Abstract
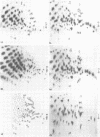
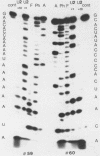
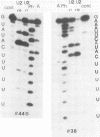
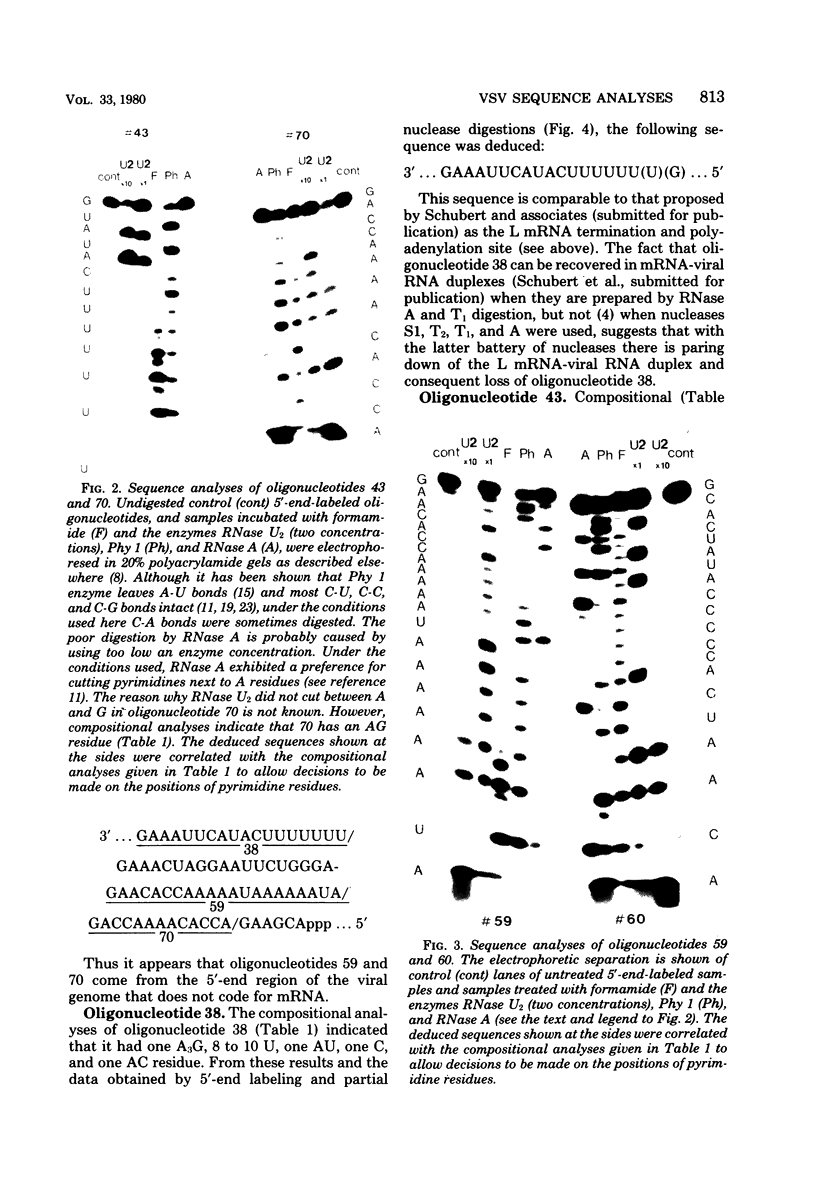
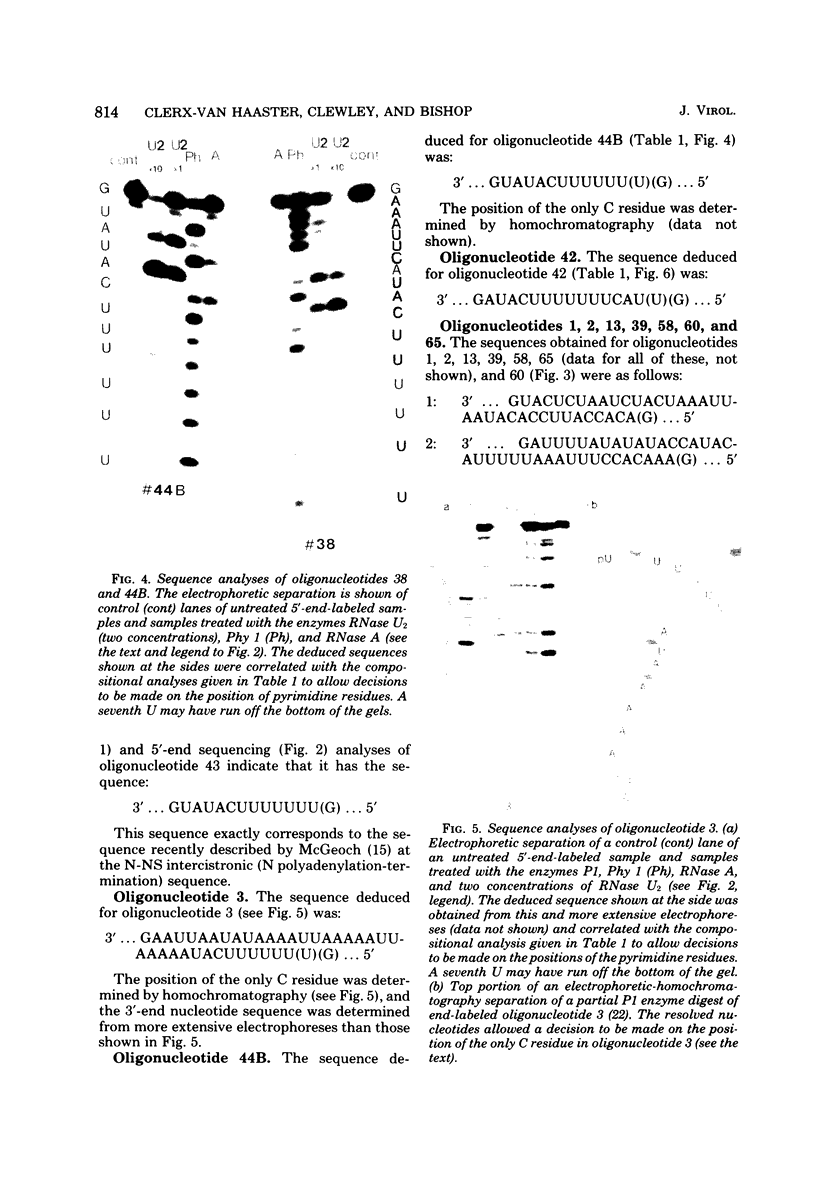
RNase T1 oligonucleotide fingerprint analyses of three vesicular stomatitis virus Indiana serotype small defective interfering (DI) particle RNA species indicate that they only have oligonucleotides derived from the 5′ region of the viral genome. These studies also indicate that these three DI RNAs have partial L gene sequences as well as two 5′ viral oligonucleotides (59 and 70) that are not transcribed into L (or other) mRNA species (J. P. Clewley and D. H. L. Bishop, J. Virol. 30:116-123, 1979). Analyses of the large DI RNA (LT DI) reveal a different origin. The LT DI RNA has oligonucleotides derived from both the 3′ end of the genome (including all the large oligonucleotides identified for N, NS, M, and G genes), in addition to at least one of the 5′-proximal L gene oligonucleotides (47), as well as all seven oligonucleotides (3, 38, 42, 43, 44B, 59, and 70) that are not protected from nuclease digestion after the formation of mRNA-viral RNA duplexes (Clewley and Bishop). It appears therefore that the genesis of LT RNA involves a deletion of internal L gene sequences from the viral RNA. Oligonucleotide sequence analyses have been undertaken on several of the vesicular stomatitis viral RNA oligonucleotides, including all seven (3, 38, 42, 43, 44B, 59, and 70) that are not transcribed into mRNA. The analyses confirm that oligonucleotides 59 [3′...GAACACCAAAAAUAAAAAAUA(G)...5′] and 70 [3′...GACCAAAACACCA(G)...5′] are at the 5′-end region of the viral genome. Oligonucleotide 38 [3′...GAAAUUCAUACUUUUUU(U)(G)...5′] may represent the termination signal for L mRNA synthesis (R. A. Lazzarini, personal communication). Oligonucleotide 43 [3′...GUAUACUUUUUUU(G)...5′] corresponds to the sequence shown to be the N gene mRNA polyadenylation signal (D. J. McGeoch, Cell 17:673-681, 1979). The other three oligonucleotides share a common feature with oligonucleotides 43 and 38, viz., a stretch of 6 or 7 U residues preceded by an AUAC sequence. Thus the sequence of oligonucleotide 3 is 3′...GAAUUAAUAUAAAAUUAAAAAUUAAAAAUACUUUUUU(U)(G)...5′, whereas that of oligonucleotide 42 is 3′...GAUACUUUUUUUCAU(U)(G)...5′, and that of oligonucleotide 44B is 3′...G(U)AUACUUUUUU(G)...5′. These sequence analyses suggest a common polyadenylation signal for the synthesis of all vesicular stomatitis virus mRNA species, i.e., the sequence (3′)...AUACUUUUUU(U)...(5′).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D. A., Herman R. C., Chien I., Lazzarini R. A. Defective interfering particle generated by internal deletion of the vesicular stomatitis virus genome. J Virol. 1980 Feb;33(2):818–829. doi: 10.1128/jvi.33.2.818-829.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Rao D. D., Huang A. S. RNA synthesis of vesicular stomatitis virus. VIII. Oligonucleotides of the structural genes and mRNA. Gene. 1979 Feb;5(2):141–157. doi: 10.1016/0378-1119(79)90099-4. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing-an enzymic method for distinguishing between cytidine and uridine residues. Nucleic Acids Res. 1977 Oct;4(10):3441–3454. doi: 10.1093/nar/4.10.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Structure of the gene N:gene NS intercistronic junction in the genome of vesicular stomatitis virus. Cell. 1979 Jul;17(3):673–681. doi: 10.1016/0092-8674(79)90274-5. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Turnbull N. T. Analysis of the 3'-terminal nucleotide sequence of vesicular stomatitis virus N protein mRNA. Nucleic Acids Res. 1978 Nov;5(11):4007–4024. doi: 10.1093/nar/5.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Pilly D., Niemeyer A., Schmidt M., Bargetzi J. P. Enzymes for RNA sequence analysis. Preparation and specificity of exoplasmodial ribonucleases I and II from Physarum polycephalum. J Biol Chem. 1978 Jan 25;253(2):437–445. [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]