Abstract
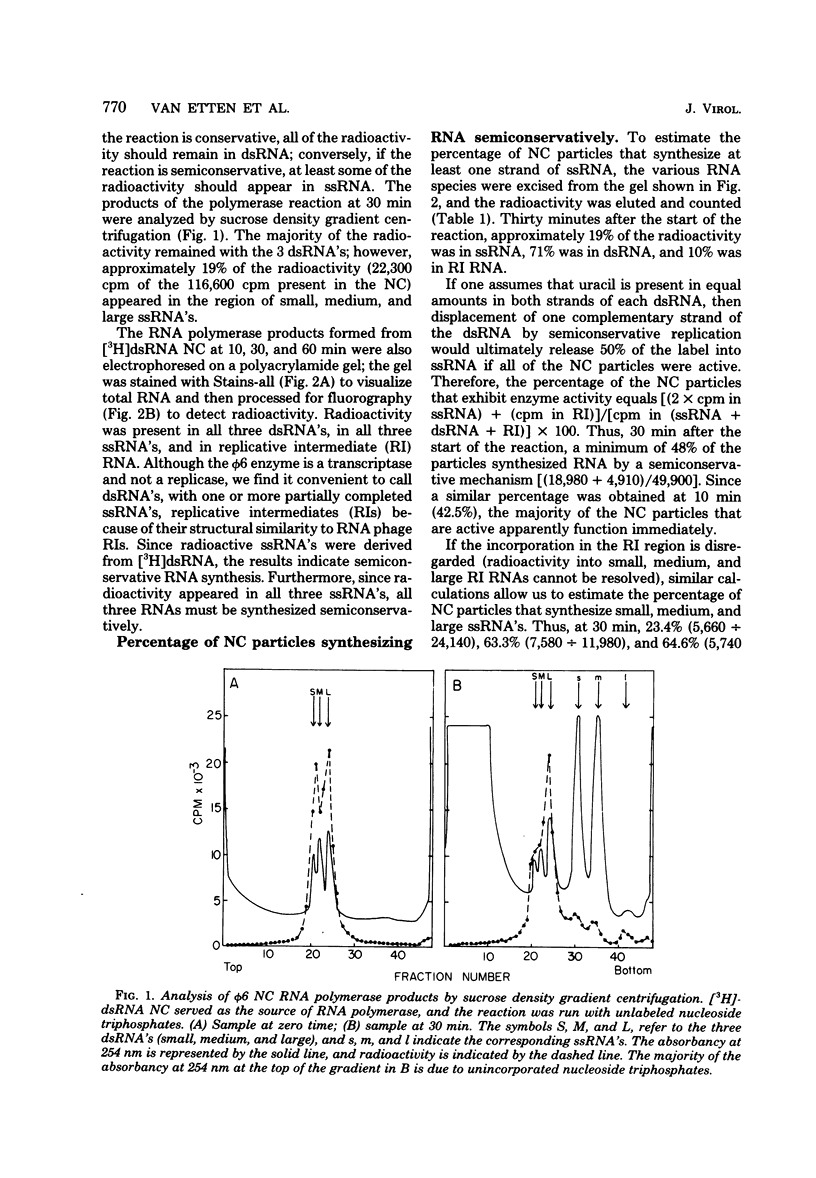
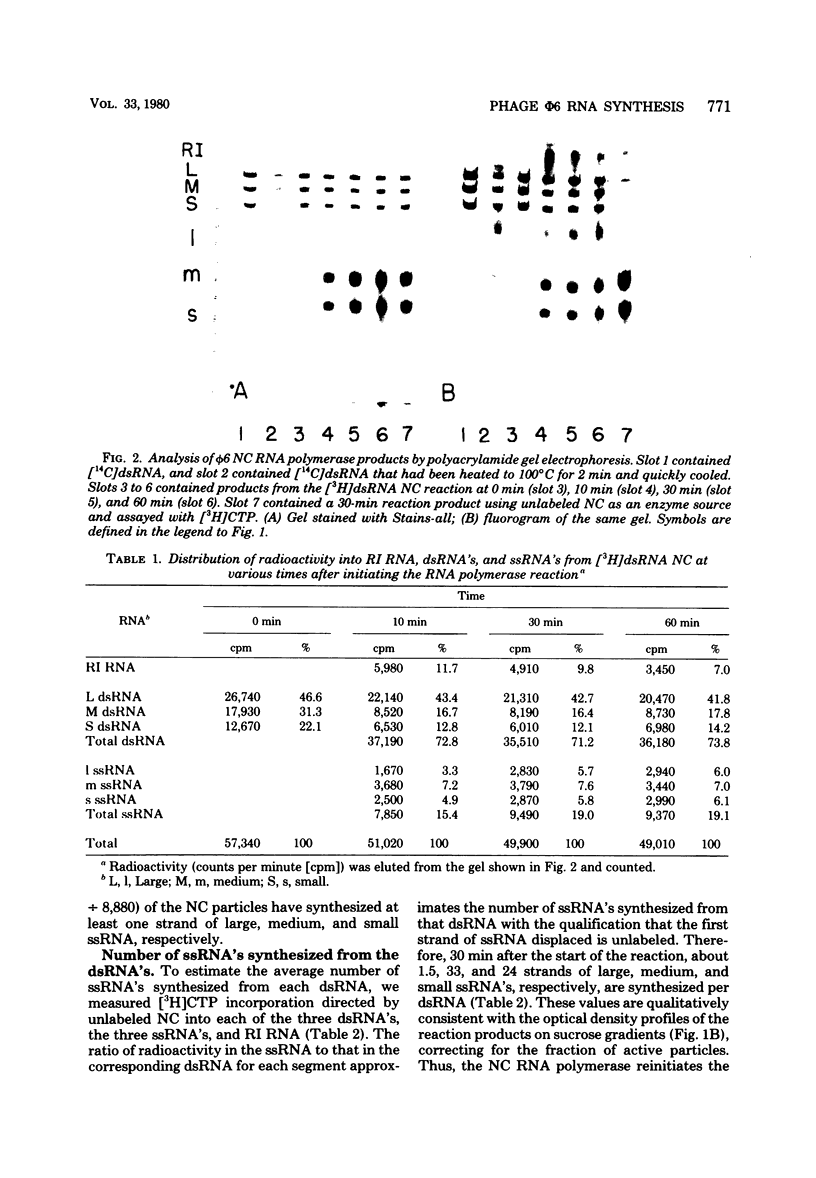
The RNA polymerase in the nucleocapsid of Pseudomonas phaseolicola bacteriophage phi 6 transcribed large, medium, and small single-stranded RNA from the viral double-stranded RNA genome by a semiconservative (displacement) mechanism. Approximately 23%, 63%, and 65% of the nucleocapsid particles in the assay mixture synthesized at least one round of large, medium, and small single-stranded RNA molecules, respectively. Some of these particles reinitiated synthesis such that an average of 1.5 large, 33 medium, and 24 small single-stranded RNAs were synthesized from each double-stranded RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Buck K. W. Semi-conservative replication of double-stranded RNA by a virion-associated RNA polymerase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):639–645. doi: 10.1016/0006-291x(78)90753-2. [DOI] [PubMed] [Google Scholar]
- Coplin D. L., Van Etten J. L., Koski R. K., Vidaver A. K. Intermediates in the biosynthesis of double-stranded ribonucleic acids of bacteriophage phi 6. Proc Natl Acad Sci U S A. 1975 Mar;72(3):849–853. doi: 10.1073/pnas.72.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin D. L., Van Etten J. L., Vidaver A. K. Synthesis of bacteriophage phi6 double-stranded ribonucleic acid. J Gen Virol. 1976 Dec;33(3):509–512. doi: 10.1099/0022-1317-33-3-509. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Etten J. V., Lane L., Gonzalez C., Partridge J., Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976 May;18(2):652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Ratti G., Buck K. W. Semi-conservative transcription in particles of a double-stranded RNA mycovirus. Nucleic Acids Res. 1978 Oct;5(10):3843–3854. doi: 10.1093/nar/5.10.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon A., Haselkorn R. Transcription and replication of bacteriophage phi6 RNA. Virology. 1978 Aug;89(1):206–217. doi: 10.1016/0042-6822(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Mindich L. RNA synthesis during infection with bacteriophage phi6. Virology. 1976 Nov;75(1):209–217. doi: 10.1016/0042-6822(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Tzagoloff A., Levine D., Mindich L. Proteins of bacteriophage phi6. J Virol. 1975 Sep;16(3):685–695. doi: 10.1128/jvi.16.3.685-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Burnett J. P. Base composition and hybridization studies of the three double-stranded RNA segments of bacteriophage phi 6. J Virol. 1974 Jun;13(6):1254–1262. doi: 10.1128/jvi.13.6.1254-1262.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Semancik J. S. RNA polymerase activity associated with bacteriophage phi 6. J Virol. 1973 Sep;12(3):464–471. doi: 10.1128/jvi.12.3.464-471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]