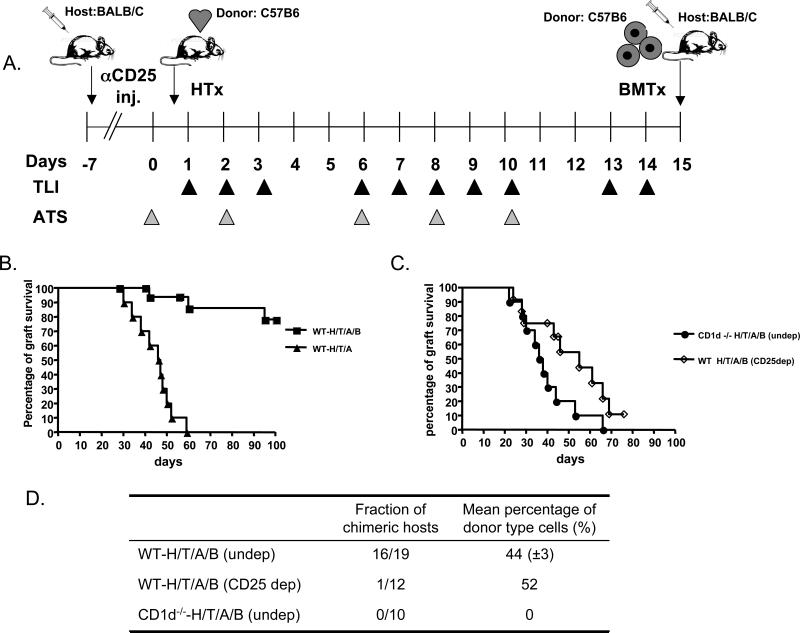
BALB/c host mice were given heart transplants from neonatal C57BL/6 donors on day 0, followed by 10 doses of TLI (240cGy each) and 5 doses of ATS over a 14 day period as shown in Figure 1A. One day after the completion of TLI, C57BL/6 bone marrow cells (50×106) were injected i.v. into the hosts. Figure 1B shows that more than 80% of the hosts that received the heart and bone marrow transplants accepted the heart grafts for at least 100 days, but hosts that did not receive the bone marrow injection all rejected their grafts by about 60 days (p<0.0001). Hosts with heart grafts surviving more than 100 days were given C3H heart transplants thereafter, and 6 of 6 were rejected within 3 weeks (data not shown). When the experimental scheme with combined heart and bone marrow transplants was repeated using NK T cell deficient CD1-/- instead of wild type hosts, then the heart grafts were all rejected by about day 65 (Figure 1C). The results indicate that the host NK T cells are required for tolerance induction, and survival of grafts in wild type versus CD1-/- hosts was significantly different (p<0.0001).
Figure 1. Dependence of Chimerism and Heart Transplant Tolerance on Residual Host CD4+ CD25+ and NK T cells.
A, experimental scheme: BALB/c host mice were given C57BL/6 neonatal heart transplants on day 0. ATS was injected i.p. on days 0, 2, 6, 8 and 10. TLI was given as 10 doses of 240cGy each over 14 days. On day 15, 50×106 C57BL/6 bone marrow cells were injected i.v. Some hosts were given a single dose of anti-CD25 mAb i.p. at day -7. B, heart graft survival in wild type hosts after TLI/ATS conditioning with or without bone marrow cell infusion (WT-H/T/A/B) (N=19) or (WT-H/T/A) (N=12). C, heart graft survival in CD1d-/- hosts after TLI/ATS conditioning and bone marrow cell infusion (CD1d-/- -H/T/A/B) (N=10) or in wild type hosts after anti-CD25 antibody injection, TLI/ATS, and bone marrow cell infusion (WT-H/T/A/B; CD25 dep) (N=12). D, peripheral blood chimerism in the latter hosts. The fraction of chimeric hosts in each group and the mean percentage of donor type cells among chimeric hosts are shown.
In order to determine whether host CD4+CD25+ Treg cells were required for tolerance induction; BALB/c hosts were given a single i.p. injection of anti-CD25 mAb, 7 days before heart transplantation (Figure 1A). The anti-CD25 mAb treatment reduced the percentage of CD4+CD25+ Treg cells among residual total CD4+ T cells from about 10% pre-treatment to less than 1% at the time of the bone marrow transplant on day 15 as judged by flow cytometry of host spleen cells (Figure 2 and 3A). The Treg depletion prevented the induction of tolerance in almost all hosts, and about 90% rejected their heart grafts by 80 days (p<0.0001), as compared to undepleted wild type hosts) (Figure 1C). Whereas 16 of 19 wild type undepleted hosts became mixed chimeras 28 days after bone marrow transplantation (Supplementary Figure 1), only 1 of 12 Treg depleted hosts developed mixed chimerism (p<0.0001) and none of 12 CD1-/- undepleted hosts became chimeric (p<0.0001) (Figure 1D). Thus, the acceptance of the bone marrow and heart grafts is dependent on both host NK T cells and Treg cells using this conditioning regimen.
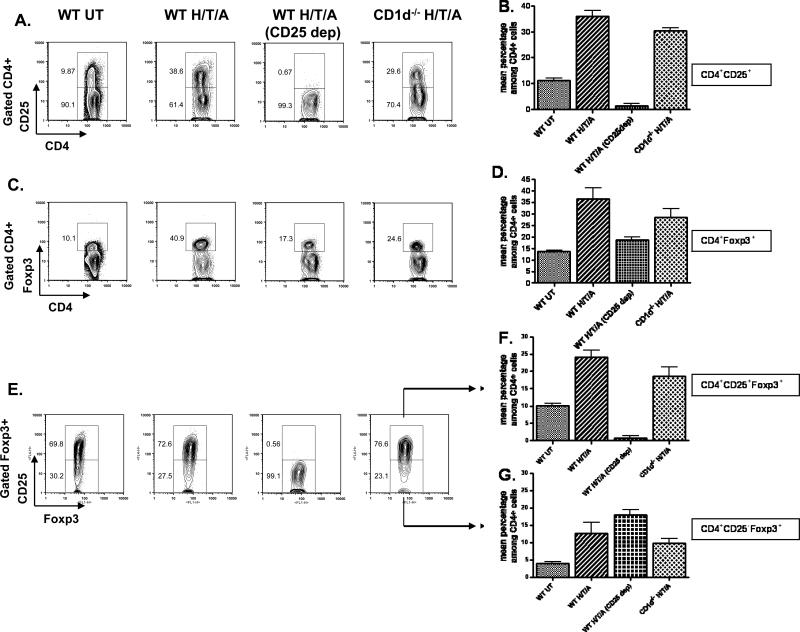
Figure 2. Heart Transplant Recipients Conditioned with TLI and ATS Have an Increased Proportion of CD4+ CD25+ Cells Among All CD4+ T Cells After Conditioning.
A, representative FACS patterns of CD25 versus CD4 among CD4+ gated splenocytes in untreated wild type BALB/c mice (WT-UT), TLI/ATS conditioned wild type mice given heart transplants (WT-H/T/A), CD25 depleted and conditioned wild type hosts (WT-H/T/A) (CD25 dep) and conditioned CD1d-/- BALB/c hosts (CD1d-/- -H/T/A) 24 hours after the completion of conditioning. B, Mean percentages (± SE) of CD4+CD25+ cells among total CD4+ cells in groups shown in panel A (N=5). C, staining of Foxp3 versus CD4 in groups shown in panel A. D, mean percentages of CD4+CD25+ cells among CD4+ cells in groups in panel C (N=5). E, staining of CD25 versus Foxp3 among Foxp3+ gated cells in groups shown in panel A. F, mean percentages of CD4+CD25+Foxp3+ cells among CD4+ cells in groups shown in panel E (N=5). G, mean percentages of CD4+CD25-Foxp3+ cells among CD4+ cells in groups shown in panel E.
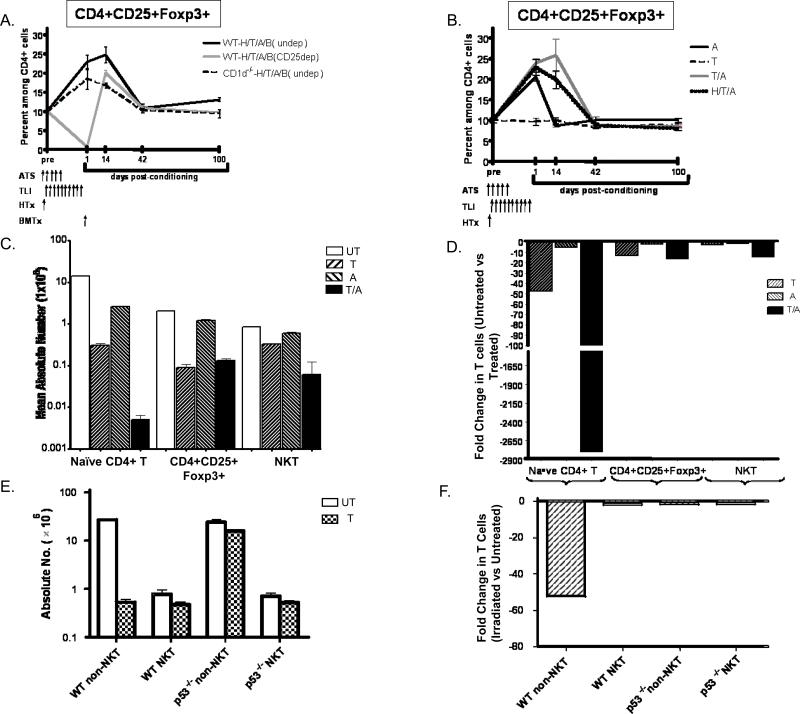
Figure 3. Changes in the Balance of T cell subsets After Combined Heart and Bone Marrow Transplantation and /or Conditioning.
A, mean percentages of host type H-2Kd+CD4+CD25+Foxp3+ cells among CD4+splenocytes before and after combined heart and bone marrow transplantation in TLI/ATS conditioned wild type BALB/c hosts (WTH/T/A/B)(undep), or in the same hosts that were given a single dose of anti-CD25 mAb on day -7 (CD25 dep) or in CD1-/- BALB/c hosts without anti-CD25 mab treatment (undep). Brackets show standard errors. N=4-5 mice for each time point B, mean percentages of CD4+CD25+Foxp3+ cells in wild type BALB/c mice given conditioning components with or without heart transplantation. N=4-5 mice for each time point. C, Mean absolute numbers of CD4+ naïve, Treg, and NKT cells in the spleen of untreated BALB/C mice or those given TLI alone, ATS alone or the combination of TLI and ATS on day 1 after conditioning (N= 5). D, Fold change in mean absolute numbers of treated and untreated mice. E, Mean absolute numbers of non-NKT and NKT cells in the spleen of wild type and p53-/- mice after conditioning with TLI alone (17 doses) compared to untreated mice (N= 6). Mice were C57BL/6, since p53-/- BALB/C were unavailable. F, Fold change in mean absolute numbers of untreated and treated mice in E.
The conditioning regimen induces marked lymphodepletion with about a 95% reduction of the absolute number of CD4+ T cells in the spleen 1 day after the completion of TLI, and 98% after completion of TLI and ATS in hosts with or without heart transplants as compared to untreated BALB/c mice (p<0.0001)(Supplementary Figure 2). Similar depletion of CD8+ T cells was observed (data not shown). TLI and ATS contributed synergistically to CD4+ T cell depletion in the spleen, as determined by experiments in which TLI alone or ATS alone or the combination, were given (Supplementary Figure 2C and D).
The balance of CD4+ T cell subsets changes after conditioning to favor CD4+ CD25+ Foxp3+ cells
In order to determine how the conditioning regimen affects the percentage of CD4+CD25+ cells among total CD4+ T cells, spleen cells from untreated wild type BALB/c mice were compared to those from mice given heart transplants and TLI/ATS conditioning. Whereas CD25+ cells were about 10% of gated CD4+ T cells in the untreated spleen, after heart transplantation and one day after the last dose of TLI the percentage of CD25+ cells increased to about 39% (Figures 2A and B). In mice given a single injection of anti-CD25 mAb before transplantation and conditioning, the CD25+ cells accounted for less than 1% of CD4+ cells (Figure 2A). There were significant increases in the mean percentages of the CD25+ cells among the CD4+ cells in transplanted and conditioned wild type (p<0.0001) or conditioned CD1-/- (p<0.0001) mice as compared to the untreated wild type mice. All three groups had means that were significantly different from the mean value of the anti-CD25 mAb treated mice (p<0.0001) that was at background staining levels (less than 1%). The difference between the wild type and CD1-/- mice given transplants was about 5% (35% versus 30%, p=0.02).
The spleen cells from the groups of BALB/c mice were stained also for the intracellular expression of Foxp3 (Figures 2C, D, E, F and G). The mean percentages of CD4+CD25+ Foxp3+ cells were similar to those of the CD25+ cells when Figure 2D is compared to Figure 2B except for mice given anti-CD25 mAb. The latter mice had a population of CD4+CD25- Foxp3+ cells that accounted for about 18% of CD4+ T cells (Figures 2C, D, E, F and G).
ATS is the Predominant Contributor to the Increased Percentage of CD4+ CD25+ Foxp3+ Cells One Day after Conditioning
Since the conditioning regimen included a combination of ATS and TLI, the contributions of each to the change in the balance of subsets were determined in additional experiments. The increase in CD4+CD25+ T cells was more marked with ATS alone or ATS and TLI as compared to TLI alone (Supplementary Figure 3). The combination of TLI and ATS induced a significantly greater increase as compared to ATS alone (p=0.0005) (Supplementary Figure 3A and B). When the same analysis was performed on CD4+Foxp3+ or CD4+ CD25+Foxp3+ cells instead of CD4+CD25+ cells, the percentages in mice with TLI alone versus untreated mice was not significantly different (p=0.8), but comparison of ATS alone or TLI and ATS conditioned mice versus untreated mice showed highly significant differences (p<0.0001)(Supplementary Figure 3C, D, E and F). ATS conditioning alone significantly increased (p=0.005) the mean percentage of CD4+CD25-Foxp3+ also compared to untreated controls (<0.0001) (Supplementary Figure 3G).
The effect of TLI and ATS on the percentage of NKT cells differed from that observed with Treg cells; whereas TLI alone increased the mean percentage of NKT cells (CD1 tetramer+ TCR+) from about 3% to 28%, ATS alone increased the percentage to about 8% (Supplementary Figure 4A). More importantly, the combination of TLI and ATS resulted in a significant reduction of the mean percentage of NKT cells from 28% with TLI alone to about 7% with the combination (p<0.0001) (Supplementary Figure 4A). Thus, the synergy between TLI and ATS in the augmentation of the percentage of Tregs was not observed with NKT cells.
Changes in the Balance of Treg, NKT, and Naïve CD4+ T Cells Are Due to Differences in Cell Depletion After Conditioning
Figure 3A shows the serial changes in the percentage of CD4+CD25+Foxp3+ cells among CD4+ cells in the spleen of wild type and CD1d-/- host mice before and after heart and bone marrow transplantation and conditioning. CD4+ cells of host type (H-2Kd+) were gated and analyzed at days 1, 14, 42, and 100 after marrow transplantation. In wild type hosts without anti-CD25 mAb treatment, the mean percentage of CD4+CD25+FopxP3+ cells rose from about 10% pre-treatment to about 22% at day 1, and about 25% at day 14 (p<0.0001). There was no significant difference from the pre-treatment level at day 42 (p>0.05). In contrast, the mean percentage in anti-CD25 mAb treated wild type hosts was less than 1% at day 1 (p<0.0001), rapidly increased to about 20% at day 14, and there was no significant difference from the pre-treatment levels at days 42 and 100 (p>0.05). Sorted host type CD4+CD25+ T cells were obtained from tolerant chimeric recipients at 100 days, and compared to sorted CD4+CD25+ T cells from from untreated BALB/C mice for the capacity to suppress the mixed leukocyte reaction (MLR). Supplementary Figure 5 shows that the stimulation index was suppressed 70 to 90% when Tregs from untreated or chimeric mice were added to responder cells cultured with either C3H or C57BL/6 stimulator cells.
Mice conditioned with TLI and ATS and given heart transplants without bone marrow transplants had similar kinetics of CD4+CD25+ T cell changes as compared to those with bone marrow transplants (Figure 3A and B). Conditioning with TLI alone induced no change in the CD4+CD25+ percentages as compared to untreated mice. Conditioning with ATS alone induced a transient increase similar to the combination conditioning on day 1, but returned to baseline at day 14 and beyond (Figure 3B). Thus, the combination of TLI and ATS prolonged the increase in Treg cells as compared to ATS alone. In order to determine the mechanisms that account for the marked changes in the percentages of CD4+ CD25+Foxp3+ Treg cells on day 1 after conditioning, we compared the changes in the absolute numbers of total CD4+ T cells, Treg cells, naïve CD4+ T cells, and NKT cells before and just after conditioning. Figures 3C and D show that the absolute number of Treg cells was reduced about 15 fold after conditioning with TLI or TLI and ATS, whereas the absolute number of total CD4+ T cells was reduced about 40 fold with TLI/ATS (Supplementary Figure 2). This resulted in about a 3 fold increase in the percentage of Tregs among CD4+ T cells (p< 0.0001). In contrast to the Tregs, the reduction in the absolute number of naïve (CD62L+CD44lo) CD4+ T cells was about 2,800 fold (p<0.0001) after the combination of TLI and ATS (Figures 3C and D). This dramatic reduction was due to the fall in the percentage of naïve CD4+ T cells from about 68% to about 1% among total CD4+ T cells (Figure 4A), and the 40 fold reduction in the absolute number of CD4+ T cells.
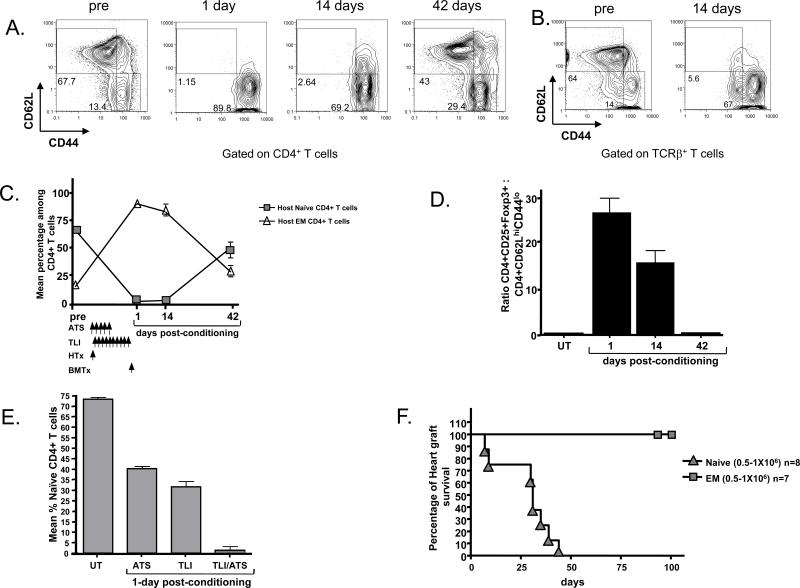
Figure 4. Reduced Proportion of Naïve T Cells in Transplant Recipients Further Alters the Balance of Effector and Regulatory T Cells.
A, representative FACS patterns of gated CD4+H-2Kd+ host type T cells for CD62L versus CD44 expression in wild type BALB/c mice before and 1 or 14 days after heart and bone marrow transplantation and TLI/ATS conditioning. Boxes show percentages of naïve (CD62L+CD44lo) and effector memory (CD62L-CD44hi) T cells. B, shows same analysis of gated host type total TCR+ T cells before and 14 days after transplantation. C, mean percentages of naïve and effector memory (EM) T cells among host CD4+ T cells before and after transplantation. N=6. D, mean ratios of host type CD4+CD25+FoxP3+ to CD4+ naïve T cells before and after transplantation. N=6. E, Mean percentages of naïve CD4+ T cells before and 5 days after conditioning with ATS alone, 24 hours after TLI alone, or 24 hours after TLI and ATS in combination without bone marrow or heart transplantation. Schedule of ATS and TLI administration was the same as in panel C. F, C57BL/6 heart graft survival in sublethally irradiated (300cGy TBI) RAG-2-/- BALB/c hosts after adoptive transfer of wild type BALB/c sorted naïve or effector memory phenotype T cells. Details of the sorting the latter cells have been described previously (31).
The reduction in the naïve CD4+ T cells was about 50 fold after TLI alone, and there was a marked synergy with TLI and ATS (Figure 3 C and D). As expected from our previous study (27) the NKT cell subset was the most resistant to depletion after TLI or TLI and ATS, and the reduction absolute number was about 10 fold with the combination (Figure 3C and D). In order to confirm that the change in balance of T cells subsets after TLI was due to the differences in resistance to radiation induced cell death based on the p53/Bcl-2 apoptotic pathway, the reduction in the absolute numbers of non-NKT cells and NKT cells was compared in wild type versus p53-/- mice. As shown in Figures 3E and F, the 50 fold decrease in non-NKT cells after TLI in wild type mice (p<0.0001) was not observed in the p53-/- mice, and the decrease was less than 2 fold (p>0.05). There was no significant difference (p>0.05) in the absolute number of NKT cells after conditioning in the wild type or p53-/- mice.
Kinetics of Naïve T Cell Depletion After Conditioning and Transplantation
Since CD4+CD25+ T cells suppress allograft rejection and GVHD (8, 17, 18, 28, 29), and naïve T cells induce allograft rejection and GVHD (30, 31, 32), the percentage of naïve (CD62LhiCD44lo) and effector memory (CD62LloCD44hi) T cells was determined before and after heart and bone marrow transplantation in recipients given TLI and ATS. As shown by the flow cytometry profiles of CD62L versus CD44 in Figure 4A, the naïve T cells accounted for about 68% of gated host type H-2Kd+CD4+ T cells before conditioning. However, the percentage was reduced to about 1-3% on days 1 and 14 after transplantation and conditioning, and returned to about 43% at day 42. A similar result was observed when spleen cells were gated on total H-2Kd+TCR+ T cells instead of CD4+ T cells, and naïve T cells were reduced from 64% to about 6% of T cells (Figure 4B). The percentage of effector memory cells increased in concert with the reduction of naïve cells at days 1 and 14 (Figures 4A and B).
The kinetics of the changes in the mean percentages of naïve and effector memory cells among CD4+ T cells was determined at serial time points before and after transplantation as shown in Figure 4C. The mean percentage of naïve cells before transplantation and conditioning was about 70% among all CD4+ T cells, and at day 1 after conditioning (at the time of bone marrow transplantation) was about 1%. The percentages of naïve CD4+ T cells were similar between days 1 and 14, but increased rapidly to about 50% by day 42. Whereas about 14×106 naïve CD4+ T cells were in the spleen before, there were about 0.01- 0.03×106 present during the first 14 days after conditioning. Thus, conditioning reduced the absolute number by about 500 -1,000 fold.
In contrast, the reduction in the mean absolute number of CD4+CD25+Foxp3+ T cells was about 8 fold. The mean absolute number was about was about 2×106 per spleen before transplantation, and about 0.25×106 during the first 14 days after. These differences resulted in a marked change in the ratio of CD4+CD25+Foxp3+ Treg cells to naïve CD4+ T cells from about 1:7 before transplantation to about 25:1 on day 1 and 17:1 on day14 (Figure 4D). However, by day 42 the ratio had returned to approximate the pre transplant value.
In order to determine the contributions of TLI and ATS alone to the dramatic reduction in naïve T cells, groups of mice without transplants were given ATS alone, TLI alone, or the combination of ATS and TLI using the time frame shown in Figure 4C. The ATS treatment reduced the mean percentage of naïve CD4+ T cells from about 70% to 40% at day 1 (p<0.0001), and the TLI reduced the percentage to about 30% (p<0.0001). However, the combination of TLI and ATS further reduced the percentage (p<0.0001) as compared to TLI or ATS alone to about 8%. A similar result was found for CD8+ T cells (supplemental Figure 2B). In summary, ATS is the main contributor to the increased percentage of Treg cells after conditioning, and both ATS and TLI contribute to the reduced percentage of naïve T cells.
Naive but not Memory Phenotype T cells Reject Allografts in Adoptive Hosts
We compared the potency of sorted naïve and effector memory phenotype T cells from untreated wild type male BALB/c mice that had not been exposed to alloantigens to reject C57BL/6 heart grafts in sublethally irradiated immunodeficient RAG-2-/- BALB/c adoptive hosts. The hosts were given transplants, 300cGy TBI, and 0.5-1×106 sorted T cells intravenously thereafter. As shown in Figure 4F, the injection of the naïve T cells induced rejection of all grafts by day 45. In contrast, the injection of the effector memory phenotype T cells failed to induce graft rejection during a 100-day observation period (p<0.0001). Hosts that received no cell injections also maintained all grafts for at least 100 days (data not shown).
Discussion
The goal of the current study was to determine the changes in the balance of regulatory and naïve T cell subsets and their contributions to graft acceptance or rejection using a TLI and ATS conditioning regimen that induces mixed chimerism and tolerance after combined heart and bone marrow transplantation (10, 11, 12). This regimen has been studied extensively in rodents, and has been used to induce persistent mixed chimerism and tolerance in humans (8). We found that host Treg and NKT cells were required for the induction of chimerism and tolerance, since depletion of wild type host Tregs with a single pretransplant injection of anti-CD25 mAb or use of CD1-/- host mice resulted in failure of the induction regimen.
One day after the completion of TLI and ATS conditioning (time point of donor bone marrow infusion) there was a marked increase in the ratio of CD4+ CD25+Foxp3+ Treg cells and NKT cells to naïve conventional T cells. The change in the balance of T cell subsets is best explained by their differential resistance to radiation induced cell death due to differential expression of the anti-apoptotic protein, Bcl-2, as reported previously (27). The current study showed that p53-/- mice had no change in the balance after TLI. T cell proliferation and thymic generation of T cells play a minimal role whereas the p53/Bcl-2 apoptotic pathway plays the dominant role in the changes (27). The considerable resistance of Treg cells to apoptosis induced by ATS as compared to conventional T cells has been reported to be due to high levels of expression of the anti-apoptotic protein, BCLXL (33). It is of interest that the altered balance of T cell subsets favoring Treg and NKT cells returned to normal about 6 weeks after conditioning, and by that time the stable mixed chimeras are tolerant to the heart allografts due to clonal deletion (12). The relationship between NKT cells and Tregs in responses to alloantigens has been reported recently (26).
Naïve but not memory phenotype CD4+ T cells from untreated mice show alloreactivity in the MLR, and alloreactivity by conventional T cells in the MLR is suppressed by Treg cells at a 1:1 ratio (28, 31). The changed ratio of these cells on day 1 was likely to have prevented the hosts from rejecting bone marrow and heart allografts acutely. The ability of naïve and memory phenotype T cells from untreated BALB/c mice to reject C57BL/6 heart allografts was compared by transferring equal numbers of sorted cells to immunodeficient RAG-2-/- BALB/c hosts bearing the grafts. Whereas the naïve cell injection resulted in rejection of all grafts, the memory phenotype cell injection obtained from donors that had not been exposed to alloantigens resulted in no rejection over a 100-day observation period.
In conclusion, the TLI and ATS tolerance induction model requires both regulatory NKT cells and Tregs for graft acceptance. Tolerance is promoted by profound but transient changes in the balance of regulatory and naive T cells of host origin favoring the regulatory cells. This study provides insights into the role of T cell subsets in clinical trials of tolerance using TLI and ATG conditioning (8).
Supplementary Material
Supplementary Figure 1. Representative FACS Patterns of T cell -, B cell - and Granulocyte/macrophage Chimerism At 28 days.
Staining was performed for H-2Kb versus TCR, B220, or Mac-1/Gran-1 on gated cell subsets after wild type BALB/c mice were given TLI/ATS conditioning and combined heart and bone marrow transplantation from C57BL/6 donors.
Supplemntary Figure 2. Marked T Cell Depletion is Present in NKT Cell or CD4+ CD25+ T Cell Deficient Hosts that Can Reject Grafts
A, mean percentages of CD4+ T cells among splenocytes in untreated wild type BALB/c mice (WT-UT), TLI/ATS conditioned wild type hosts given heart transplants (WT-H/T/A), CD25 depleted and conditioned wild type hosts with transplants (WT-H/T/A; CD25 dep) and conditioned CD1d-/- hosts with transplants (CD1d-/- -H/T/A) 24 hours after the completion of conditioning (N=5 per group). B, mean absolute number of CD4+ cells in groups of mice shown in panel A. C, mean percentage of total CD4+ cells among splenocytes in wild type mice without heart transplants 24 hours after TLI conditioning (WT-T), or 5 days after ATS conditioning (WTA) or 24 hours after TLI and ATS conditioning (WT T/A) (N=5). D, mean absolute number of CD4+ cells among splenocytes in groups of mice shown in panel C.
Supplementary Figure 3. Dominant Influence of ATS on Increase in Proportion of CD4+ Foxp3+ T Cells After Conditioning
A, representative FACS patterns of CD25 versus CD4 among CD4+ wild type BALB/c splenocytes in untreated mice (UT), 24 hours after TLI (10 doses) (T), 5 days after ATS (5 doses) (A), and 24 hours after combined TLI/ATS (T/A) conditioning. B, mean percentages of CD25+CD4+ cells among CD4+ T cells shown in panel A (N=5). C, staining of Foxp3 versus CD4 in groups shown in panel A. D, mean percentages of CD4+Foxp3+ cells among CD4+ cells in groups in panel C. E, staining of CD25 versus Foxp3 among gated Foxp3+ cells in groups from panel C. F and G, mean percentages of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells respectively among total CD4+ T cells in groups from panel E.
Supplementary Figure 4: Conditioning with TLI Increases the Percentage of NKT Cells and Decreases the Percentage of Naive CD8+ T cells More Effectively Than ATS.
A, BALB/c mice were either untreated or treated with TLI alone, ATS alone, or TLI and ATS. Spleen cells were stained for TCR+CD1-tetramer+ cells 24 hours after TLI alone, 5 days after ATS alone, or 24 hours after TLI and ATS. Bars show the mean percentages of tetramer+ cells among gated TCR+ cells. N=8 for untreated mice, and N=5 for treated mice. UT vs TLI, p<0.0001; TLI vs. ATS or TLI/ATS, p<0.0001.
B, BALB/c mice were either untreated or treated with TLI and/or ATS, and the percentage of CD622hi CD44lo (naive) cells among gated CD8+ T cells in the spleen was determined after conditioning. Bars show mean percentages of naive cells. UT vs ATS, p<0.0001; UT vs TLI, p<0.0001; UT vs TLI/ATS, p<0.0001. N=5 for UT, N=7 for ATS and TLI alone, N=5 for TLI/ATS.
Supplementary Figure 5: CD4+ CD25+ T Cells from Untreated and Chimeric BALB/c Mice Suppress the MLR to C57BL/6 and C3H Third Party Stimulator Cells
Stimulation index was calculated from 3H-thymidine incorporation with or without stimulator cells. BALB/c sorted TCR+ splenocytes were used as responders, irradiated (20Gy) C57B/6 (donor type) splenocytes or C3H (third party) splenocytes as stimulators with and without addition of sorted untreated (UT BALB/c) wild type BALB/c CD4+CD25+ Treg cells (Panel A) or H-2Kd+ Treg cells (CHIM BALB/c) from BALB/c hosts given combined C57BL/6 heart and bone marrow transplants 100 days earlier (Panel B). Panel C shows the percentage suppression of 3H-thymidine incorporation in Panels A and B. Bars show means of triplicate wells. One of 2 replicate experiments is shown. There were 1×105 responder, stimulator, and/ or Treg cells in each well.
Acknowledgments
We thank Glenna Letsinger for assistance in the submission of the manuscript. In addition, we also thank the NIH tetramer facility, Rockville Md., for providing CD1d-tetramer.
Grant Support:This work was supported by grants from the National Institutes of Health RO1- AI-037683 and PO1- CA-49605
Abbreviations
- TLI
Total lymphoid irradiation
- NKT
Natural Killer T cells
- ATS
Anti-thymocyte serum
Footnotes
Disclosures
The authors have no commercial or financial conflict of interest.
References
- 1.Slavin S, Strober S, Fuks Z, Kaplan HS. Long-term survival of skin allografts in mice treated with fractionated total lymphoid irradiation. Science. 1976;193(4259):1252–1254. doi: 10.1126/science.785599. [DOI] [PubMed] [Google Scholar]
- 2.Slavin S, Reitz B, Bieber CP, Kaplan HS, Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978;147(3):700–707. doi: 10.1084/jem.147.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146(1):34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73(11):1757–1764. doi: 10.1097/00007890-200206150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14(4):417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 7.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6(9):2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 8.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayamizu K, Lan F, Huie P, Sibley RK, Strober S. Comparison of chimeric acid and non-chimeric tolerance using posttransplant total lymphoid irradiation: cytokine expression and chronic rejection. Transplantation. 1999;68(7):1036–1044. doi: 10.1097/00007890-199910150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Lan F, Hayamizu K, Strober S. Cyclosporine facilitates chimeric and inhibits nonchimeric tolerance after posttransplant total lymphoid irradiation. Transplantation. 2000;69(4):649–655. doi: 10.1097/00007890-200002270-00029. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169(10):5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 13.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6(4):464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 14.Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, et al. Cutting edge: administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000;165(1):1–4. doi: 10.4049/jimmunol.165.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JF, Colvin GA, Zhong S, Wang H, D'Hondt L, Abedi M, et al. H2-mismatched transplantation with repetitive cell infusions and CD40 ligand antibody infusions without myeloablation. Br J Haematol. 2002;119(1):155–163. doi: 10.1046/j.1365-2141.2002.03801.x. [DOI] [PubMed] [Google Scholar]
- 16.Graca L, Daley S, Fairchild PJ, Cobbold SP, Waldmann H. Co-receptor and co-stimulation blockade for mixed chimerism and tolerance without myelosuppressive conditioning. BMC Immunol. 2006;7:9. doi: 10.1186/1471-2172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 18.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 19.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187(12):2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai JG, Xue SA, Coe D, Addey C, Bartok I, Scott D, et al. Regulatory T cells, derived from naive CD4+CD25- T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation. 2005;79(10):1310–1316. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 21.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 23.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7(6):1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 24.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174(6):3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 25.Trager DK, Banks BA, Rosenbaum GE, Holm BI, Shizuru JA, Strober S, et al. Cardiac allograft prolongation in mice treated with combined posttransplantation total-lymphoid irradiation and anti-L3T4 antibody therapy. Transplantation. 1989;47(4):587–591. doi: 10.1097/00007890-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an IL-4 dependent expansion of donor CD4+CD25+Foxp3+ Tregs that protects against graft-versus-host disease. Blood. 2009 doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. 2009;39(3):763–775. doi: 10.1002/eji.200838657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiopu A, Wood KJ. Regulatory T cells: hypes and limitations. Curr Opin Organ Transplant. 2008;13(4):333–338. doi: 10.1097/MOT.0b013e3283061137. [DOI] [PubMed] [Google Scholar]
- 29.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104(50):19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutt S, Tseng D, Ermann J, George TI, Liu YP, Davis CR, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179(10):6547–6554. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 32.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minamimura K, Gao W, Maki T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol. 2006;176(7):4125–4132. doi: 10.4049/jimmunol.176.7.4125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative FACS Patterns of T cell -, B cell - and Granulocyte/macrophage Chimerism At 28 days.
Staining was performed for H-2Kb versus TCR, B220, or Mac-1/Gran-1 on gated cell subsets after wild type BALB/c mice were given TLI/ATS conditioning and combined heart and bone marrow transplantation from C57BL/6 donors.
Supplemntary Figure 2. Marked T Cell Depletion is Present in NKT Cell or CD4+ CD25+ T Cell Deficient Hosts that Can Reject Grafts
A, mean percentages of CD4+ T cells among splenocytes in untreated wild type BALB/c mice (WT-UT), TLI/ATS conditioned wild type hosts given heart transplants (WT-H/T/A), CD25 depleted and conditioned wild type hosts with transplants (WT-H/T/A; CD25 dep) and conditioned CD1d-/- hosts with transplants (CD1d-/- -H/T/A) 24 hours after the completion of conditioning (N=5 per group). B, mean absolute number of CD4+ cells in groups of mice shown in panel A. C, mean percentage of total CD4+ cells among splenocytes in wild type mice without heart transplants 24 hours after TLI conditioning (WT-T), or 5 days after ATS conditioning (WTA) or 24 hours after TLI and ATS conditioning (WT T/A) (N=5). D, mean absolute number of CD4+ cells among splenocytes in groups of mice shown in panel C.
Supplementary Figure 3. Dominant Influence of ATS on Increase in Proportion of CD4+ Foxp3+ T Cells After Conditioning
A, representative FACS patterns of CD25 versus CD4 among CD4+ wild type BALB/c splenocytes in untreated mice (UT), 24 hours after TLI (10 doses) (T), 5 days after ATS (5 doses) (A), and 24 hours after combined TLI/ATS (T/A) conditioning. B, mean percentages of CD25+CD4+ cells among CD4+ T cells shown in panel A (N=5). C, staining of Foxp3 versus CD4 in groups shown in panel A. D, mean percentages of CD4+Foxp3+ cells among CD4+ cells in groups in panel C. E, staining of CD25 versus Foxp3 among gated Foxp3+ cells in groups from panel C. F and G, mean percentages of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells respectively among total CD4+ T cells in groups from panel E.
Supplementary Figure 4: Conditioning with TLI Increases the Percentage of NKT Cells and Decreases the Percentage of Naive CD8+ T cells More Effectively Than ATS.
A, BALB/c mice were either untreated or treated with TLI alone, ATS alone, or TLI and ATS. Spleen cells were stained for TCR+CD1-tetramer+ cells 24 hours after TLI alone, 5 days after ATS alone, or 24 hours after TLI and ATS. Bars show the mean percentages of tetramer+ cells among gated TCR+ cells. N=8 for untreated mice, and N=5 for treated mice. UT vs TLI, p<0.0001; TLI vs. ATS or TLI/ATS, p<0.0001.
B, BALB/c mice were either untreated or treated with TLI and/or ATS, and the percentage of CD622hi CD44lo (naive) cells among gated CD8+ T cells in the spleen was determined after conditioning. Bars show mean percentages of naive cells. UT vs ATS, p<0.0001; UT vs TLI, p<0.0001; UT vs TLI/ATS, p<0.0001. N=5 for UT, N=7 for ATS and TLI alone, N=5 for TLI/ATS.
Supplementary Figure 5: CD4+ CD25+ T Cells from Untreated and Chimeric BALB/c Mice Suppress the MLR to C57BL/6 and C3H Third Party Stimulator Cells
Stimulation index was calculated from 3H-thymidine incorporation with or without stimulator cells. BALB/c sorted TCR+ splenocytes were used as responders, irradiated (20Gy) C57B/6 (donor type) splenocytes or C3H (third party) splenocytes as stimulators with and without addition of sorted untreated (UT BALB/c) wild type BALB/c CD4+CD25+ Treg cells (Panel A) or H-2Kd+ Treg cells (CHIM BALB/c) from BALB/c hosts given combined C57BL/6 heart and bone marrow transplants 100 days earlier (Panel B). Panel C shows the percentage suppression of 3H-thymidine incorporation in Panels A and B. Bars show means of triplicate wells. One of 2 replicate experiments is shown. There were 1×105 responder, stimulator, and/ or Treg cells in each well.