Abstract
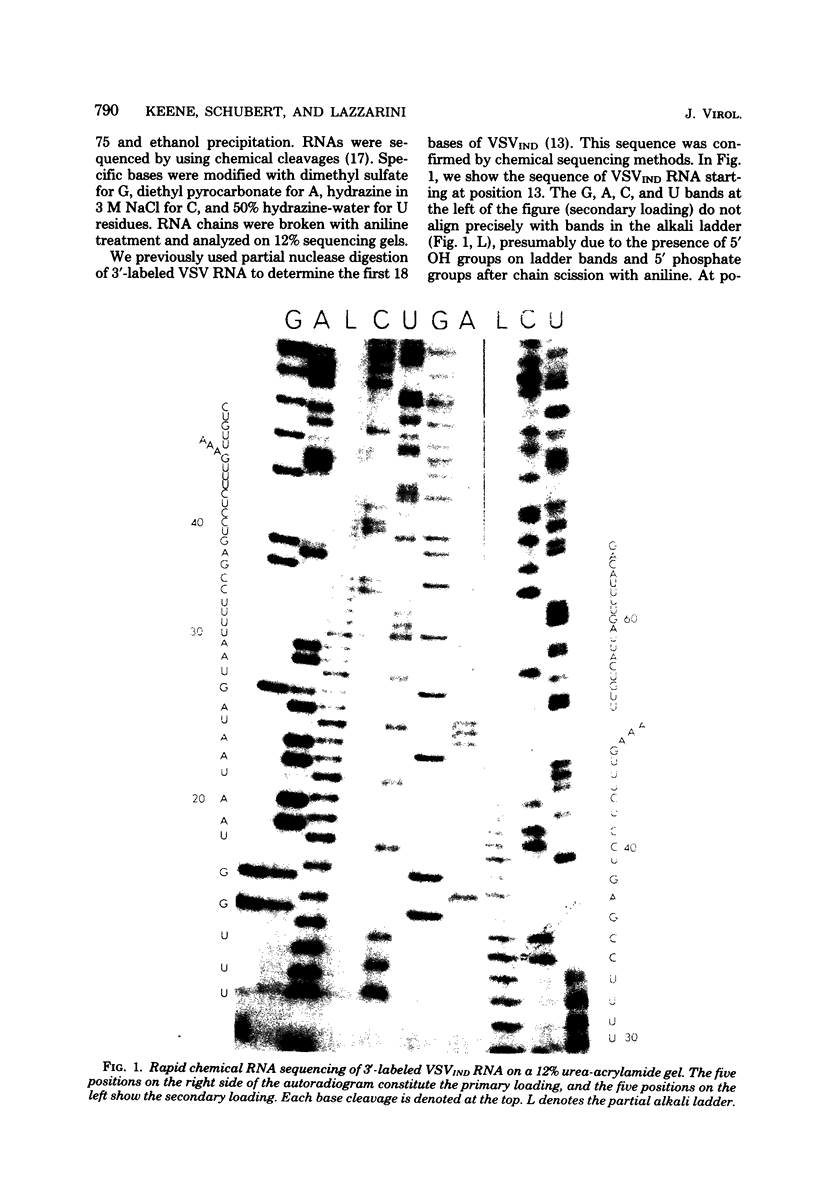
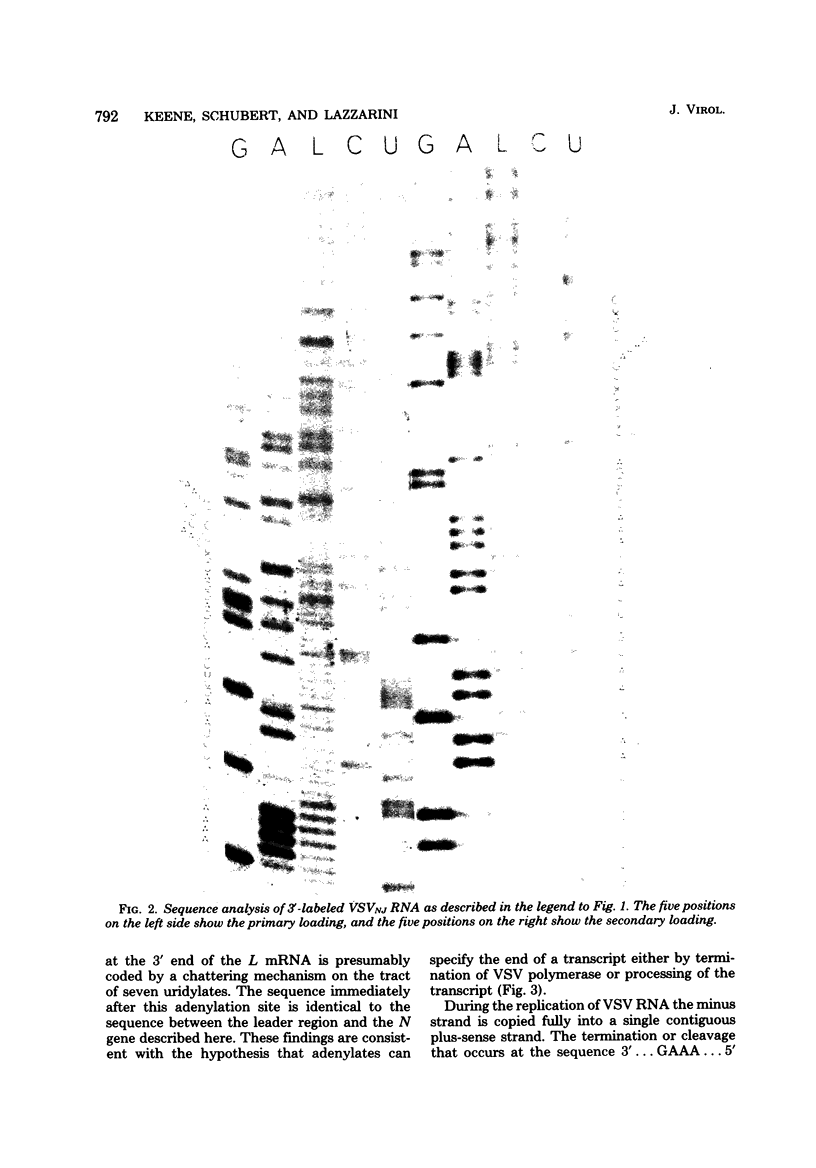
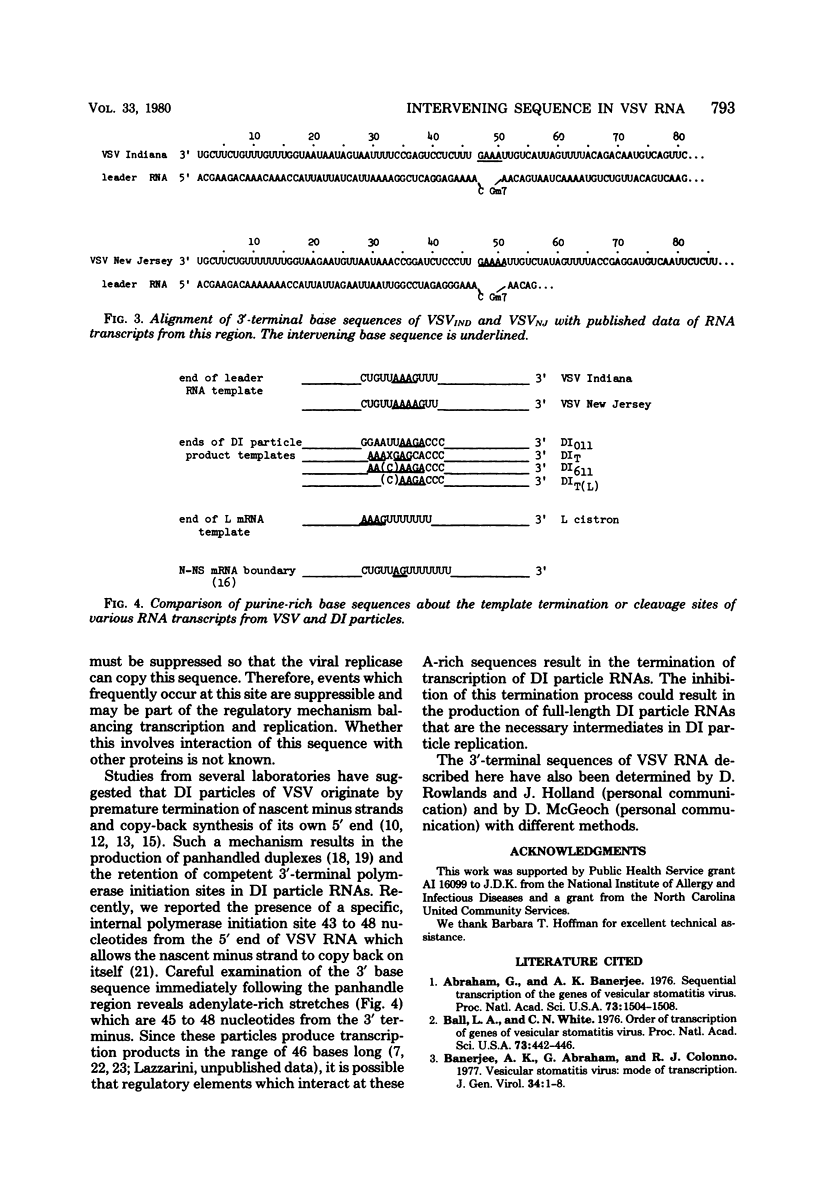
The base sequence at the 3' end of vesicular stomatitis virus RNA was determined by using terminal labels and chemical RNA sequencing. The leader RNA was complementary to 47 bases at the 3' terminus, whereas the nucleocapsid gene (N) began 51 nucleotides from the 3' end of the genomic RNA. The intervening bases were 3'...GAAA...5' for the Indiana serotype and 3'...GAAAA...5' for the New Jersey serotype. The complements of these bases did not appear in either the leader RNA or the N mRNA. This sequence may function as a stop signal or cleavage site during transcription. Furthermore, processing or termination at this sequence must be inhibited during the production of full-length RNA plus-sense strands (replication). We recently found similar sequences approximately 46 to 48 nucleotides from the 3' ends of several defective interfering particle RNAs where the short defective interfering particle transciption products terminate. This sequence is present also at the end of the polymerase (L) gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Nucleotide sequence of the leader RNA of the New Jersey serotype of vesicular stomatitis virus. Nucleic Acids Res. 1978 Nov;5(11):4165–4176. doi: 10.1093/nar/5.11.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Dierks P. M., Parsons J. T. In vitro synthesis of a unique RNA species by a T particle of vesicular stomatitis virus. J Virol. 1977 Sep;23(3):708–716. doi: 10.1128/jvi.23.3.708-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze-Fernandez M. T., Banerjee A. K. In vitro RNA transcription by the New Jersey serotype of vesicular stomatitis virus. I. Characterization of the mRNA species. J Virol. 1978 Apr;26(1):179–187. doi: 10.1128/jvi.26.1.179-187.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Adler S., Lazzarini R. A., Colonno R. J., Banerjee A. K., Westphal H. Intervening polyadenylate sequences in RNA transcripts of vesicular stomatitis virus. Cell. 1978 Oct;15(2):587–596. doi: 10.1016/0092-8674(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Rosenberg M., Lazzarini R. A. Characterization of the 3' terminus of RNA isolated from vesicular stomatitis virus and from its defective interfering particles. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1353–1357. doi: 10.1073/pnas.74.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Structure of the gene N:gene NS intercistronic junction in the genome of vesicular stomatitis virus. Cell. 1979 Jul;17(3):673–681. doi: 10.1016/0092-8674(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete sequences of the ribosome recognition sites in vesicular stomatitis virus mRNAs: recognition by the 40S and 80S complexes. Cell. 1978 Jun;14(2):345–353. doi: 10.1016/0092-8674(78)90120-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]