Abstract
Purpose
Oxaliplatin effect in the treatment of colorectal cancer is improved upon combination with thymidylate synthase (TS) inhibitors. Pemetrexed is polyglutamated by the folylpolyglutamate synthase (FPGS) and blocks folate metabolism and DNA synthesis by inhibiting TS, dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyltransferase (GARFT). The present study evaluates the pharmacological interaction between oxaliplatin and pemetrexed in colorectal cancer cells.
Methods
Human HT29, WiDr, SW620 and LS174T cells were treated with oxaliplatin and pemetrexed. Drug interaction was studied using the combination index method, while cell cycle was investigated with flow cytometry. The effects of drugs on Akt phosphorylation and apoptosis were studied with ELISA and fluorescence microscopy, respectively. RT-PCR analysis was performed to assess whether drugs modulated the expression of pemetrexed targets and of genes involved in DNA repair (ERCC1 and ERCC2). Finally, platinum–DNA adduct levels were detected by ultra-sensitive multi-collector inductively coupled plasma mass spectrometry (ICP-MS).
Results
A dose-dependent inhibition of cell growth was observed after drug exposure, while a synergistic interaction was detected preferentially with sequential combinations. Oxaliplatin enhanced cellular population in the S-phase. Drug combinations increased apoptotic indices with respect to single agents, and both drugs inhibited Akt phosphorylation. RT-PCR analysis showed a correlation between the FPGS/(TS × DHFR × GARFT) ratio and pemetrexed sensitivity, as well as a downregulation of ERCC1, ERCC2, TS, DHFR and GARFT after drug exposure. In addition, pretreatment with pemetrexed resulted in an increase of oxaliplatin–DNA adducts.
Conclusion
These data demonstrate that oxaliplatin and pemetrexed synergistically interact against colon cancer cells, through modulation of cell cycle, inhibition of Akt phosphorylation, induction of apoptosis and modulation of gene expression.
Keywords: Colon cancer, Oxaliplatin, Pemetrexed, Gene expression, DNA repair, DNA adducts
Introduction
Colorectal cancer is the second leading cause of cancer-related deaths in the western world [1]. During the last 4 decades the thymidylate synthase (TS) inhibitor 5-fluorouracil (5-FU) has been the treatment of choice for colorectal cancer, in both adjuvant and palliative settings, despite the fact that as a single agent it produces responses in only 15–20% of metastatic patients [2]. In recent years, the combinations of 5-FU or its oral prodrug capecitabine with irinotecan and oxaliplatin and with novel biological agents bevacizumab and cetuximab have increased responses in first-line therapy up to 40% [3].
Oxaliplatin (L-OHP) is a platinum analogue that differs from cisplatin by the presence of a diaminocyclohexane ligand in its chemical structure; it forms mainly intrastrand links between two adjacent guanine residues, disrupting DNA replication and transcription [4]. The ability of cells to repair these platinum-induced DNA lesions or adducts is known to play an important role in oxaliplatin cytotoxicity. One of the major DNA repair systems in mammalian cells is the nucleotide excision repair (NER), which removes bulky helix distorting adducts produced by oxaliplatin [5]. The excision repair cross-complementing group 1 (ERCC1) protein is an important factor in the incision process, which is the rate-limiting step of the pathway. ERCC1 forms a heterodimer with XPF, and the ERCC1/XPF complex is responsible for the cleavage of the damaged strand [6]. A functional ERCC1 is essential to repair the platinum–DNA adducts and it is involved in drug sensitivity in vitro [7, 8]. Moreover, low ERCC1 gene expression levels correlated with improved overall survival following 5-FU/oxaliplatin therapy in advanced colorectal cancer patients [9]. Another molecular determinant of alkylating agents resistance/sensitivity is the helicase excision repair cross-complementing group 2 (ERCC2), whose protein levels correlated with drug cytotoxicity in a panel of cancer cell lines [10].
Fluoropyrimidine resistance in several tumors, including colorectal cancer may be related to TS expression [11]; in addition, high levels of dUMP have been found in colorectal tumor samples from patients unresponsive to 5-FU/leucovorin [12]. More potent inhibitors of TS and newer antifolates may overcome these resistance mechanisms and lead to improved drug efficacy. Furthermore, these drugs would be beneficial to treat patients with dihydropyrimidine dehydrogenase deficiency, which results in severe adverse reactions to 5-FU [13].
Pemetrexed is a multitarget antifolate (MTA) whose mechanism of action relies mainly on the inhibition of TS, with weaker secondary effects on glycinamide ribonucleotide formyltransferase (GARFT) and dihydrofolate reductase (DHFR), leading to impairment of DNA synthesis and repair [14]. Pemetrexed is rapidly polyglutamated by the enzyme folylpolyglutamate synthase (FPGS) and its polyglutamate derivatives are retained within the cell; this accumulation represents a substantial pharmacological advantage, since it produces a prolonged suppression of target enzymes [15]. Combinations of pemetrexed with agents such as gemcitabine and platinum compounds are currently under investigation and have shown in vitro and in the clinical setting promising results in several chemoresistant tumors [16, 17], including colorectal cancer [18].
In the present study, we have evaluated the pharmacological interaction between oxaliplatin and pemetrexed and established the optimal combination schedule in four human colorectal cancer cell lines. Additional experiments have explored the molecular and cellular basis of the pharmacological effects observed with respect to cell cycle distribution, induction of apoptosis, Akt phosphorylation, modulation of gene expression and the formation of oxaliplatin–DNA adducts.
Materials and methods
Drugs and chemicals
Oxaliplatin, 5-fluorouracil and methotrexate (MTX) were obtained from Sigma Chemical Co. (St. Louis, MO, USA), while pemetrexed and GARFT inhibitor LY309887 were generous gifts from Eli Lilly (Indianapolis, IN, USA). Drugs were dissolved in sterile distilled water and diluted in culture medium immediately before use. McCoy’s 5A medium, Leibovitz L-15 medium, Minimum Essential Medium Eagle (MEME), fetal bovine serum (FBS), l-glutamine, penicillin and streptomycin were obtained from Gibco (Gaithersburg, MD). All other chemicals were from Sigma Chemical Co. (St. Louis, MO, USA).
Cell lines
Human colon cancer cells HT29, SW620, WiDr and LS174T were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained as monolayer cultures in McCoy’s 5A (HT29), Leibovitz L-15 (SW620) and MEME (WiDr and LS174T) medium, supplemented with 10% fetal bovine serum, l-glutamine (2 mM), penicillin (50 IU/ml) and streptomycin (50 μg/ml). Cells were cultivated in 75 cm2 tissue culture flasks (Costar, Cambridge, MA, USA), at 37°C in 5% CO2 and 95% air, and harvested with trypsin–EDTA when they were in logarithmic growth.
Drug incubations and cytotoxicity assay
Cytotoxicity was assessed by the CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) based on the cellular metabolism of the tetrazolium compound MTT. Cells were seeded at 5 × 104 cells/ml in 12-well plates and allowed to attach for 24 h. Cells were treated with each drug (24 h), either alone or in combination, as follows: (1) oxaliplatin 0.001 ng/ml–100 μg/ml; (2) pemetrexed 0.001 ng/ml–100 μg/ml; (3) oxaliplatin and pemetrexed, simultaneously;( 4) oxaliplatin, followed by pemetrexed; (5) pemetrexed, followed by oxaliplatin. Concentrations for drug combination studies were based on IC50 values, representing concentrations resulting in 50% inhibition of cell growth, obtained from experiments with single agents. Cells were also treated with 5-fluorouracil, methotrexate and LY309887 (0.001 ng/ml–100 μg/ml) for gene expression experiments. For cytotoxicity experiments cell cultures were allowed to grow in drug free medium for an additional 24 h following drug exposure and growth inhibition was expressed as the percentage absorbance, relative to untreated control cultures, measured at 570 nm with a microplate reader (Multiskan Spectrum, Vantaa, Finland). IC50 values were calculated using the sigmoid inhibition model (GraphPad PRISM version 4.0, Intuitive Software for Science, San Diego, CA, USA).
Drug interaction between oxaliplatin and pemetrexed was assessed, at a fixed concentration ratio (see “Results”), using the combination index, where CI < 1, CI = 1, and CI > 1 indicate synergistic, additive and antagonistic effects, respectively. Data analysis was performed using the Calcusyn software (Biosoft, Oxford, UK).
Cell cycle analysis
Cells were plated at a density of 1 × 105 cells/ml in 100 mm Petri dishes (Costar) and treated with oxaliplatin and pemetrexed at concentrations corresponding to IC50 levels. Cells were harvested immediately after the end of drug exposure, washed twice with PBS and DNA was stained with propidium iodide (25 μg/ml), RNase (1 mg/ml) and Nonidet-P40 (0.1%). Samples were kept on ice for 30 min and cytofluorimetry was performed using a FACScan (Becton–Dickinson, San José, CA, USA). Data analysis was carried out with CELLQuest (Becton–Dickinson) and Modfit software (Verity Software, Topsham, ME, USA).
Analysis of apoptosis
Apoptosis was evaluated in cells treated as described above for drug incubations and cytotoxicity assays, with oxaliplatin, pemetrexed and their combinations at IC50 levels. At the end of the incubation period, cells were washed twice with PBS and fixed in 4% buffered paraformaldehyde for 15 min. Cells were resuspended and incubated for a further 15 min in a solution containing 8 μg/ml bisbenzimide HCl in PBS. A total of 200 cells spotted on glass slides from randomly chosen microscopic fields were examined and counted by fluorescence microscopy (Leica, Berlin, Germany) and the percentage of cells displaying apoptotic degeneration relative to the total number was calculated as the apoptotic index.
Assay of Akt phosphorylation
The effect of oxaliplatin and pemetrexed treatment on cellular content of activated Akt was assayed in cells, treated as described for cell cycle analysis, with a P-Ser473 specific ELISA and normalized to the total Akt content (BioSource International, Camarillo, CA, USA). P-Ser473 Akt and Akt total concentrations were calculated from standard curves and values of P-Ser473 Akt were then normalized for total Akt and protein content, measured with the Lowry reagent (Sigma), as described previously [19].
Quantitative PCR analysis in cell lines
Cells were plated at a density of 1 × 105 cells/ml in 100 mm Petri dishes (Costar) and treated with oxaliplatin, pemetrexed, 5-fluorouracil, methotrexate and LY309887 at concentrations corresponding to IC50 levels. Cells were harvested immediately after the end of drug exposure, washed twice with PBS and RNA was extracted using the QiaAmp RNA Mini Kit (Qiagen, San Diego, CA, USA). The RNA obtained was dissolved in 10 mM dithiothreitol containing 200 U/ml RNase inhibitor in RNase free-water, and the absorbance measured at 260/280 nm, using the Uvikon-940 spectrophotometer (Kontron, Milan, Italy). RNA (1 μg) was reverse transcribed at 37°C for 1 h in a 50 μl reaction volume containing 0.8 mM dNTPs, 200 U of MMLV-RT, 40 U of RNase inhibitor, and 0.05 μg/ml of random primers. The resulting cDNA was amplified by quantitative PCR with the Applied Biosystems 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). PCR reactions were performed in triplicate using 5 μl of cDNA, 12.5 μl of TaqMan Universal PCR Master Mix, 2.5 μl of probe and 2.5 μl of forward and reverse primers in a final volume of 25 μl. Samples were amplified using the following thermal profile: 50°C for 2 min, 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s followed by annealing and extension at 60°C for 1 min.
Forward and reverse primers and probes for FPGS (NM_004957), TS (NM_0010711), DHFR (NM_000791) and GARFT (NM_000819) were designed with the Primer Express version 2.0 software (Applied Biosystems) on the basis of the gene sequence obtained by the GeneBank database, while primers and probes for ERCC1 (NM_001983) and for ERCC2 (NM_000400) were obtained from Applied Biosystems Assay-on-Demand® products (Hs00157415 and Hs00361161).
Amplifications were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Preliminary experiments were carried out with dilutions of cDNA obtained from Quantitative PCR Human Reference Total RNA (Stratagene, La Jolla, CA, USA) to demonstrate that the efficiencies of amplification of the target and reference genes are approximately equal and to determine the absolute value of the slope of standard cDNA concentration versus CT, where CT is the threshold cycle, as previously reported [20].
Results of basal expression analysis of single genes (TS, DHFR, GARFT and FPGS) or combined genes [FPGS/(TS × DHFR × GARFT)] were related to pemetrexed chemosensitivity, while quantification of gene expression in treated cells was performed using the ΔΔCT calculation. The amount of target gene, normalized to GAPDH and relative to the calibrator (untreated control cells), was expressed as 2−ΔΔCT and reported as percent variation with respect to control.
Oxaliplatin–DNA adduct formation in cell lines
Cells were plated at a density of 1 × 105 cells/ml in 100 mm Petri dishes (Costar) and allowed to attach for 24 h prior to drug treatment. Cells were pretreated with pemetrexed, based on IC50 values determined for each cell line, prior to incubation with oxaliplatin as follows: (1) no drug control (26 h incubation); (2) no drug (24 h), followed by oxaliplatin 300 μM (2 h); (3) pemetrexed 10× below IC50 (24 h), followed by oxaliplatin 300 μM (2 h); (4) pemetrexed at IC50 (24 h), followed by oxaliplatin 300 μM (2 h); (5) pemetrexed 10× above IC50 (24 h), followed by oxaliplatin 300 μM (2 h). Cells were harvested immediately after the end of drug exposure, washed twice with PBS and DNA extracted using the Qiagen DNA Mini kit (Qiagen, San Diego, USA). The concentration of DNA in each sample was quantified by UV absorption (A260). DNA samples were diluted in 3.5% nitric acid and hydrolyzed overnight at 70°C. Platinum–DNA adduct levels were determined by ultra-sensitive multi-collector inductively coupled plasma mass spectrometry (ICP-MS) as previously described [21, 22]. The limits of detection and quantitation using this methodology were 0.3 and 1 attomoles platinum/ml (1 attomole = 1 × 10−18 mole/ml), respectively. Final platinum–DNA adduct levels were calculated as nmoles/g DNA and the results of duplicate analyzes were within 10% of each other in all cases.
Statistical methods
All experiments were performed in triplicate and data were expressed as mean values ±SD and were analysed by Student’s t test or ANOVA, followed by the Tukey’s test for multiple comparisons. Statistical significance was set at P < 0.05.
Results
Cytotoxicity
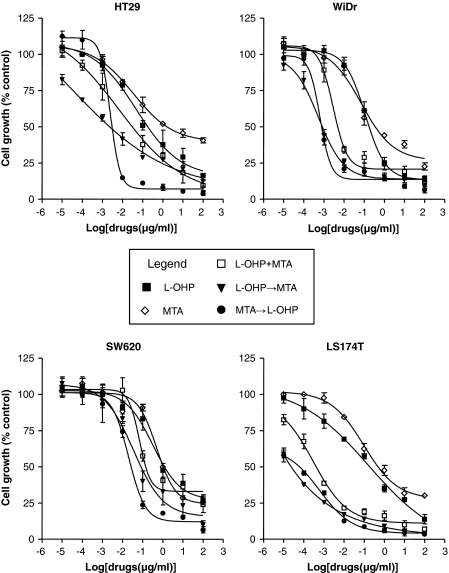
Oxaliplatin was cytotoxic against colon cancer cell lines with IC50 values of 0.33 ± 0.02 (HT29), 0.13 ± 0.01 (WiDr), 1.13 ± 0.35 (SW620) and 0.19 ± 0.01 (LS174T) μg/ml, after 24 h exposure. A dose-dependent inhibition of cell growth by pemetrexed was also observed in all cell lines, with IC50 values of 5.10 ± 0.42, 1.14 ± 0.15, 0.87 ± 0.23, and 1.05 ± 0.36 μg/ml in HT29, WiDr, SW620, and LS174T cells, respectively (Fig. 1).
Fig. 1.
Dose-response cytotoxicity of oxaliplatin (L-OHP), pemetrexed (MTA) and their combinations in HT29, WiDr, SW620 and LS174T colon cancer cells. Data represent the mean percentage of proliferating cells (±SD) from three independent experiments. In combination experiments the values refer to oxaliplatin concentration
Pharmacological interaction between oxaliplatin and pemetrexed
Because the CI method recommends a ratio of IC50 values at which drugs are equipotent, the combination studies were performed at fixed concentrations ratios (oxaliplatin:pemetrexed) of 1:15 (HT29), 1:9 (WiDr), 1:1 (SW620) and 1:5 (LS174T). The three combination schedules (oxaliplatin + pemetrexed, oxaliplatin → pemetrexed and pemetrexed → oxaliplatin) reduced the IC50 values of oxaliplatin in all cell lines (Fig. 1). Indeed, the analysis of drug interaction revealed synergistic effects (CI < 1) with both simultaneous and sequential treatments; the sequences oxaliplatin → pemetrexed and pemetrexed → oxaliplatin proved to be the most effective in all cell lines studied (Table 1).
Table 1.
Combination index values for oxaliplatin and pemetrexed at 50, 75 and 90% growth inhibition
| Cell line | Treatment | IC50 (ng/ml)a | Combination Indexb | ||
|---|---|---|---|---|---|
| 50%c | 70% | 90% | |||
| HT29 | L-OHP + MTA | 46.38 ± 7.42 | 0.28 | 0.17 | 0.15 |
| L-OHP → MTA | 3.05 ± 0.87 | 0.02 | 0.04 | 0.14 | |
| MTA → L-OHP | 9.26 ± 1.12 | 0.06 | 0.01 | <0.01 | |
| WiDr | L-OHP + MTA | 13.30 ± 1.54 | 0.22 | 0.27 | 0.36 |
| L-OHP → MTA | 1.80 ± 0.24 | 0.03 | 0.03 | 0.03 | |
| MTA → L-OHP | 2.70 ± 0.56 | 0.04 | 0.01 | <0.01 | |
| SW620 | L-OHP + MTA | 317.40 ± 23.10 | 0.51 | 0.29 | 0.12 |
| L-OHP → MTA | 168.30 ± 13.50 | 0.18 | 0.14 | 0.09 | |
| MTA → L-OHP | 75.40 ± 8.97 | 0.04 | 0.03 | 0.01 | |
| LS174T | L-OHP + MTA | 0.36 ± 0.08 | 0.04 | 0.05 | 0.09 |
| L-OHP → MTA | 0.06 ± 0.01 | <0.01 | <0.01 | <0.01 | |
| MTA → L-OHP | 0.04 ± 0.01 | <0.01 | <0.01 | <0.01 | |
aIC50 values calculated with respect to oxaliplatin
bMean data from three independent experiments (SD < 10% in all cases)
cInhibition levels
Cell cycle perturbations
Treatment with oxaliplatin significantly increased the percentage of HT29, SW620, and LS174T cells in the S-phase with respect to controls (Table 2); whereas a minimal enhancement was detected in WiDr cells. The cell cycle effects of pemetrexed were also evaluated; DNA content histograms of all cell lines showed an accumulation of cells at the G1–S-phase, determining an increase in the percentage of cells scored for G1 phase (Table 2).
Table 2.
Cell cycle modulation after 24 h drug exposure
| Cell line | Treatment | G1a phase (%) | ∆G1b | S-phase (%) | ∆S | G2/M phase (%) | ∆G2/M |
|---|---|---|---|---|---|---|---|
| HT 29 | Control | 69.3 | 26.2 | 4.5 | |||
| L-OHP | 48.9 | −20.4* | 40.9 | +14.7* | 10.2 | +5.7* | |
| MTA | 75.5 | +6.2 | 24.5 | −1.7 | 0 | −4.5* | |
| WiDr | Control | 69.7 | 24.5 | 5.8 | |||
| L-OHP | 61.9 | −7.8 | 27.5 | +3.0 | 10.5 | +4.7 | |
| MTA | 72.2 | +2.5 | 27.8 | +3.3 | 0 | −5.8* | |
| SW620 | Control | 49.5 | 44.9 | 5.5 | |||
| L-OHP | 37.5 | −12.0* | 57.1 | +12.2* | 5.4 | −0.1 | |
| MTA | 67.2 | +17.7* | 30.2 | −14.7* | 2.6 | −2.9 | |
| LS174T | Control | 56.4 | 33.5 | 10.1 | |||
| L-OHP | 41.1 | −15.3* | 50.0 | +16.5* | 8.9 | −1.2 | |
| MTA | 65.1 | +8.7 | 34.6 | +1.1 | 0.4 | −9.7* |
aMean data from three independent experiments (SD < 10% in all cases)
bDifferences (Δ) calculated with respect to controls
* P < 0.05
Induction of apoptosis
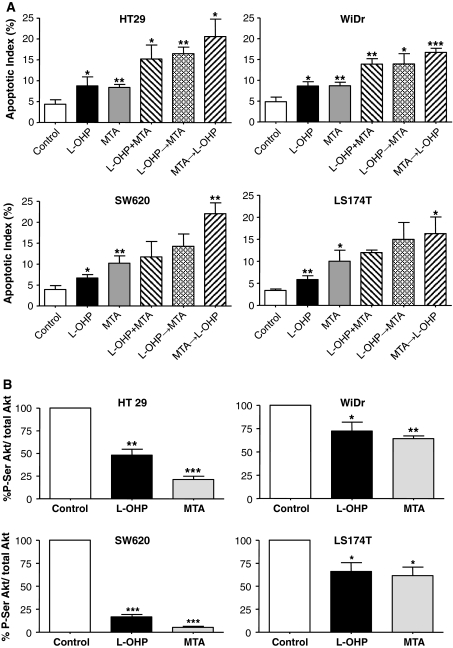
The four colon cancer cell lines exposed to oxaliplatin, pemetrexed and drug combinations presented typical apoptotic morphology with cell shrinkage, nuclear condensation and fragmentation, and rupture of cells into debris. The occurrence of apoptosis was significantly higher in oxaliplatin and pemetrexed-treated cells with respect to controls (apoptotic index 8.8 ± 2.1% and 8.4 ± 0.7% vs. 4.4 ± 1.1% in HT29 cells, respectively). Simultaneous administration and sequential combinations of oxaliplatin and pemetrexed further increased the apoptotic index with respect to controls (Fig. 2a). In particular, in HT29 and WiDr cells, all combinations significantly increased the apoptotic index as compared to cells treated with oxaliplatin and pemetrexed alone (P < 0.05), whereas in SW620 and LS174T cell lines, a significantly higher percentage of apoptotic cells was detected only after treatment with the pemetrexed → oxaliplatin combination sequence.
Fig. 2.
a Percentage of cells with apoptotic morphology evaluated following treatment with oxaliplatin (L-OHP), pemetrexed (MTA) and their combinations. Data represent mean values ±SD from three independent experiments. For single drug incubations, *P < 0.05, **P < 0.01 versus control; for drug combinations *P < 0.05, **P < 0.01, ***P < 0.001 versus oxaliplatin and pemetrexed alone. b Reduction of P-Ser473 Akt by oxaliplatin (L-OHP) and pemetrexed (MTA) in HT29, WiDr, SW620 and LS174T colon cancer cells. Data represent mean values ±SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 with respect to control
Inhibition of Akt phosphorylation
Oxaliplatin and pemetrexed were able to significantly reduce the amount of phosphorylated Akt in all cell lines, with pemetrexed being more potent than oxaliplatin, particularly in HT29 and SW620 cells. The amount of phosphorylated Akt was decreased up to 51.9 ± 6.5% and 83.4 ± 0.5% by oxaliplatin, and up to 78.7 ± 3.5% and 94.7 ± 1.1% by pemetrexed, in HT29 and SW620 cells, respectively (Fig. 2b).
Gene expression analysis
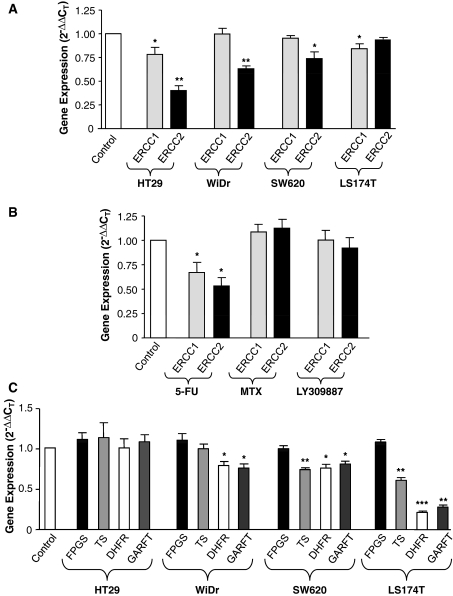
Pemetrexed modulated gene expression levels of ERCC1 and ERCC2 are shown in Fig. 3a. In particular, pemetrexed significantly reduced ERCC1 gene expression in HT29 and LS174T cell lines. Similar results were observed for ERCC2, whose expression was significantly decreased by pemetrexed up to 59.8 ± 5.0% in HT29 cells, 36.8 ± 3.0% in WiDr cells and 22.6 ± 7.4% in SW620 cells. Additional experiments were designed to demonstrate which of the target enzymes of pemetrexed contributed to the modulation of ERCC1 and ERCC2. For this proposal, mRNA levels of ERCC1 and ERCC2 were analyzed after exposure to selective inhibitors of TS (5-FU), DHFR (MTX) and GARFT (LY309887). No significant changes in ERCC1/2 levels were detected with 5-FU, MTX or LY309887 in WiDr, SW620 and LS174T cells, while 5-FU treatment reduced ERCC1 and ERCC2 expression in HT29 cells by 34.1 ± 3.6% and 47.2 ± 8.3%, respectively (Fig. 3b).
Fig. 3.
Gene expression analysis of selected genes involved in the activity of oxaliplatin and pemetrexed. a modulation of ERCC1 and ERCC2 mRNA expression by pemetrexed, at IC50 levels. b Modulation of ERCC1 and ERCC2 mRNA expression by 5-fluorouracil (5-FU), methotrexate, LY309887, at IC50 levels, in HT29 cells. c FPGS, TS, DHFR and GARFT mRNA modulation after oxaliplatin exposure at IC50 levels. Data represent mean values ±SD from three independent experiments*P < 0.05, **P < 0.01, ***P < 0.001 with respect to control. In particular, the results of the repeated measures ANOVA followed by the Tukey’s test were 0.001, 0.007, 0.033 and 0.045 for the modulation of ERCC1 and ERCC2 after pemetrexed exposure in the HT29, WiDR, SW620 and LS174T cells, respectively; 0.023 for the modulation of ERCC1 and ERCC2 after 5-FU exposure in the HT29 cells; and 0.023, 0.003 and <0.001 for the modulation of FPGS, TS, DHFR and GARFT after oxaliplatin exposure, in the WiDr, SW620 and LS1574T cells, respectively
Basal mRNA levels of FPGS, TS, DHFR and GARFT were analyzed in all cell lines (Table 3) and related to pemetrexed sensitivity. None of the target genes, taken individually, were clearly related to cell sensitivity to pemetrexed. However, a good correlation was found between the ratio of FPGS/(TS × DHFR × GARFT) and IC50 values of pemetrexed (R 2 = 0.954, P = 0.024). Finally, oxaliplatin significantly modulated basal gene expression of pemetrexed target enzymes in WiDr, SW620 and LS174T cells (Fig. 3c). In particular, TS mRNA expression was significantly decreased by up to 25.7 ± 4.0% and 39.0 ± 6.0% in SW620 and LS174T cells, respectively. DHFR and GARFT mRNA expression was markedly reduced in LS174T cells (78.5 ± 3.5% and 72.2 ± 4.4%, respectively) with smaller decreases observed in WiDr and SW620 cells.
Table 3.
Gene expression of FPGS and pemetrexed targets
| Cell line | FPGSa | TS | DHFR | GARFT | Ratiob |
|---|---|---|---|---|---|
| HT29 | 8.41 ± 0.34 | 7.97 ± 0.22 | 16.55 ± 0.83 | 5.28 ± 0.19 | 0.01 |
| WiDr | 11.32 ± 0.44 | 6.12 ± 0.20 | 6.93 ± 0.27 | 4.00 ± 0.15 | 0.07 |
| SW620 | 29.31 ± 1.05 | 6.32 ± 0.24 | 7.07 ± 0.30 | 8.18 ± 0.42 | 0.08 |
| LS174T | 3.61 ± 0.11 | 3.98 ± 0.16 | 5.32 ± 0.21 | 1.93 ± 0.09 | 0.09 |
aMean values calculated from standard curves, normalized with respect to expression values of the housekeeping gene GAPDH
bRatio calculated with the formula FPGS/(TS × DHFR × GARFT); SD < 10% in all cases
Oxaliplatin–DNA adduct formation
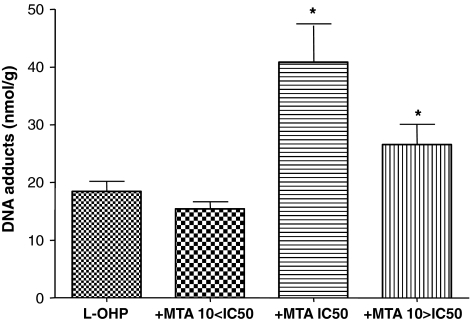
Following treatment of cells with oxaliplatin (300 μM) for 2 h, oxaliplatin–DNA adduct levels of 18.5 ± 4.3, 76.5 ± 31.6, 90.8 ± 47.5, and 103.8 ± 39.2 nmol/g DNA were observed in SW620, WiDr, HT29 and LS174T cells, respectively (mean ± SD, n = 3). An inverse correlation was observed between oxaliplatin–DNA adduct formation and oxaliplatin IC50 values for the cell lines studied (R 2 = 0.851), with higher levels of platinum–DNA adduct formation being associated with decreased IC50 values. There was a trend towards higher levels of oxaliplatin–DNA adducts being formed following pretreatment with pemetrexed, with increased adduct levels observed at pemetrexed concentrations ≥IC50 concentrations in all four cell lines studied. Significant increases in adduct formation were observed at IC50 pemetrexed concentrations in SW620 and LS174T cells and at 10× IC50 pemetrexed concentrations in SW620 and HT29 cells, as compared to control cells incubated with oxaliplatin in the absence of pemetrexed (P < 0.05 in all cases). Figure 4 shows the extent of oxaliplatin–DNA adduct formation in SW620 cells in the presence of increasing concentrations of pemetrexed; a similar pattern was observed in the other cell lines studied.
Fig. 4.
Formation of oxaliplatin–DNA adducts in SW620 cells. Cells were treated with oxaliplatin (L-OHP) at a concentration of 300 μM for 2 h, either alone or following a 24 h preincubation with pemetrexed (MTA) at the IC50 concentration, 10× below IC50 or 10× above IC50. Data represent mean values ±SD from three independent experiments. *P < 0.05 with respect to control (oxaliplatin alone)
Discussion
The present study demonstrates that oxaliplatin and pemetrexed exhibit synergistic interactions in a panel of colorectal cancer cell lines, supporting the role of oxaliplatin combinations with TS inhibitors. Limited published preclinical research focusing on this issue has shown synergistic activity following simultaneous exposure to pemetrexed and oxaliplatin in parental and 5-FU-resistant human HT29 colon cancer cells, with additive antitumor effects and minimal toxicity in athymic mice bearing HT29 xenografts [23]. Despite the limited availability of preclinical data, the association of pemetrexed (500 mg/m2) and oxaliplatin (120 mg/m2), every 21 days for 6 cycles, was studied as first-line therapy in a phase II trial, reporting a clinical response rate of 29.6% in patients with metastatic adenocarcinoma of the colon or rectum [18].
However, a recent multi-institutional phase II study showed that pemetrexed in combination with oxaliplatin is active and well tolerated as a first-line therapy for advanced gastric cancer, with response rates comparable to those achieved in studies using different 5-FU-oxaliplatin combinations, without the inconvenience of prolonged 5-FU schedules [24].,
The in vitro data obtained in our study demonstrate that sequential administration of oxaliplatin and pemetrexed (i.e. oxaliplatin → pemetrexed and pemetrexed → oxaliplatin) results in greater cytotoxic effects than those observed following simultaneous combination of these two drugs. Generally consistent results were obtained in HT29, WiDr, SW620, and LS174T colon cancer cells, although some cell line-dependent effects were observed.
The significant synergy observed between oxaliplatin and pemetrexed in sequential combination may be attributed, at least in part, to cell cycle perturbations, induction of apoptosis and expression modulation of target enzymes. Indeed, cell cycle analysis demonstrated a shift towards the S-phase after oxaliplatin exposure, potentially facilitating pemetrexed activity. These findings are in agreement with previous data showing an S-phase delay after oxaliplatin treatment, followed by G2/M accumulation, in various cell lines, including colorectal cancer cells [25–28]. Pemetrexed has been shown to induce cell cycle alterations that may depend on a change in nucleotide pools through inhibition of its target enzymes TS, DHFR, and GARFT in different tumor cell lines [16, 19, 29]; the cell cycle effects of pemetrexed in the studied colon cancer cell lines were in agreement with previously published data in HT29 cells [30].
The phosphatidylinositol-3 kinase (PI3K)/Akt pathway provides a potent antiapoptotic signal in cancer cells which might result in chemoresistance to several anticancer agents [31]. A recent study has shown that endogenous phosphorylated Akt levels are much higher in the RKO cells (resistant to oxaliplatin) than in the HCT116 cells (sensitive to oxaliplatin) and Sphingosine kinase isoforms and neutral sphingomyelinase contribute to the regulation of chemosensitivity by controlling ceramide formation and the downstream Akt pathway in human colon cancer cells [32]. Furthermore the activation of apoptosis by oxaliplatin mainly relies on Bax relocalization to the mitochondria and cytochrome c release in the cytoplasm [33]. Since Akt prevents cytochrome c-induced cleavage of pro-caspase-9 [34], the inhibition of PI3K/Akt pathway may be an effective molecular target for oxaliplatin-based combinations. Therefore, in the present work, we investigated the modulation of Akt phosphorylation, demonstrating that pemetrexed significantly decreased the amount of the activated form of Akt in all tested colon cancer cell lines, most notably observed in HT29 and SW620 cells. Moreover, our study also showed that oxaliplatin exposure reduced Akt activation; thus, the reciprocal improvement of oxaliplatin and pemetrexed therapeutic potential may depend on suppression of Akt activity and triggering drug-induced apoptosis, as suggested for pemetrexed–gemcitabine combinations in lung cancer cells [20]. Indeed, exposure of all studied cell lines to oxaliplatin and pemetrexed resulted in a significant induction of apoptosis with the sequence pemetrexed → oxaliplatin resulting in a significantly higher increase in apoptotic index as compared to single agents.
Studies on antifolate-resistant cells overexpressing TS have shown that resistance to pemetrexed is lower than to other antifolates, possibly because pemetrexed can inhibit several folate-dependent reactions. Preclinical findings have suggested that mRNA levels of TS, DHFR, and GARFT are related to IC50 values of pemetrexed in lung and bladder cancer cell lines [19, 20, 29]. In contrast, RT-PCR analysis of TS, GARFT, DHFR, and genes involved in pemetrexed transport, failed to establish a relation between gene expression level and chemosensitivity in gastric cancer cell lines [35]. In the present study, gene expression was not directly related to pemetrexed cytotoxicity for any of the genes analyzed. However, a low value of the FPGS/(TS × DHFR × GARFT) ratio was associated with a decreased cell sensitivity, suggesting that the ratio between activating enzymes and target enzymes was more discriminating than either target independently, as previously described for methotrexate in leukemia cells [36].
Several drugs can modulate TS expression, potentially influencing the activity of TS inhibitors. For example, vinorelbine has been shown to suppress TS expression in PC14 cells, resulting in a synergistic interaction with 5-FU treatment [37], while several other drugs, such as platinum derivatives and irinotecan, can also down-regulate TS levels [38, 39]. Yeh and co-workers [40] provided important data explaining why a combination of oxaliplatin and 5-FU resulted in better objective responses in 5-FU-resistant patients than oxaliplatin alone. Indeed, oxaliplatin treatment caused a significant reduction of free TS protein expression most likely due to TS downregulation at the transcriptional level. Our findings confirm the modulation of TS mRNA levels in SW620 and LS174T cells, and also provide evidence for the suppression of DHFR and GARFT by oxaliplatin, thus increasing the FPGS/(TS × DHFR × GARFT) ratio and potentially favoring pemetrexed activity.
Due to the accelerated rate of DNA replication in neoplastic cells, interruption of folate metabolism and depletion in nucleotide pools causes ineffective DNA synthesis and repair; furthermore, folate deficiency has been shown to act synergistically with alkylating agents to increase DNA strand breaks and mutations as a result of impaired DNA excision repair [41]. This effect may depend on drug-induced inhibition of gene expression of key enzymes in DNA damage repair systems. In particular, our results have demonstrated a substantial reduction of transcriptional levels of ERCC1 and ERCC2 in HT29 cells, whereas LS174T showed a significant decrease of ERCC1, and a significant reduction of ERCC2 was detected in WiDr and SW620 cell lines. In the HT29 cell line 5-FU exposure induced a similar effect to that of pemetrexed on ERCC1 and ERCC2 gene expression, as previously detected in a cisplatin-resistant HST-1 human squamous carcinoma cell line and colorectal cancer cell lines [42, 43]. However, in WiDr, SW620 and LS174T cells, inhibition of TS by 5-FU was not sufficient to modulate mRNA levels of these enzymes affected by pemetrexed treatment. Thus, it may be hypothesized that TS inhibition and thymidine depletion are necessary for downregulation of studied NER genes, but the simultaneous inhibition of other folate-dependent enzymes seems to be required in different cells. These gene expression data may go some way to explaining the synergistic interaction observed with the oxaliplatin → pemetrexed and pemetrexed → oxaliplatin combinations.
In addition to these findings, preliminary experiments were carried out to determine the potential impact of pemetrexed on the direct binding of oxaliplatin to DNA in the form of oxaliplatin–DNA adducts. These studies showed a clear trend towards higher levels of oxaliplatin–DNA adducts being formed following pretreatment with pemetrexed, providing supportive mechanistic data for the synergistic effects observed with this sequential drug administration. An induced stimulation of oxaliplatin–DNA adduct formation has previously been correlated with a potentiation of oxaliplatin cytotoxicity by cetuximab in HCT-8 colorectal cancer cells. This upregulation was associated with similarly reduced expression of ERCC1 at both the mRNA and protein levels [44].
In conclusion, our results indicate that sequential administrations of oxaliplatin and pemetrexed result in the most effective cytotoxic effects in colon cancer cell lines. These findings may be explained by favorable modulation of several molecular determinants involved in chemosensitivity/chemoresistance to these cytotoxic agents, including signal transduction pathways, drug targets and DNA repair systems. These data add to an increasing number of publications highlighting the potential clinical benefits of using oxaliplatin in combination with other anticancer drugs. For example, promising preclinical data have recently been published with respect to the combination of oxaliplatin and Src inhibitors such as dasatinib [45]. Although the extrapolation of in vitro data to the clinical setting should be considered with caution, our results may have implications for rational development of chemotherapeutic regimens including oxaliplatin and pemetrexed for the treatment of colorectal cancer.
Acknowledgments
This work was supported in part by a research grant from Lilly (Sesto Fiorentino, Italy), by the Italian Ministry of University (MIUR, PRIN 2005; R.D. and M.D.T) and by Cancer Research UK (G.J.V.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM. Advances in the treatment of metastatic colorectal cancer. Oncologist. 2005;10:40–48. doi: 10.1634/theoncologist.10-90003-40. [DOI] [PubMed] [Google Scholar]
- 3.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 4.Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1:227–235. [PubMed] [Google Scholar]
- 5.Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev. 2005;31:90–105. doi: 10.1016/j.ctrv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Reardon JT, Vaisman A, Chaney SG, Sancar A. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and Bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res. 1999;59:3968–3971. [PubMed] [Google Scholar]
- 7.Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer. 2003;39:112–119. doi: 10.1016/S0959-8049(02)00411-2. [DOI] [PubMed] [Google Scholar]
- 8.Chang IY, Kim MH, Kim HB, Lee DY, Kim SH, Kim HY, You HJ. Small interfering RNA-induced suppression of ERCC1 enhances sensitivity of human cancer cells to cisplatin. Biochem Biophys Res Commun. 2005;327:225–233. doi: 10.1016/j.bbrc.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Chen ZP, Malapetsa A, Alaoui-Jamali M, Bergeron J, Monks A, Myers TG, Mohr G, Sausville EA, Scudiero DA, Aloyz R, Panasci LC. DNA repair protein levels vis-a-vis anticancer drug resistance in the human tumor cell lines of the National Cancer Institute drug screening program. Anticancer Drugs. 2002;13:511–519. doi: 10.1097/00001813-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 12.Adjei AA. A review of the pharmacology and clinical activity of new chemotherapy agents for the treatment of colorectal cancer. Br J Clin Pharmacol. 1999;48:265–277. doi: 10.1046/j.1365-2125.1999.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bocci G, Danesi R, Allegrini G, Innocenti F, Di Paolo A, Falcone A, Conte PF, Del Tacca M. Severe 5-fluorouracil toxicity associated with a marked alteration of pharmacokinetics of 5-fluorouracil and its catabolite 5-fluoro-5, 6-dihydrouracil: a case report. Eur J Clin Pharmacol. 2002;58:593–595. doi: 10.1007/s00228-002-0534-6. [DOI] [PubMed] [Google Scholar]
- 14.Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ, Patel VF, Andis SL, Bewley JR, Rayl EA, Moroson BA, Beardsley GP, Kohler W, Ratnam M, Schultz RM. LY231514, a pyrrolo[2, 3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–1123. [PubMed] [Google Scholar]
- 15.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 16.Giovannetti E, Mey V, Danesi R, Mosca I, Del Tacca M. Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res. 2004;10:2936–2943. doi: 10.1158/1078-0432.CCR-03-0520. [DOI] [PubMed] [Google Scholar]
- 17.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 18.Atkins JN, Jacobs SA, Wieand HS, Smith RE, John WJ, Colangelo LH, Vogel VG, Kuebler JP, Cescon TP, Miller BJ, Geyer CE, Jr, Wolmark N. Pemetrexed/oxaliplatin for first-line treatment of patients with advanced colorectal cancer: a phase II trial of the National Surgical Adjuvant Breast and Bowel Project Foundation Research Program. Clin Colorectal Cancer. 2005;5:181–187. doi: 10.3816/CCC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 19.Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G, Peters GJ. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol. 2008;73:1290–1300. doi: 10.1124/mol.107.042382. [DOI] [PubMed] [Google Scholar]
- 20.Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del Tacca M, Danesi R. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 21.Cooper B, Veal GJ, Radivoyevitch T, Tilby MJ, Meyerson HJ, Lazarus HM, Koc ON, Creger RJ, Pearson G, Nowell G, Gosky D, Ingalls ST, Hoppel CL, Gerson SL. A Phase I and pharmacodynamic study of fludarabine, carboplatin and topotecan in patients with relapsed, refractory, or high-risk acute leukemia. Clin Cancer Res. 2004;10:6830–6839. doi: 10.1158/1078-0432.CCR-04-0097. [DOI] [PubMed] [Google Scholar]
- 22.Veal GJ, Errington J, Tilby MJ, Pearson ADJ, Foot ABM, McDowell H, Ellershaw C, Pizer B, Nowell GM, Pearson DG, Boddy AV. Adaptive dosing and platinum–DNA adduct formation in children receiving high dose carboplatin for the treatment of solid tumours. Br J Cancer. 2007;96:725–731. doi: 10.1038/sj.bjc.6603607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond E, Louvet C, Tournigand C, Coudray AM, Faivre S, De Gramont A, Gespach C. Pemetrexed disodium combined with oxaliplatin, SN38, or 5-fluorouracil, based on the quantitation of drug interactions in human HT29 colon cancer cells. Int J Oncol. 2002;21:361–367. [PubMed] [Google Scholar]
- 24.Celio L, Sternberg CN, Labianca R, La Torre I, Amoroso V, Barone C, Pinotti G, Cascinu S, Di Costanzo F, Cetto GL, Bajetta E. Pemetrexed in combination with oxaliplatin as a first-line therapy for advanced gastric cancer: a multi-institutional phase II study. Ann Oncol. 2009;20:1062–1067. doi: 10.1093/annonc/mdn766. [DOI] [PubMed] [Google Scholar]
- 25.Arnould S, Guichard S, Hennebelle I, Cassar G, Bugat R, Canal P. Contribution of apoptosis in the cytotoxicity of the oxaliplatin-irinotecan combination in the HT29 human colon adenocarcinoma cell line. Biochem Pharmacol. 2002;64:1215–1226. doi: 10.1016/S0006-2952(02)01291-1. [DOI] [PubMed] [Google Scholar]
- 26.Faivre S, Chan D, Salinas R, Woynarowska B, Woynarowski JM. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem Pharmacol. 2003;66:225–237. doi: 10.1016/S0006-2952(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 27.Troiani T, Lockerbie O, Morrow M, Ciardiello F, Eckhardt SG. Sequence-dependent inhibition of human colon cancer cell growth and of prosurvival pathways by oxaliplatin in combination with ZD6474 (Zactima), an inhibitor of VEGFR and EGFR tyrosine kinases. Mol Cancer Ther. 2006;5:1883–1894. doi: 10.1158/1535-7163.MCT-06-0055. [DOI] [PubMed] [Google Scholar]
- 28.Temmink OH, Hoebe EK, van der Born K, Ackland SP, Fukushima M, Peters GJ. Mechanism of trifluorothymidine potentiation of oxaliplatin-induced cytotoxicity to colorectal cancer cells. Br J Cancer. 2007;96:231–240. doi: 10.1038/sj.bjc.6603549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mey V, Giovannetti E, De Braud F, Nannizzi S, Curigliano G, Verweij F, De Cobelli O, Pece S, Del Tacca M, Danesi R. In vitro synergistic cytotoxicity of gemcitabine and pemetrexed and pharmacogenetic evaluation of response to gemcitabine in bladder cancer patients. Br J Cancer. 2006;95:289–297. doi: 10.1038/sj.bjc.6603242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonkinson JL, Worzalla JF, Teng CH, Mendelsohn LG. Cell cycle modulation by a multitargeted antifolate, LY231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res. 1999;59:3671–3676. [PubMed] [Google Scholar]
- 31.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, Banno Y. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arango D, Wilson AJ, Shi Q, Corner GA, Aranes MJ, Nicholas C, Lesser M, Mariadason JM, Augenlicht LH. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer. 2004;91:1931–1946. doi: 10.1038/sj.bjc.6602215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Lee KW, Jung Y, Kim TY, Ham HS, Jong HS, Jung KH, Im SA, Kim TY, Kim NK, Bang YJ. Cytotoxic effects of pemetrexed in gastric cancer cells. Cancer Sci. 2005;96:365–371. doi: 10.1111/j.1349-7006.2005.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rots MG, Willey JC, Jansen G, Van Zantwijk CH, Noordhuis P, DeMuth JP, Kuiper E, Veerman AJ, Pieters R, Peters GJ. mRNA expression levels of methotrexate resistance-related proteins in childhood leukemia as determined by a standardized competitive template-based RT-PCR method. Leukemia. 2000;14:2166–2175. doi: 10.1038/sj.leu.2401943. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto S, Igishi T, Hashimoto K, Kodani M, Shigeoka Y, Nakanishi H, Touge H, Kurai J, Makino H, Takeda K, Yasuda K, Hitsuda Y, Shimizu E. Schedule-dependent synergism of vinorelbine and 5-fluorouracil/UFT against non-small cell lung cancer. Int J Oncol. 2004;25:1311–1318. [PubMed] [Google Scholar]
- 38.van der Wilt CL, van Laar JA, Gyergyay F, Smid K, Peters GJ. Biochemical modification of the toxicity and the anti-tumour effect of 5-fluorouracil and cis-platinum by WR-2721 in mice. Eur J Cancer. 1992;28A:2017–2024. doi: 10.1016/0959-8049(92)90251-V. [DOI] [PubMed] [Google Scholar]
- 39.Guichard S, Hennebelle I, Bugat R, Canal P. Cellular interactions of 5-fluorouracil and the camptothecin analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell line. Biochem Pharmacol. 1998;55:667–676. doi: 10.1016/S0006-2952(97)00541-8. [DOI] [PubMed] [Google Scholar]
- 40.Yeh KH, Cheng AL, Wan JP, Lin CS, Liu CC. Down-regulation of thymidylate synthase expression and its steady-state mRNA by oxaliplatin in colon cancer cells. Anticancer Drugs. 2004;15:371–376. doi: 10.1097/00001813-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Novakovic P, Stempak JM, Sohn KJ, Kim YI. Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis. 2006;27:916–924. doi: 10.1093/carcin/bgi312. [DOI] [PubMed] [Google Scholar]
- 42.Fujishima H, Nakano S, Masumoto N, Esaki T, Tatsumoto T, Kondo T, Niho Y. Inhibition by 5-fluorouracil of ERCC1 and gamma-glutamylcysteine synthetase messenger RNA expression in a cisplatin-resistant HST-1 human squamous carcinoma cell line. Oncol Res. 1997;9:167–172. [PubMed] [Google Scholar]
- 43.Ojima E, Inoue Y, Watanabe H, Hiro J, Toiyama Y, Miki C, Kusunoki M. The optimal schedule for 5-fluorouracil radiosensitization in colon cancer cell lines. Oncol Rep. 2006;16:1085–1091. [PubMed] [Google Scholar]
- 44.Balin-Gauthier D, Delord JP, Pillaire MJ, Rochaix P, Hoffman JS, Bugat R, Cazaux C, Canal P, Allal BC. Cetuximab potentiates oxaliplatin cytotoxic effect through a defect in NER and DNA replication initiation. Br J Cancer. 2008;98:120–128. doi: 10.1038/sj.bjc.6604134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopetz S, Lesslie DP, Dallas NA, Park SI, Johnson M, Parikh NU, Kim MP, Abbruzzese JL, Ellis LM, Chandra J, Gallick GE. Synergistic activity of the Src family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]