Abstract
Species of Calonectria and their Cylindrocladium anamorphs are important plant pathogens worldwide. At present 52 Cylindrocladium spp. and 37 Calonectria spp. are recognised based on sexual compatibility, morphology and phylogenetic inference. The polyphasic approach of integrating Biological, Morphological and Phylogenetic Species Concepts has revolutionised the taxonomy of fungi. This review aims to present an overview of published research on the genera Calonectria and Cylindrocladium as they pertain to their taxonomic history. The nomenclature as well as future research necessary for this group of fungi are also briefly discussed.
Keywords: Calonectria, Cylindrocladium, species concepts, nomenclature, pathogenicity
INTRODUCTION
The genus Calonectria (Ca.) was erected in 1867 by De Notaris, based on Ca. daldiniana collected on leaves of Magnolia grandiflora (Magnoliaceae), in Daldini, Italy (Rossman 1979a). Rossman (1979a) later reduced Ca. daldiniana to synonymy under Ca. pyrochroa, and defined this nectrioid fungus as having an ascocarp wall structure that is brightly coloured, changing to blood-red in 3 % KOH solution, warty to scaly and with a Cylindrocladium (Cy.) anamorph (Rossman 1993, Rossman et al. 1999). However, due to the restricted morphological characteristics of the teleomorph (Rossman 1979b, 1983), specimens can in many cases only be identified to species level if the anamorph is present (Schoch et al. 2000b, Crous 2002).
The anamorph genus Cylindrocladium, which is based on Cy. scoparium, was first described by Morgan (1892) in the U.S.A., where it was found growing as saprobe on a pod of Gleditsia triacanthos. Although Morgan (1892) failed to mention the stipe extension terminating in a vesicle of characteristic shape, he defined the genus as having branched conidiophores producing cylindrical conidia. This fungus has a wide distribution in sub-tropical and tropical regions of the world, and species are pathogenic to numerous plants (Crous 2002).
The aim of this review is to present an overview of published research on the genus Calonectria and their Cylindrocladium anamorphs. More specifically, the application of three types of species concepts is considered as they pertain to the taxonomic history of this genus. Although several species concepts (Mayden 1997) have been proposed, only the Morphological Species Concept (MSC), the Biological Species Concept (BSC) and the Phylogenetic Species Concept (PSC) are treated, as these have been most widely applied to Calonectria. Several reviews (Rossman 1996, Brasier 1997, Harrington & Rizzo 1999, Taylor et al. 1999, 2000, Seifert et al. 2000, Kohn 2005) have treated the various species concepts applied to the taxonomy of fungi and this topic is not treated other than in the manner in which it applies to Calonectria.
TAXONOMIC HISTORY
Calonectria resides in the Nectriaceae, one of three families in Hypocreales, an order that has been reviewed extensively (Rogerson 1970, Rossman 1983, Rossman et al. 1996, 1999). The Nectriaceae is circumscribed as having uniloculate ascomata that are orange to purple and not immersed in well-developed stromata (Rossman et al. 1999). The family includes approximately 20 genera of socio-economic importance and of these, Calonectria is most clearly distinguished from the others by its Cylindrocladium anamorphs and relevance as plant pathogens.
The first monograph of Cylindrocladium by Boedijn & Reitsma (1950), introduced seven Cylindrocladium species with one Calonectria connection. Later, in her treatment of Calonectria, Rossman (1983) recognised five species including the novel Ca. ophiospora. However, this species description did not include the anamorph state. The circumscribed type, Ca. pyrochoa, was also incorrectly reduced to synonymy with several other species based only on the teleomorph morphology. Peerally (1991a) highlighted this in a monograph of Cylindrocladium, where he regarded the anamorph morphology as important in distinguishing species of Calonectria. He subsequently recognised 10 Calonectria species with their Cylindrocladium anamorphs, including an additional 16 Cylindrocladium species not associated with a teleomorph. However, he mistakenly reduced Cylindrocladiella, a genus that accommodates Cylindrocladium-like species with small conidia (Boesewinkel 1982) and Nectricladiella teleomorphs, to synonymy with Cylindrocladium (Schoch et al. 2000b).
The monograph of Cylindrocladium by Crous & Wingfield (1994) entrenched the importance of anamorph characteristics in the taxonomy of Calonectria spp. In this monograph, 22 Cylindrocladium species and one variety were recognised, associated with 16 Calonectria species. Five species were assigned to the genus Cylindrocladiella based on morphological characters of the holomorph. The focus on anamorph characteristics is perpetuated in the most recent monograph (Crous 2002), which recognised 28 Calonectria species, all associated with Cylindrocladium anamorphs and an additional 18 Cylindrocladium species for which teleomorph states were not known. Of the latter group, seven taxa were of doubtful authenticity. Presently, 37 Calonectria and 52 Cylindrocladium species are recognised (Table 1; Crous 2002, Crous et al. 2004b, 2006a; Gadgil & Dick 2004, Lombard et al. 2009, 2010).
Table 1.
List of recognised Calonectria species and their respective Cylindrocladium anamorphs.
| Teleomorph | Reference | Anamorph | Reference |
|---|---|---|---|
| Calonectria acicola Gadgil & M.A. Dick | Gadgil & Dick 2004 | Cylindrocladium acicola Gadgil & M.A. Dick | Gadgil & Dick 2004 |
| Calonectria asiatica Crous & Hywel-Jones | Crous et al. 2004b | Cylindrocladium asiaticum Crous & Hywel-Jones | Crous et al. 2004b |
| Calonectria avesiculata T.S. Schub., Ell-Gholl, Alfieri & Schoult. | Schubert et al. 1989 | Cylindrocladium avesiculatum D.L. Gill, Alfieri & Sobers | Gill et al. 1971 |
| Calonectria brassicae (Panwar & Bohra) L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2009 | ||
| Calonectria brachiatica L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2009 | ||
| Calonectria cerciana L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2010 | ||
| Calonectria clavata Alfieri, El-Gholl & E.L. Barnard | El-Gholl et al. 1993b | Cylindrocladium flexuosum Crous | Crous et al. 1995 |
| Calonectria colhounii Peerally | Peerally 1973 | Cylindrocladium colhounii Peerally | Peerally 1973 |
| Calonectria colombiensis Crous | Crous et al. 2004b | Cylindrocladium colombiense Crous | Crous et al. 2004b |
| Calonectria gracilipes Crous & Mchau | Crous et al. 1997a | Cylindrocladium graciloideum Crous & Mchau | Crous et al. 1997a |
| Calonectria gracilis Crous, M.J. Wingf. & Alfenas | Crous et al. 1997b | Cylindrocladium pseudogracile Crous | Crous et al. 1997b |
| Calonectria hederae G. Arnaud ex C. Booth | Booth & Murray 1960 | Cylindrocladium hederae G. Arnaud ex Peerally | Peerally 1991a |
| Calonectria hongkongensis Crous | Crous et al. 2004b | Cylindrocladium hongkongense Crous | Crous et al. 2004b |
| Calonectria ilicicola Boedijn & Reitsma | Boedijn & Reitsma 1950 | Cylindrocladium parasiticum Crous, M.J. Wingf. & Alfenas | Crous et al. 1993d |
| Calonectria indusiata (Seaver) Crous | Crous 2002 | Cylindrocladium theae (Petch) Subram | Alfieri et al. 1972 |
| Calonectria insularis C.L. Schoch & Crous | Schoch et al. 1999 | Cylindrocladium insulare C.L. Schoch & Crous | Schoch et al. 1999 |
| Calonectria kyotensis Terash. | Terashita 1968 | Cylindrocladium floridanum Sobers & C.P. Seym. | Sobers & Seymour 1967 |
| Calonectria leguminum (Rehm) Crous | Crous 2002 | Cylindrocladium leguminum Crous | Crous 2002 |
| Calonectria macroconidialis (Crous, M.J. Wingf. & Alfenas) Crous | Crous et al. 1999 | Cylindrocladium macroconidiale (Crous, M.J. Wingf. & Alfenas) Crous | Crous et al. 1999 |
| Calonectria madagascariensis Crous | Crous 2002 | Cylindrocladium madagascariense Crous | Crous 2002 |
| Calonectria mexicana C.L. Schoch & Crous | Schoch et al. 1999 | Cylindrocladium mexicanum C.L. Schoch & Crous | Schoch et al. 1999 |
| Calonectria morganii Crous, Alfenas & M.J. Wingf. | Crous et al. 1993a | Cylindrocladium scoparium Morgan | Morgan 1892 |
| Calonectria multiseptata Crous & M.J. Wingf. | Crous et al. 1998b | Cylindrocladium multiseptatum Crous & M.J. Wingf. | Crous et al. 1998b |
| Calonectria naviculata Crous & M.J. Wingf. | Crous et al. 1994 | Cylindrocladium naviculatum Crous & M.J. Wingf. | Crous et al. 1994 |
| Calonectria ovata D. Victor & Crous | Victor et al. 1997 | Cylindrocladium ovatum El-Gholl, Alfenas, Crous & T.S. Schub. | El-Gholl et al. 1993a |
| Calonectria pauciramosa C.L. Schoch & Crous | Schoch et al. 1999 | Cylindrocladium pauciramosum C.L. Schoch & Crous | Schoch et al. 1999 |
| Calonectria pseudoreteaudii L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2010 | ||
| Calonectria pseudospathiphylli J.C. Kang, Crous & C.L. Schoch | Kang et al. 2001b | Cylindrocladium pseudospathiphylli J.C. Kang, Crous & C.L. Schoch | Kang et al. 2001b |
| Calonectria pteridis Crous, M.J. Wingf. & Alfenas | Crous et al. 1993c | Cylindrocladium pteridis F.A. Wolf | Wolf 1926 |
| Calonectria pyrochroa (Desm.) Sacc. | Rossman 1979a | Cylindrocladium ilicicola (Hawley) Boedijn & Reitsma | Boedjin & Reitsma 1950 |
| Calonectria queenslandica L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2010 | ||
| Calonectria reteaudii (Bugnic.) C. Booth | Booth 1966 | Cylindrocladium reteaudii (Bugnic.) Boesew. | Boesewinkel 1982 |
| Calonectria rumohrae El-Gholl & Alfenas | El-Gholl et al. 1997 | Cylindrocladium rumohrae El-Gholl & Alfenas | El-Gholl et al. 1997 |
| Calonectria scoparia Ribeiro & Matsuoka ex Peerally | Peerally 1991a | Cylindrocladium candelabrum Viégas | Crous 2002 |
| Calonectria spathiphylli El-Gholl, J.Y. Uchida, Alfenas, T.S. Schub., Alfieri & A.R. Chase | El-Gholl et al. 1992 | Cylindrocladium spathiphylli Schoult., El-Gholl & Alfieri | Schoulties et al. 1982 |
| Calonectria spathulata El-Gholl, Kimbr., E.L. Barnard, Alfieri & Schoult. | Crous & Wingfield 1994 | Cylindrocladium spathulatum El-Gholl, Kimbr., E.L. Barnard, Alfieri & Schoult. | Crous & Wingfield 1994 |
| Calonectria terrae-reginae L. Lombard, M.J. Wingf. & Crous | Lombard et al. 2010 | ||
| Calonectria variabilis Crous, B.J.H. Janse, D. Victor, G.F. Marais & Alfenas | Crous et al. 1993b | Cylindrocladium variabile Crous, B.J.H. Janse, D. Victor, G.F. Marais & Alfenas | Crous et al. 1993b |
| Cylindrocladium angustatum Crous & El-Gholl | Crous et al. 2000 | ||
| Cylindrocladium australiense Crous & K.D. Hyde | Crous et al. 2006a | ||
| Cylindrocladium canadense J.C. Kang, Crous & C.L. Schoch | Kang et al. 2001b | ||
| Cylindrocladium chinense Crous | Crous et al. 2004b | ||
| Cylindrocladium citri (H.S. Fawc. & Klotz) Boedijn & Reitsma | Boedjin & Reitsma 1950 | ||
| Cylindrocladium curvatum Boedijn & Reitsma | Boedjin & Reitsma 1950 | ||
| Cylindrocladium curvisporum Crous & D. Victor | Victor et al. 1997 | ||
| Cylindrocladium ecuadoriae Crous & M.J. Wingf. | Crous et al. 2006a | ||
| Cylindrocladium gordoniae Leahy, T.S. Schub. & El-Gholl | Leahy et al. 2000 | ||
| Cylindrocladium hawksworthii Peerally | Peerally 1991b | ||
| Cylindrocladium hurae (Linder & Whetzel) Crous | Crous 2002 | ||
| Cylindrocladium indonesiae Crous | Crous et al. 2004b | ||
| Cylindrocladium leucothoës El-Gholl, Leahy & T.S. Schub. | El-Gholl et al. 1989 | ||
| Cylindrocladium malesianum Crous | Crous et al. 2004b | ||
| Cylindrocladium multiphialidicum Crous, Simoneau & Risède | Crous et al. 2004b | ||
| Cylindrocladium pacificum J.C. Kang, Crous & C.L. Schoch | Kang et al. 2001b | ||
| Cylindrocladium penicilloides (Tubaki) Tubaki | Tubaki 1958 | ||
| Cylindrocladium pseudonaviculatum Crous, J.Z. Groenew. & C.F. Hill | Crous et al. 2002 | ||
| Cylindrocladium sumatrense Crous | Crous et al. 2004b |
A general search on MycoBank (www.mycobank.org; Crous et al. 2004a, Robert et al. 2005) and Index Fungorum (www.indexfungorum.org) resulted in a total of 291 and 261 name records respectively for Calonectria. A similar search for Cylindrocladium species on both electronic databases indicated a total of 98 and 93 names respectively.
NOMENCLATURE OF CALONECTRIA
The nomenclature of pleomorphic fungi has been a topic of substantial debate during the course of the past two decades (Gams 1991, Cannon & Kirk 2000, Hawksworth 2004, 2005). The separate naming of anamorphs (mitotic morphs) and teleomorphs (meiotic morphs) has resulted in confusion, especially for non-taxonomists (Cannon & Kirk 2000). This is especially evident where teleomorph species epithets are different to those of their anamorphs and also where more than one anamorph (synanamorph) is found. The naming of fungal morphs based on the International Code of Botanical Nomenclature (ICBN; McNeill et al. 2005) and in particular following strict interpretation of Article 59 of the Code has now been unsatisfactory for many fungal groups due to our ability to connect morphs using molecular evidence, and there are increasing calls for further changes to be made.
Recent alterations to the Code at the ICBN meeting in Vienna allows for anamorphic fungi to be named in teleomorph genera, but these are vulnerable to be superseded by a connected teleomorph name in the future (Hawksworth 2004, McNeill et al. 2005, P. Cannon pers. comm.). Although there are several Cylindrocladium species without Calonectria connections (Crous 2002, Crous et al. 2004b, 2006a), we believe that new species should be described in Calonectria irrespective of whether a teleomorph is known or not. This follows a clear view based on phylogenetic inference that Cylindrocladium spp. all have Calonectria states (Schoch et al. 1999, 2000a, 2000b, Crous 2002, Crous et al. 2004b, 2006a). Following the approach of Crous et al. (2006b, 2008, 2009a, b) with other fungal groups, Lombard et al. (2009, 2010) recently described five new species in the genus Calonectria, irrespective whether the teleomorph was observed or not. Thus, for taxonomic purposes, Cylindrocladium species with known teleomorph states are referred to as Calonectria in this review.
IMPORTANCE OF CALONECTRIA
The genus Calonectria was initially regarded as a saprobe as no disease symptoms could be induced by inoculating a suspected host (Graves 1915). The first proof of pathogenicity of these fungi was provided by Massey (1917), and subsequently by Anderson (1919), who proved pathogenicity of Ca. morganii (as Cy. scoparium). Subsequently, Calonectria species have been associated with a wide range of disease symptoms on a large number of hosts worldwide (Crous 2002; Table 2; Figs 1, 2). In the past, several authors have indicated that Calonectria species cause disease on plants residing in approximately 30 plant families (Booth & Gibson 1973, French & Menge 1978, Peerally 1991a, Wiapara et al. 1996, Schoch et al. 1999). Upon closer inspection, the number of plant families is actually closer to 100 (Table 2) and approximately 335 plant host species (Crous 2002). The plant hosts include important forestry, agricultural and horticultural crops and the impact of these plant pathogens has likely been underestimated.
Table 2.
Plant families that are host to Calonectria species and number of known plant host species in each family (Crous 2002).
| Host Plant family | Host species | Host Plant family | Host species | Host Plant family | Host species | Host Plant family | Host species |
|---|---|---|---|---|---|---|---|
| Actinidiaceae | 2 | Cornaceae | 1 | Malipighiaceae | 2 | Pteridaceae | 1 |
| Altingiaceae | 1 | Crassulaceae | 1 | Malvaceae | 6 | Rhamnaceae | 1 |
| Anacardiaceae | 3 | Cupressaceae | 4 | Meliaceae | 2 | Rhizophoraceae | 1 |
| Annonaceae | 4 | Curcurbitaceae | 3 | Moraceae | 2 | Rosaceae | 10 |
| Aparagaceae | 1 | Cycadaceae | 1 | Musaceae | 2 | Rubiaceae | 2 |
| Apiaceae | 1 | Davalliaceae | 1 | Myristicaceae | 1 | Ruscaceae | 1 |
| Apocynaceae | 2 | Dennstaedtiaceae | 1 | Myrsinaceae | 1 | Rutaceae | 3 |
| Aquifoliaceae | 4 | Dilleniaceae | 1 | Myrtaceae | 31 | Salicaceae | 3 |
| Araceae | 5 | Dipterocarpaceae | 1 | Nelumbonaceae | 1 | Sapindaceae | 4 |
| Araliaceae | 2 | Dryopteridaceae | 2 | Nepenthaceae | 1 | Sapotaceae | 3 |
| Arecaceae | 21 | Ebenaceae | 1 | Nothofagaceae | 1 | Sarraceniaceae | 1 |
| Araucariaceae | 2 | Ericaceae | 14 | Nymphaeaceae | 1 | Saxifragaceae | 1 |
| Aspleniaceae | 1 | Euphorbiaceae | 6 | Oleaceae | 1 | Solanaceae | 4 |
| Asteraceae | 5 | Fabaceae | 57 | Onagraceae | 2 | Sterculiaceae | 2 |
| Berberidaceae | 2 | Fagaceae | 4 | Orchidaceae | 1 | Strelilziaceae | 2 |
| Betulaceae | 1 | Ginkgoaceae | 1 | Phytolaccaceae | 1 | Theaceae | 1 |
| Bixaceae | 1 | Juglandaceae | 2 | Pinaceae | 17 | Ulmaceae | 1 |
| Bromeliaceae | 3 | Lauraceae | 6 | Piperaceae | 1 | Verbenaceae | 1 |
| Buxaceae | 1 | Laxmanniaceae | 1 | Platanaceae | 1 | Vitaceae | 2 |
| Caricaceae | 2 | Lecythidaceae | 1 | Plumbaginaceae | 1 | Vochysiaceae | 1 |
| Caryophyllaceae | 1 | Leeaceae | 1 | Poaceae | 6 | Xanthorrhoeaceae | 1 |
| Celastraceae | 1 | Linaceae | 1 | Polygalaceae | 1 | Zingiberaceae | 1 |
| Chenopodiaceae | 1 | Lomariopsidaceae | 1 | Polygonaceae | 3 | ||
| Combretaceae | 3 | Lythraceae | 1 | Polypodiaceae | 1 | ||
| Convolvulaceae | 1 | Magnoliaceae | 2 | Proteaceae | 7 |
Fig. 1.
Disease symptoms associated with Calonectria (Cylindrocladium). A. Cutting rot of Vallea stipolaris. B. Cutting rot of Eucalyptus sp. C. Defoliated Eucalyptus trees in a plantation. D. Leaf and shoot blight of a Eucalyptus sp. E. Cylindrocladium leaf blight of a Eucalyptus sp. F. Leaf spots on a Eucalyptus sp. G–H. Stem cankers on twigs of a Eucalyptus sp. I–J. Root and collar rot of Pinus spp. K. Root rot of Eucalyptus sp. with conidiophores on the root surface.
Fig. 2.
Disease symptoms associated with Calonectria (Cylindrocladium). A–D. Defoliation and yellowing associated with Calonectria pseudonaviculata infection on Buxus sp. at Paleis Het Loo in the Netherlands (upper part of hedge in A, arrows). B–D. Leaf yellowing and defoliation (note detaching leaves in D, arrows). E–H. Calonectria ilicicola causing Cylindrocladium black rot (CBR) on Arachis hypogaea in Georgia, U.S.A. F. Perithecia forming at the basal plant parts. G. Pods infected with tomato spotted wilt virus (left), healthy pods (middle), and pods infected with CBR (right). H. Field symptoms associated with CBR (photos with permission of T. Brenneman). I. Avocado roots infected with Ca. ilicicola (photo with permission of L. Forsberg). J. Seeding blight of Callistemon citrinus associated with Ca. morganii (photo with permission of G. Polizzi). K. Seedling rot of Drosera sp. associated with Ca. pteridis infection. L. Leaf spots of Callistemon citrinus associated with Ca. pauciramosa (photo with permission of G. Polizzi). M. Arbutus unedo associated with Ca. pauciramosa infection (photo with permission of G. Polizzi). N–O. Root rot and petiole lesions of Spathiphyllum sp. associated with Ca. spathiphylli infection (photo with permission from the late N.E. El-Gholl). P. Potato tuber infected with Ca. brassicae. Q–R. Leaf blight of Eucalyptus sp. associated with a mixed infection of Ca. pteridis and Ca. ovata.
The majority of disease reports associated with Calonectria species in forestry include hosts in five plant families, of which the most important are associated with Fabaceae (Acacia spp.), Myrtaceae (Eucalyptus spp.) and Pinaceae (Pinus spp.). Disease symptoms (Figs 1, 2) include cutting rot (Crous et al. 1991, Crous 2002, Lombard et al. 2009, 2010), damping-off (Batista 1951, Cox 1953, Terashita & Itô 1956, Sharma & Mohanan 1982, Sharma et al. 1984, Crous et al. 1991, Brown & Ferreira 2000, Crous 2002, Taniguchi et al. 2008) leaf diseases (Cox 1953, Hodges & May 1972, Barnard 1984, Sharma et al. 1984, El-Gholl et al. 1986, Peerally et al. 1991a, Crous et al. 1993b, Crous & Wingfield 1994, Crous et al. 1998b, Schoch & Crous 1999, Schoch et al. 1999, Booth et al. 2000, Park et al. 2000, Crous & Kang 2001, Gadgil & Dick 2004), shoot blight (Sharma et al. 1984, Crous et al. 1991, 1998b, Crous & Kang 2001), stem cankers (Cox 1953, Sharma et al. 1984, 1985, Crous et al. 1991, Lombard et al. 2009) and root rot (Cox 1953, Hodges & May 1972, Cordell & Skilling 1975, Mohanan & Sharma 1985, Crous et al. 1991, Lombard et al. 2009). The majority of these diseases is associated with seedling and cutting production in forestry nurseries, but in a few cases Cylindrocladium species have also been reported from older, established commercial plantations. In these cases the pathogens have been reported to cause leaf diseases and shoot blight resulting in defoliation of trees leading to loss of vigour (Hodges & May 1972, Sharma et al. 1985, Booth et al. 2000, Park et al. 2000, Crous & Kang 2001, Crous 2002, Old et al. 2003, Rodas et al. 2005).
In agriculture, Calonectria species have been reported to cause diseases on several economically important crops. Several plant families of agricultural importance are susceptible to Calonectria infections, including Fabaceae and Solanaceae. Important diseases in these families are Cylindrocladium black rot of Arachis hypogea (peanut) and red crown rot of Glycine max (soybean) caused by Ca. ilicicola and Ca. pyrochroa in the USA (Bell & Sobers 1966, Beute & Rowe 1973, Rowe et al. 1973, Sobers & Littrell 1974, Rowe & Beute 1975, Phipps et al. 1976, Johnson 1985, Dianese et al. 1986, Berner et al. 1988, 1991, Culbreath et al. 1991, Porter et al. 1991, de Varon 1991, Hollowell et al. 1998, Kim et al 1998) and Cylindrocladium tuber rot of Solanum tuberosum (potato) (Boedijn & Reitsma 1950, Bolkan et al. 1980, 1981) by Ca. brassicae (as Cy. gracile) in Brazil. Other diseases associated with Calonectria species on agricultural crops include root rot and leaf diseases of fruit bearing and spice plants (Jauch 1943, Wormald 1944, Sobers & Seymour 1967, Nishijima & Aragaki 1973, Milholland 1974, Krausz & Caldwell 1987, Hutton & Sanewski 1989, Anandaraj & Sarma 1992, Risède 1994, Jayasinghe & Wijesundera 1996, Risède & Simoneau 2001, Vitale & Polizzi 2008), post-harvest diseases of fruits (Fawcett & Klotz 1937, Boedijn & Reitsma 1950, Sepiah 1990, Fitzell & Peak 1992, Vaidya & Roa 1992, Sivapalan et al. 1998), root and crown rot of Medicago sativa (alfalfa) (Ooka & Uchida 1982, Hwang & Flores 1987), and sheath net blotch of Oryza sativa (rice) (Crous 2002).
On horticultural crops, Calonectria species have been reported mostly from the Northern Hemisphere, especially in gardens and ornamental commercial nurseries in Europe and Asia (Polizzi & Crous 1999, Polizzi 2000, Crous 2002, Henricot & Culham 2002, Pérez-Sierra et al. 2007, Polizzi et al. 2007a, b, Hirooka et al. 2008, Polizzi et al. 2009, Vitale et al. 2009). Hosts in this sector include ornamental trees, shrubs and cut-flowers in several plant families, most commonly in Arecaceae, Asteraceae, Ericaceae and Rosaceae. A wide range of disease symptoms are recorded including crown-, collar- and root rot, leaf spots, and cutting rot (Massey 1917, Anderson 1919, Aragaki et al. 1972, 1988, Peerally 1991b, Uchida & Kadooka 1997, Polizzi & Crous 1999, Polizzi 2000, Crous 2002, Henricot & Culham 2002, Henricot & Beales 2003, Poltronieri et al. 2004, Lane et al. 2006, Pérez-Sierra et al. 2006, 2007, Polizzi et al. 2006a, b, 2007a, b, Vitale & Polizzi 2007, Aghajani et al. 2008, Hirooka et al. 2008, Vitale et al. 2008, Polizzi et al. 2009, Vitale et al. 2009).
MORPHOLOGY
Morphological or phenotypic characters have played a major role in the description of fungal species (Brasier 1997, Taylor et. al. 2000) and form the basis of new fungal descriptions as required by the ICBN (McNeill et al. 2005). In recent years, the use of morphological characters alone to delimit new species has been set aside to a large extent, with more focus being placed on biological and phylogenetic characters (Rossman 1996, Brasier 1997, Taylor et al. 2000). This trend is also evident in recent studies on Calonectria species (Crous et al. 2004b, 2006a).
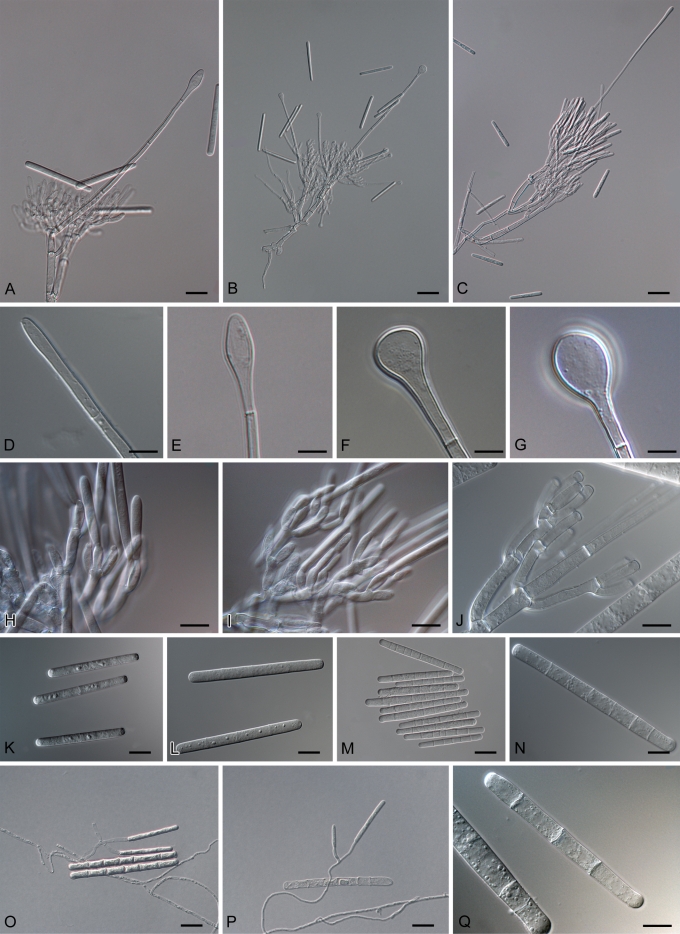
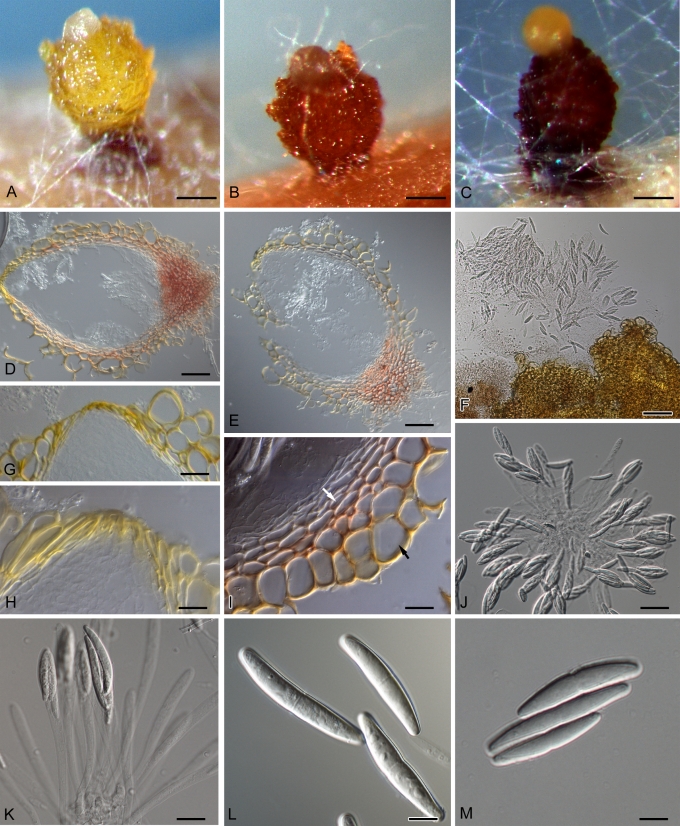
The morphology of Calonectria and to a greater extent its anamorph, Cylindrocladium, has been important in the taxonomic history of these fungi. Prior to the 1990s, identification of species was based on morphological characteristics and to a lesser extent on sexual compatibility using standardised media (Boedijn & Reitsma 1950, Peerally 1991a, Crous et al. 1992, Crous & Wingfield 1994, Crous 2002). This resulted in the establishment of several species complexes, as many Cylindrocladium species are morphologically very similar. These include the Ca. scoparia complex (Schoch et al. 1999), Ca. brassicae (as Cy. gracile) complex (Crous et al. 2004b) and Ca. kyotensis complex (Crous et al. 2006a). Characteristics of the anamorphs that are extensively employed in identifications include vesicle shape, stipe extension length and macroconidial septation and dimensions (Fig. 3) (Boesewinkel 1982, Peerally 1991a, Crous & Wingfield 1994, Crous 2002). The morphological characteristics of the teleomorph (Fig. 4) that are important for identifications are ascospore septation and dimensions, ascospore number within the asci and perithecial colour. Perithecia of Calonectria species are morphologically very similar and these are not typically useful in identifications (Crous & Wingfield 1994, Crous 2002).
Fig. 3.
Anamorph structures of Calonectria. A. Macroconidiophore of Ca. pauciramosa. B. Macroconidiophore of Ca. hongkongensis. C. Macroconidiophore of Ca. brassicae. D. Clavate vesicle of Ca. reteaudii. E. Obpyriform vesicle of Ca. pauciramosa. F. Sphaeropedunculate vesicle of Ca. hongkongensis. G. Pyriform vesicle of Ca. morganii. H. Fertile branches of Ca. pauciramosa with doliiform to reniform phialides. I. Fertile branches of a Calonectria sp. with elongate-doliiform to reniform phialides. J. Fertile branches of Ca. reteaudii with cylindrical to allantoid phialides. K. One-septate macroconidia of Ca. pauciramosa. L. Three-septate macroconidia of Ca. colhounii. M–N. Five to eight-septate macroconidia of Ca. reteaudii. O–P. Microconidiophores of Ca. reteaudii. Q. three-septate microconidium of Ca. reteaudii. Scale bars: B–C, M = 50 μm; A, O–P = 20 μm; D–L, N, Q = 10 μm.
Fig. 4.
Teleomorph structures of Calonectria spp. A. Yellow perithecium of Ca. colhounii. B. Orange to red perithecium of Ca. pauciramosa. C. Dark red perithecium of Calonectria sp. D–E. Vertical sections through perithecia. F. Squashed perithecium exuding ascospores. G–H. Ostiolar regions of perithecia. I. Vertical section through the wall of a perithecium showing the textura globulosa (black arrow) and textura angularis (white arrow) wall layers. J. Asci containing eight ascospores. K. Asci containing four ascospores. L–M. One-septate ascospores. Scale bars: A–C = 100 μm; F = 50 μm; J–K = 20 μm; D–E, G–I, L–M = 10 μm.
The use of biochemical techniques can also be used in phenotypic characterisation. These include substrate utilisation and cell wall polysaccharide analysis. The use of aminopeptidase specificity (Stevens et al. 1990) and utilisation of nitrogen and carbon (Hunter & Barnett 1978, Sharma et al. 1992) have been used successfully to separate several Cylindrocladium species. The use of polysaccharides obtained from cell walls of Cylindrocladium positively identified linkages between asexual species and their respective Calonectria teleomorphs (Ahrazem et al. 1997). However, this method has been found to have limited value as some species in complexes could not be distinguished (Crous 2002).
MATING COMPATIBILITY
Mating strategies have been employed in the taxonomy of Calonectria and have played an important role in identifying new species of the genus (Schoch et al. 1999, Crous 2002). Based on these studies, there are approximately 18 homothallic and 34 heterothallic species of Calonectria (Crous 2002, Crous et al. 2004b, Gadgil & Dick 2004, Crous et al. 2006a), with the heterothallic species showing a biallelic mating system (Schoch et al. 1999). Studies in the female fertility of Cylindrocladium by Schoch et al. (1999, 2000a, 2001a) have also shown that several species are self-sterile hermaphrodites requiring fertilisation from an opposite mating type. This is typical of heterothallic ascomycetes (Leslie & Klein 1996).
Several difficulties associated with applying the BSC have been highlighted (Brasier 1997, Taylor et al. 1999, 2000, Kohn 2005). The most relevant underlying problem occurs where genetically isolated fungal strains retain the ancestral ability to recombine to produce viable progeny (Brasier 1997). This phenomenon has also been found with several phylogenetic species that are closely related in Calonectria. Crous (2002), for example, showed that Cy. hawksworthii, Ca. insularis and Ca. morganii were capable of recombining, but that the progeny had low levels of fertility. Other mating studies done by Overmeyer et al. (1996) and Neubauer & Zinkernagel (1995) have found that induction of fertile perithecia requires the presence of an additional isolate that, however, does not contribute to the genetic make-up of the progeny. This clearly highlights the need for further studies regarding the mechanism of perithecial formation and recombination in Calonectria.
PHYLOGENY
Phylogenetic studies on Calonectria and its Cylindrocladium anamorphs have substantially influenced the taxonomy of these genera. Application of molecular techniques and particularly DNA sequence comparisons to distinguish between species has resulted in the recognition of numerous cryptic species. Several molecular approaches have been employed that include total protein electrophoresis (Crous et al. 1993a, El-Gholl et al. 1993a), isozyme electrophoresis (El-Gholl et al. 1992, 1997, Crous et al. 1998a), random amplification of polymorphic DNA (RAPD) (Overmeyer et al. 1996, Victor et al. 1997, Schoch et al. 2000a, Risède & Simoneau 2004) restriction fragment length polymorphisms (RFLP) (Crous et al. 1993b, 1995, 1997b, Jeng et al. 1997, Victor et al. 1997; Risède & Simoneau 2001) and DNA hybridisation (Crous et al. 1993b, 1995, 1997a, Victor et al. 1997). Although the above-mentioned techniques have been useful, DNA sequence comparisons and associated phylogenetic inference have had the most dramatic impact on the taxonomy of Calonectria and are most widely applied today.
In the first study using 5.8S ribosomal RNA gene and flanking internally transcribed spacers (ITS) sequences Jeng et al. (1997) were able to distinguish between Cy. scoparium and Cy. floridanum isolates. Subsequently, it was found that this gene region contains few informative characters (Crous et al. 1999, Schoch et al. 1999, Risède & Simoneau 2001, Schoch et al. 2001b). Therefore, the β-tubulin (Schoch et al. 2001b) and histone H3 (Kang et al. 2001a) gene regions have been applied in order to allow for improved resolution in separating species.
The first complete DNA sequence-based phylogenetic study using partial β-tubulin gene sequences (Schoch et al. 2001b) compared phenotypic, biological and phylogenetic concepts used in the taxonomy of Cylindrocladium. This also highlighted the fact that Calonectria represents a monophyletic lineage (Schoch et al. 2000b, 2001b). Subsequently, combined DNA sequence data for the ITS, β-tubulin and histone H3 gene regions have been widely used in studies relating to taxonomic issues surrounding Cylindrocladium and Calonectria (Crous et al. 1999, Schoch et al. 2000a, 2000b, Crous & Kang 2001, Kang et al. 2001a, 2001b, Henricot & Culham 2002, Crous et al. 2004b, 2006a, Lombard et al. 2009, 2010). Other partial gene sequences recently used include translation elongation 1-alpha (TEF-1α) and calmodulin (Crous et al. 2004b, Lombard et al. 2010). However, insufficient data are currently available for these gene regions on GenBank (www.ncbi.nlm.nih.gov) to make them particularly valuable for comparative analysis.
A recent search in GenBank (March 2010) revealed a total of 734 partial gene sequences for Calonectria and Cylindrocladium. These include 311 for β-tubulin, 177 for histone H3, 159 for ITS, 39 for calmodulin, 36 for TEF-1α, five for large subunit RNA gene (LSU), three each for the high mobility group (HMG) box and peptidase synthetase and one for the small subunit RNA (SSU) gene. For Cylindrocladium and Calonectria, there are only six studies (Kang et al. 2001a, 2001b; Crous et al. 2004b, 2006a, Lombard et al. 2009, 2010) that provide files on TreeBase (www.treebase.org).
FUTURE RESEARCH
Population biology
Most studies on Calonectria have focused on the taxonomy, phylogeny and pathology of species. There have in contrast been relatively few studies treating the population biology of these fungi. This is unfortunate as population dynamics contributes considerable knowledge to a better understanding of population structure, distribution of genetic diversity, gene flow, centres of origin and mating strategies (McDonald 1997, Linde et al. 2002, Grünwald et al. 2003). An understanding of the population dynamics of Calonectria would contribute in determining the natural spread of these fungi as well as assist in phytosanitary and quarantine regulations. Another important aspect surrounding knowledge of Calonectria population dynamics is that this would contribute to plant breeding programmes and thus control of the many diseases that are caused by these fungi (McDonald 1997, Wright et al. 2006, 2007).
Limited research has been conducted on the population dynamics of Calonectria. To date only two studies (Wright et al. 2006, 2007) have reported on the development of polymorphic markers to characterise simple sequence repeats (SSRs) in loci of Ca. ilicicola (Wright et al. 2006) and Ca. pauciramosa (Wright et al. 2007). However, no study has yet been published on the population biology of either of these important pathogens using these markers. There is clearly a gap in this area of research concerning Calonectria spp. and future research on this topic should be encouraged.
Whole genome sequences
A relatively new and innovative technology employed in fungal genetics is the use of whole genome sequences of filamentous fungi. Whole genome sequencing has become relatively inexpensive and thus common in recent years. This revolutionary technology will promote our understanding of the mechanisms of gene function, conidiation, pathogenesis and sexual reproduction at the genotype level (Kupfer et al. 1997, Prade 1998, Yoder & Turgeon 2001, Foster et al. 2006, Cuomo et al. 2007). It is estimated that most filamentous fungi have a genome size of 30 to 40 Mb, containing approximately 8000 to 9000 genes (Kupfer et al. 1997, Prade 1998, Foster et al. 2006). There are currently several completed fungal genome sequences (http://www.broad.mit.edu/annotation/fungi/fgi/, Foster et al. 2006, Baker et al. 2008), including the model yeast Saccharomyces cerevisiae (Goffeau et al. 1996), plant pathogens and spoilage fungi such as Aspergillus flavus (Payne et al. 2006), Fusarium graminearum (http://www.broad.mit.edu, Cuomo et al. 2007), Magnaporthe grisea (Dean et al. 2005) and the model filamentous fungus Neurospora crassa (Galagan et al. 2003). Although there are currently over 300 ongoing filamentous fungal genome sequencing projects (http://www.genomesonline.org, Baker et al. 2008, Liolios et al. 2008), none include species of Calonectria.
The most closely related plant pathogen to Calonectria species currently being sequenced is Haematonectria haematococca (http://www.ncbi.nlm.gov). When the first Calonectria species is selected for whole genome sequencing, comparisons with H. haematococca could help to identify important genes in pathogenesis and sexual reproduction. Some Calonectria species that could be considered for genome sequencing include Ca. pauciramosa, based on its pathogenicity and importance on several plant hosts worldwide (Crous 2002), and Ca. reteaudii, one of the most important forest pathogens of South East Asia (Booth et al. 2000, Old et al. 2003).
CONCLUSIONS
Early studies on the taxonomy of Calonectria and Cylindrocladium focused on the use of MSC in combination with BSC. More recently, the wide availability of molecular techniques and particularly DNA sequence data have revolutionised the taxonomy of Calonectria and Cylindrocladium. Today, it is well accepted that the morphology of the Cylindrocladium state contributes most information to naming species and that these fungi all reside in Calonectria.
The first study to combine MSC, BSC and PSC concepts by Schoch et al. (1999) resulted in the identification of four species within a single species complex. Subsequently, several studies including the MSC, BSC and PSC have elucidated cryptic species in the genus (Kang et al. 2001a, 2001b, Henricot & Culham 2002, Crous et al. 2004b, 2006a, Lombard et al. 2009, 2010). Application of the BSC in the taxonomy of Calonectria has been found to be unreliable in some instances (Crous 2002). However, the implementation of MSC and PSC in combination provides powerful tool for taxonomic studies of these genera and it is likely that this will continue in future studies. Although several species complexes have been identified in Calonectria, more research is needed on the population level in order to study the gene flow between populations. Additional to this, more gene regions need to be identified and widely used in PSC. With the identification of several new species since 2002, an updated monograph is required to facilitate ease of identification.
References
- Aghajani MA, Alizadeh A, Rahimian H (2008). First report of brown patch on bristle basket grass in Iran. Plant Pathology 57: 384. [Google Scholar]
- Ahrazem O, Prieto A, Leal JA, Gomez-Miranda B, Domenech J, Jimenez-Barbero J, Bernabe M (1997). Structural elucidation of acidic fungal polysaccharides isolated from the cell-wall of genera Cylindrocladium and Calonectria. Carbohydrate Research 303: 67–72. [DOI] [PubMed] [Google Scholar]
- Alfieri SA, Linderman RG, Morrison RH, Sobers EK (1972). Comparative pathogenicity of Calonectria theae and Cylindrocladium scoparium to leaves and roots of azalea. Phytopathology 62: 647–650. [Google Scholar]
- Anandaraj M, Sarma YR (1992). A new leaf rot in Pimenta dioica. Indian Phytopathology 45: 276–277. [Google Scholar]
- Anderson PJ (1919). Rose canker and its control. Massachusetts Agricultural Experiment Station Bulletin 183: 11–46. [Google Scholar]
- Aragaki M, Laemmlen FF, Nishijima WT (1972). Collar rot of Koa caused by Calonectria crotalariae. Plant Disease Reporter 56: 73–74. [Google Scholar]
- Aragaki M, Yahata PS, Uchida JY (1988). Heliconia root rot caused by Cylindrocladium spathiphylli f. sp. heliconiae. Phytopathology 78: 1614. [Google Scholar]
- Baker SE, Thykaer J, Adney WS, Brettin TS, Brockman FJ, D'Haeseleer P, Martinez AD, Miller RM, Rokhsar DS, Schadt CW, Torok T, Tuskan G, Bennett J, Berka RM, Briggs SP, Heitman J, Taylor J, Turgeon BG, Werner-Washburne M, Himmel ME (2008). Fungal genome sequencing and bioenergy. Fungal Biology Reviews 22: 1–5. [Google Scholar]
- Barnard EL (1984). Occurrence, impact and fungicide control of girdling stem cankers caused by Cylindrocladium scoparium on Eucalyptus seedlings in a south Florida nursery. Plant Disease 68: 471–473. [Google Scholar]
- Batista AC (1951). Cylindrocladium scoparium Morgan var. brasiliensis Batista & Ciferri, a new fungus on Eucalyptus. Boletim da Secretaria de Agricultura, Industria e Comercio do Estado de Pernambuco 18: 188–191. [Google Scholar]
- Bell DK, Sobers EK (1966). A peg, pod and root necrosis of peanuts caused by a species of Calonectria. Phytopathology 56: 1361–1364. [Google Scholar]
- Berner DK, Berggren GT, Snow JP, White EP (1988). Distribution and management of red crown rot of soybean in Louisiana, U.S.A. Applied Agricultural Research 3: 160–166. [Google Scholar]
- Berner DK, Berggren GT, Snow JP (1991). Effects of glyphosate on Calonectria crotalariae and red crown rot of soybean. Plant Disease 75: 809–813. [Google Scholar]
- Beute MK, Rowe RC (1973). Studies on the biology and control of Cylindrocladium black rot (CBR) of peanut. Journal of the American Peanut Research Educational Associations 5: 197. [Google Scholar]
- Boedijn KB, Reitsma J (1950). Notes on the genus Cylindrocladium. Reinwardtia 1: 51–60. [Google Scholar]
- Boesewinkel HJ (1982). Heterogeneity within Cylindrocladium and its teleomorphs. Transactions of the British Mycological Society 78: 553–556. [Google Scholar]
- Bolkan HA, Dianese JC, Ribeiro WRC, Almeida OC de (1980). Disease caused by Cylindrocladium on potato tubers in Brazil. Plant Disease 64: 225. [Google Scholar]
- Bolkan HA, Ribeiro WRC, Almeida OC de (1981). Pathogenicity of Cylindrocladium clavatum causing potato tuber rot. Plant Disease 65: 47–49. [Google Scholar]
- Booth C (1966). The genus Cylindrocarpon. Mycological Papers 104: 1–56. [Google Scholar]
- Booth C, Gibson IAS (1973). Cylindrocladium scoparium. CMI Descriptions of Pathogenic Fungi and Bacteria No. 362.
- Booth TH, Jovanovic T, Old KM, Dubzinski MJ (2000). Climatic mapping to identify high – risk areas for Cylindrocladium quinqueseptatum leaf blight on eucalypts in mainland South East Asia and around the world. Environmental Pollution 108: 365–372. [DOI] [PubMed] [Google Scholar]
- Booth C, Murray JS (1960). Calonectria hederae Arnaud and its Cylindrocladium conidial state. Transactions of the British Mycological Society 43: 69–72. [Google Scholar]
- Brasier CM (1997). Fungal species in practice: identifying species units in fungi. In: Species: The units of Biodiversity (Claridge MF, Dawah HA, Wilson MR, eds). Chapman & Hall, U.K.: 135–170.
- Brown BB, Ferreira FA (2000). Disease during propagation of eucalypts. In: Diseases and pathogens of eucalypts. (Keane PJ, Kile GA, Podger FD, Brown BN, eds). CSIRO publishing, Australia: 119–151.
- Cannon PF, Kirk PM (2000). The philosophy and practicalities of amalgamating anamorph and teleomorph concepts. Studies in Mycology 45: 19–25. [Google Scholar]
- Cordell CE, Skilling DD (1975). Forest nursery diseases in the U.S.A. 7. Cylindrocladium root rot. U.S.D.A. Forest Service Agricultural Handbook No. 470: 23–26. [Google Scholar]
- Cox RS (1953). Etiology and control of a serious complex of diseases of conifer seedlings. Phytopathology 43: 469. [Google Scholar]
- Crous PW (2002). Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, St. Paul, Minnesota, U.S.A.
- Crous PW, Alfenas AC, Junghans TG (1998a). Variability within Calonectria ovata and its anamorph Cylindrocladium ovatum from Brazil. Sydowia 50: 1–13. [Google Scholar]
- Crous PW, Alfenas AC, Wingfield MJ (1993a). Calonectria scoparia and Calonectria morganii sp. nov., and variation among isolates of their Cylindrocladium anamorphs. Mycological Research 97: 701–708. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ, Hill CF (2002) Cylindrocladium pseudonaviculatum sp. nov. from New Zealand, and new Cylindrocladium records from Vietnam. Sydowia 54: 23–33. [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD (2006a). Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hywel-Jones NL (2004b). Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Janse BJH, Victor D, Marais GF, Alfenas AC (1993b). Molecular characterization of Cylindrocladium spp. with three-septate conidia and ovoid-like vesicles. Systematic and Applied Microbiology 16: 266–273. [Google Scholar]
- Crous PW, Kang JC (2001). Phylogenetic confirmation of Calonectria spathulata and Cylindrocladium leucothoes based on morphology, and sequence data of the β-tubulin and ITS rRNA genes. Mycoscience 42: 51–57. [Google Scholar]
- Crous PW, Kang JC, Schoch CL, Mchau GRA (1999). Phylogenetic relationships of Cylindrocladium pseudogracile and Cylindrocladium rumohrae with morphologically similar taxa, based on morphology and DNA sequences of internal transcribed spacers and β-tubulin. Canadian Journal of Botany 77: 1813–1820. [Google Scholar]
- Crous PW, Krof A, Zyl WH van (1995). Nuclear DNA polymorphisms of Cylindrocladium species with 1-septate conidia and clavate vesicles. Systematic and Applied Microbiology 18: 224–250. [Google Scholar]
- Crous PW, Mchau GRA, Zyl WH van, Wingfield MJ (1997a). New species of Calonectria and Cylindrocladium isolated from soil in the tropics. Mycologia 89: 653–660. [Google Scholar]
- Crous PW, Phillips AJL, Wingfield MJ (1991). The genera Cylindrocladium and Cylindrocladiella in South Africa, with special reference to forestry nurseries. South African Forestry Journal 157: 69–85. [Google Scholar]
- Crous PW, Phillips AJL, Wingfield MJ (1992). Effects of cultural conditions on vesicle and conidium morphology in species of Cylindrocladium and Cylindrocladiella. Mycologia 84: 497–504. [Google Scholar]
- Crous PW, Schoch CL, El-Gholl NE, Schubert TS, Leahy RM (2000). Cylindrocladium angustatum sp. nov., a new leaf spot pathogen of Tillandsia capitata from Florida U.S.A. Mycosience 41: 521–526. [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006b). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Groenewald JZ (2009a). Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia 23: 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ (2009b). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Theron L, Zyl WH van (1997b). Delineation of Cylindrocladium species with 1–3-septate conidia and clavate vesicles based on morphology and rDNA RFLPs. Mycological Research 101: 210–214. [Google Scholar]
- Crous PW, Wingfield MJ (1994). A monograph of Cylindrocladium, including anamorphs of Calonectria. Mycotaxon 51: 341–435. [Google Scholar]
- Crous PW, Wingfield MJ, Alfenas A (1993c). Additions to Calonectria. Mycotaxon 46: 217–234. [Google Scholar]
- Crous PW, Wingfield MJ, Alfenas AC (1993d). Cylindrocladium parasiticum sp. nov., a new name for C. crotalariae. Mycological Research 97: 889–896. [Google Scholar]
- Crous PW, Wingfield MJ, Alfenas AC, Silveira SF (1994). Cylindrocladium naviculatum sp. nov., and two new vesciculate hyphomycete genera, Falcocladium and Vesiculomyces. Mycotaxon 50: 441–458. [Google Scholar]
- Crous PW, Wingfield MJ, Mohammed C, Yuan ZQ (1998b). New foliar pathogens of Eucalyptus from Australia and Indonesia. Mycological Research 102: 527–532. [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ (2008). Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreath AK, Beute MK, Campbell CL (1991). Spatial and temporal aspects of epidemics of Cylindrocladium black rot in resistant and susceptible peanut genotypes. Phytopathology 81: 144–150. [Google Scholar]
- Cuomo CA, Güldener U, Xu J-R, Trial F, Turgeon BG, et al. (2007). The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317: 1400–1402. [DOI] [PubMed] [Google Scholar]
- Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, et al. (2005). The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434: 980–986. [DOI] [PubMed] [Google Scholar]
- Dianese JC, Ribeiro WRC, Urben AF (1986). Root rot of soybean caused by Cylindrocladium clavatum in central Brazil. Plant Disease 70: 977–980. [Google Scholar]
- El-Gholl NE, Alfenas AC, Crous PW, Schubert TS (1993a). Description and pathogenicity of Cylindrocladium ovatum sp. nov. Canadian Journal of Botany 71: 466–470. [Google Scholar]
- El-Gholl NE, Alfenas AC, Junghans, DT, Schubert TS, Miller JW, Leahy EM (1997). Description of Calonectria rumohrae sp. nov. (anamorph = Cylindrocladium rumohrae sp. nov.) Mycotaxon 64: 467–484. [Google Scholar]
- El-Gholl NE, Alfieri SA, Barnard EL (1993b). Description and pathogenicity of Calonectria clavata sp. nov. Mycotaxon 48: 201–216. [Google Scholar]
- El-Gholl NE, Kimbrough JW, Barnard EL, Alfieri SA, Schoulties CL (1986). Calonectria spathulata sp. nov. Mycotaxon 26: 151–164. [Google Scholar]
- El-Gholl NE, Leahy RM, Schubert TS (1989). Cylindrocladium leucothoeae sp. nov. Canadian Journal of Botany 67: 2529–2532. [Google Scholar]
- El-Gholl NE, Uchida JY, Alfenas AC, Schubert T S, Alfieri SA, Chase AR (1992). Induction and description of perithecia of Calonectria spathiphylli sp. nov. Mycotaxon 45: 285–300. [Google Scholar]
- Fawcett HS, Klotz LJ (1937). A new species of Candelospora causing decay of citrus fruit. Mycologia 29: 207–215. [Google Scholar]
- Fitzell RD, Peak CM (1992). Field evaluation of benomyl to control Cylindrocladium fruit spot of custard apple. Australasian Plant Pathology 21: 16–17. [Google Scholar]
- Foster SJ, Monahan BJ, Bradshaw RE (2006). Genomics of the filamentous fungi – moving from the shadow of the baker's yeast. Mycologist 20: 10–14. [Google Scholar]
- French DW, Menge JA (1978). Survival of Cylindrocladium floridanum in naturally and artificially infested forest tree nurseries. Plant Disease Reporter 62: 806–810. [Google Scholar]
- Gadgil PD, Dick MA (2004). Fungi silvicolae novazelandiae: 5. New Zealand Journal of Forestry Science 34: 316–323. [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, et al. (2003). The genome sequence of the filamentous fungus Neurospora crassa. Nature 422: 859–868. [DOI] [PubMed] [Google Scholar]
- Gams W (1991). What are names in current use? Mycotaxon 40: 319–322. [Google Scholar]
- Gill DL, Alfieri SA, Sobers EK (1971). A new leaf disease of Ilex spp. caused by Cylindrocladium avesiculatum sp. nov. Phytopathology 61: 58–60. [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, et al. (1996). Life with 6000 genes. Science 274: 546–567. [DOI] [PubMed] [Google Scholar]
- Graves AH (1915). Root rot of coniferous seedlings. Phytopathology 5: 213–217. [Google Scholar]
- Grünwald NJ, Goodwinj SB, Milgroom MG, Fry WE (2003). Analysis of genotypic diversity data for populations of microorganisms. Phytopathology 93: 738–746. [DOI] [PubMed] [Google Scholar]
- Harrington TC, Rizzo DM (1999). Defining species in the fungi. In: Structure and Dynamics of Fungal Populations. (Worrall JJ, ed.). Kluwer Academic Press, Dordrecht: 43–70.
- Hawksworth DL (2004). Limitation of dual nomenclature for pleomorphic fungi. Taxon 53: 596–598. [Google Scholar]
- Hawksworth DL (2005). Two major changes in fungal nomenclature enacted in Vienna. Mycological Research 109: 1061–1062. [Google Scholar]
- Henricot B, Beales P (2003). First record of Cylindrocladium pauciramosum on myrtle (Myrtus communis) in Portugal. Plant Pathology 52: 420. [Google Scholar]
- Henricot B, Culham A (2002). Cylindrocladium buxicola, a new species affecting Buxus spp., and its phylogenetic status. Mycologia 94: 980–997. [PubMed] [Google Scholar]
- Hirooka Y, Takeuchi J, Horie H, Natsuaki KT (2008). Cylindrocladium brown leaf spot on Howea belmoreana caused by Calonectria ilicicola (anamorph: Cylindrocladium parasiticum) in Japan. Journal of General Plant Pathology 74: 66–70. [Google Scholar]
- Hodges CS, May LC (1972). A root disease of pine, Araucaria, and Eucalyptus in Brazil caused by a new species of Cylindrocladium. Phytopathology 62: 898–901. [Google Scholar]
- Hollowell JE, Shew BB, Beute MK, Abad ZG (1998). Occurrence of pod rot pathogens in peanuts grown in North Carolina. Plant Disease 82: 1345–1349. [DOI] [PubMed] [Google Scholar]
- Hunter BB, Barnett HL (1978). Growth and sporulation of species and isolates of Cylindrocladium in culture. Mycologia 70: 614–635. [Google Scholar]
- Hutton DG, Sanewski GM (1989). Cylindrocladium leaf and fruit spot of custard apple in Queensland. Australasian Plant Pathology 18: 15–16. [Google Scholar]
- Hwang SF, Flores G (1987). Effects of Cylindrocladium gracile, Fusarium roseum and Plenodomus meliloti on crown and root rot, foliage yield and winterkill of alfalfa in north-eastern Alberta. Canadian Plant Disease Survey 67: 31–33. [Google Scholar]
- Jauch C (1943). The presence of Cylindrocladium scoparium in Argentina. Revista Argentina de Agronomia 10: 355–360. [Google Scholar]
- Jayasinghe CK, Wijesundera RLC (1996). Morphological, cultural and pathogenic variation among the clove isolates of Cylindrocladium quinqueseptatum. Journal of Plantation Crops 24: 34–42. [Google Scholar]
- Jeng RS, Dumas M, Liu FH, Wang CL, Hubbes M (1997). DNA analysis of Cylindrocladium floridanum isolates from selected forest nurseries. Mycological Research 101: 285–291. [Google Scholar]
- Johnson GI (1985). Occurrence of Cylindrocladium crotalariae on peanut (Arachis hypogaea) seed. Plant Disease 69: 434–436. [Google Scholar]
- Kang JC, Crous PW, Old KM, Dubzinski MJ (2001a). Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on a β-tubulin gene phylogeny and morphology. Canadian Journal of Botany 79: 1241–1247. [Google Scholar]
- Kang JC, Crous PW, Schoch CL (2001b). Species concepts in the Cylindrocladium floridanum and Cy. spathiphylli complexes (Hypocreaceae) based on multi-allelic sequence data, sexual compatibility and morphology. Systematic and Applied Microbiology 24: 206–217. [DOI] [PubMed] [Google Scholar]
- Kim KD, Russin JS, Snow JP (1998). Susceptibility to Calonectria ilicicola in soybean grown in greenhouse and field. Korean Journal of Crop Science 43: 239–244. [Google Scholar]
- Kohn LM (2005). Mechanisms of fungal speciation. Annual Review of Phytopathology 43: 279–308. [DOI] [PubMed] [Google Scholar]
- Krausz JP, Caldwell JD (1987). Cylindrocladium root rot of kiwifruit. Plant Disease 71: 374–375. [Google Scholar]
- Kupfer DM, Reece CA, Clifton SW, Roe BA, Prade RA (1997). Multicellular ascomycetous fungal genomes contain more than 8000 genes. Fungal Genetics and Biology 21: 364–372. [DOI] [PubMed] [Google Scholar]
- Lane CR, Beales PA, Henricot B, Holden A (2006). First record of Cylindrocladium pauciramosum on Ceanothus in the UK. Plant Pathology 55: 582. [Google Scholar]
- Leahy RM, Schubert TS, El-Gholl NE (2000). Cylindrocladium gordoniae sp. nov. Mycotaxon 76: 77–83. [Google Scholar]
- Leslie JF, Klein KK (1996). Female fertility and mating type effects on effective population size and evolution in filamentous fungi. Genetics 144: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liolios K, Mavromatis K, Tavernarakis N, Kyrpides NC (2008). The Genome On Line Database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Research 36: D475–D479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde CC, Zhan J, McDonald BA (2002). Population structure of Mycosphaerella graminicola: from lesions to continents. Phytopathology 92: 946–955. [DOI] [PubMed] [Google Scholar]
- Lombard L, Rodas CA, Crous PW, Wingfield BD, Wingfield MJ (2009). Calonectria (Cylindrocladium) species associated with dying Pinus cuttings. Persoonia 23: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Zhou XD, Crous PW, Wingfield BD, Wingfield MJ (2010). Calonectria species associated with cutting rot of Eucalyptus. Persoonia 24: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey LM (1917). The crown canker disease of rose. Phytopathology 7: 408–417. [Google Scholar]
- Mayden RL (1997). A hierarchy of species concepts: the denouement in the saga of the species problem. In: Species: The units of Biodiversity (Claridge MF, Dawah HA, Wilson MR, eds). Chapman & Hall, U.K.: 381–424.
- McDonald BA (1997). The population genetics of fungi: tools and techniques. Phytopathology 87: 448–453. [DOI] [PubMed] [Google Scholar]
- McNeill J, Stuessy TF, Turland NJ, Hörandl E (2005). XVII International Botanical Congress: preliminary mail vote and report of Congress action on nomenclature proposals. Taxon 54: 1057–1064. [Google Scholar]
- Milholland RD (1974). Stem and root rot of blueberry caused by Calonectria crotalariae. Phytopathology 64: 831–834. [Google Scholar]
- Mohanan C, Sharma JK (1985). Cylindrocladium causing seedling diseases of Eucalyptus in Kerala, India. Transactions of the British Mycological Society 84: 538–539. [Google Scholar]
- Morgan AP (1892). Two new genera of hyphomycetes. Botanical Gazette 17: 190–192. [Google Scholar]
- Neubauer C, Zinkernagel V (1995). Calonectria morganii (Crous, Alfenas and Wingfield), the sexual stage of Cylindrocladium scoparium Morgan. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 102: 323–325. [Google Scholar]
- Nishijima WT, Aragaki M (1973). Pathogenicity and further characterization of Calonectria crotalariae causing collar rot of papaya. Phytopathology 63: 553–558. [Google Scholar]
- Old KM, Wingfield MJ, Yuan ZQ (2003). A manual of diseass of eucalypts in South – East Asia. Center for International Forestry Research, Jakarta, Indonesia. Pp. 98.
- Ooka JJ, Uchida JY (1982). Cylindrocladium root and crown rot of alfalfa in Hawaii. Plant Disease 66: 947–948. [Google Scholar]
- Overmeyer C, Lünneman S, Wallburnn C von, Meinhardt F (1996). Genetic variability among isolates and sexual offspring of the plant pathogenic fungus Calonectria morganii on the basis of random amplification of polymorphic DNA (RAPD) and restriction fragment length polymorphism (RFLP). Current Microbiology 33: 249–255. [DOI] [PubMed] [Google Scholar]
- Park RF, Keane PJ, Wingfield MJ, Crous PW (2000). Fungal diseases of eucalypt foliage. In: Diseases and pathogens of eucalypts. (Keane PJ, Kile GA, Podger FD, Brown BN, eds). CSIRO publishing, Australia: 153–239.
- Payne GA, Nierman WC, Wortman JR, Pritchard BL, Brown D, et al. (2006). Whole genome comparison of Aspergillus flavus and A. oryzae. Medical Mycology 44: S9–S11. [DOI] [PubMed] [Google Scholar]
- Peerally A (1973). Calonectria colhounii sp. nov., a common parasite of tea in Mauritius. Transactions of the British Mycological Society 61: 89–93. [Google Scholar]
- Peerally A (1991a). The classification and phytopathology of Cylindrocladium species. Mycotaxon 40: 323–366. [Google Scholar]
- Peerally A (1991b). Cylindrocladium hawksworthii sp. nov. pathogenic to water-lilies in Mauritius. Mycotaxon 40: 367–376. [Google Scholar]
- Pérez-Sierra A, Alvarez LA, Henricot B, Garcia-Jimenez J, Armengol J (2006). Cylindrocladium pauciramosum causes root and collar rot of Polygala myrtifolia in Spain. Plant Pathology 55: 298. [Google Scholar]
- Pérez-Sierra A, Alvarez LA, Leon M, Abad-Campos P, Armengol J, Garcia-Jimenez J (2007). First report of leaf spot, blight and stem lesions caused by Cylindrocladium pauciramosum on Callistemon in Spain. Plant Disease 91: 1057. [DOI] [PubMed] [Google Scholar]
- Phipps PM, Beute MK, Barker KR (1976). An elutriation method for quantitative isolation of Cylindrocladium crotalariae microsclerotia from peanut field soil. Phytopathology 66: 1255–1259. [Google Scholar]
- Polizzi G (2000). Prime esperience di lotta chimica nei confrontidel marciume del colletto e delle radici di Polygala myrtifolia causato da Cylindrocladium pauciramosum. Informatore Fitopatologico 11: 39–47. [Google Scholar]
- Polizzi G, Crous PW (1999). Root and collar of milkwort caused by Cylindrocladium pauciromosum, a new record for Europe. European Journal of Plant Pathology 105: 407–411. [Google Scholar]
- Polizzi G, Grasso FM, Vitale A, Aiello D (2007b). First occurrence of Calonectria leaf spot on mexican blue palm in Italy. Plant Disease 91: 1057. [DOI] [PubMed] [Google Scholar]
- Polizzi G, Vitale A, Aiello D, Castello I, Guarnaccia V, Parlavecchio G (2009). First record of crown and root rot caused by Cylindrocladium pauciramosum on brush cherry in Italy. Plant Disease 93: 547. [DOI] [PubMed] [Google Scholar]
- Polizzi G, Vitale A, Aiello D, Dimartino MA, Parlavecchio G (2007a). First report of damping-off and leaf spot caused by Cylindrocladium scoparium on different accessions of bottlebrush cuttings in Italy. Plant Disease 91: 769. [DOI] [PubMed] [Google Scholar]
- Polizzi G, Vitale A, Aiello D, Parlavecchio G (2006a). First record of crown and root rot caused by Cylindrocladium pauciramosum on California lilac in Italy. Plant Disease 90: 1459. [DOI] [PubMed] [Google Scholar]
- Polizzi G, Vitale A, Castello I, Groenewald JZ, Crous PW (2006b). Cylindrocladium leaf spot, blight and crown rot, new diseases of mastic tree seedlings caused by Cylindrocladium scoparium. Plant Disease 90: 1110. [DOI] [PubMed] [Google Scholar]
- Poltronieri LS, Silva JF da, Alfenas AC, Zauza EAV, Trindade DR (2004). Eugenia brachypoda, new host of Cylindrocladium pteridis in the State of Pará, Brazil. Fitopatologia Brasileira 29: 102–103. [Google Scholar]
- Porter DM, Wright FS, Taber RA, Smith DH (1991). Colonization of peanut seed by Cylindrocladium crotalariae. Phytopathology 81: 896–900. [Google Scholar]
- Prade RA (1998). Fungal genomics – one per week. Fungal Genetics and Biology 25: 76–78. [DOI] [PubMed] [Google Scholar]
- Risède J-M (1994). Partial characterization of Cylindrocladium sp., a root pathogen of banana in Martinique. Fruits (Paris) 49: 167–178. [Google Scholar]
- Risède J-M, Simoneau P (2001). Typing Cylindrocladium species by analysis of ribosomal DNA spacers polymorphism: application to field isolates from the banana rhizosphere. Mycologia 93: 494–504. [Google Scholar]
- Risède J-M, Simoneau P (2004). Pathogenic and genetic diversity of soilborne isolates of Cylindrocladium from banana cropping systems. European Journal of Plant Pathology 110: 139–154. [Google Scholar]
- Robert V, Stegehuis G, Stalpers J (2005). The MycoBank engine and related databases. http://www.mycobank.org
- Rodas CA, Lombard L, Gryzenhout M, Slippers B, Wingfield MJ (2005). Cylindrocladium blight of Eucalyptus grandis in Colombia. Australasian Plant Pathology 34: 134–149. [Google Scholar]
- Rogerson CT (1970). The Hypocrealean fungi (Ascomycetes, Hypocreales). Mycologia 62: 865–910. [PubMed] [Google Scholar]
- Rossman AY (1979a). Calonectria and its type species, C. daldiniana, a later synonym of C. pyrochroa. Mycotaxon 8: 321–328. [Google Scholar]
- Rossman AY (1979b). A preliminary account of the taxa described in Calonectria. Mycotaxon 8: 485–558. [Google Scholar]
- Rossman AY (1983). The phragmosporous species of Nectria and related genera. Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY (1993). Holomorphic hypocrealean fungi: Nectria sensu stricto and telemorphs of Fusarium. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. (Reynolds DR, Taylor JW, eds.). CAB International, Wallingford, U.K.: 149–160.
- Rossman AY (1996). Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 88: 1–19. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rowe RC, Beute MK (1975). Variability in virulence of Cylindrocladium crotalariae isolates on peanut. Phytopathology 65: 422–425. [Google Scholar]
- Rowe RC, Beute MK, Wells JC (1973). Cylindrocladium black rot of peanuts in North Carolina – 1972. Plant Disease Reporter 57: 387–389. [Google Scholar]
- Schoch CL, Crous PW (1999). First report of Cylindrocladium root and petiole rot on Spathiphyllum in South Africa. South African Journal of Botany 65: 67–72. [Google Scholar]
- Schoch CL, Crous PW, Polizzi G, Koike ST (2001a). Female fertility and single nucleotide polymorphism comparisons in Cylindrocladium pauciramosum. Plant Disease 85: 941–946. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ (1999). The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286–298. [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ (2001b). Phylogeny of Calonectria based on comparisons of β-tubulin DNA sequences. Mycological Research 105: 1045–1052. [Google Scholar]
- Schoch CL, Crous PW, Cronwright G, Witthuhn RC, El-Gholl NE, Wingfield BD (2000a). Recombination in Calonectria morganii and phylogeny with other heterothallic small-spored Calonectria species. Mycologia 92: 665–673. [Google Scholar]
- Schoch CL, Crous PW, Wingfield MJ, Wingfield BD (2000b). Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Studies in Mycology 45: 45–62. [Google Scholar]
- Schoulties CL, El-Gholl NE, Alfieri SA (1982). Cylindrocladium spathiphylli sp. nov. Mycotaxon 16: 265–272. [Google Scholar]
- Schubert TS, El-Gholl NE, Alfieri SA, Schoulties CL (1989). Calonectria avesiculata sp. nov. Canadian Journal of Botany 67: 2414–2419. [Google Scholar]
- Seifert KA, Gams W, Crous PW, Samuels GJ (2000). Molecules, morphology and classification: Towards monophyletic genera in the Ascomycetes. Studies in Mycology 45: 1–4. [Google Scholar]
- Sepiah M (1990). New storage disease of guava fruit caused by Cylindrocladium scoparium. Plant Disease 74: 253. [Google Scholar]
- Sharma JK, Mohanan C (1982). Cylindrocladium spp. associated with various diseases of Eucalyptus in Kerala. European Journal of Forest Pathology 12: 129–136. [Google Scholar]
- Sharma JK, Mohanan C, Maria Florence EJ (1984). Nursery diseases of Eucalyptus in Kerala. European Journal of Forest Pathology 14: 77–89. [Google Scholar]
- Sharma JK, Mohanan C, Maria Florence EJ (1985). Disease survey in nurseries and plantations of forest tree species grown in Kerala. Kerala Forest Research Institute, Research Report 36: 1–268. [Google Scholar]
- Sharma JK, Mohanan C, Rugimini P (1992). Cultural characters and growth of Cylindrocladium quinqueseptatum isolates. European Journal of Forest Pathology 22: 217–226. [Google Scholar]
- Sivapalan A, Metussin R, Hamdan F, Zain RM (1998). Fungi associated with postharvest fruit rots of Durio graveolens and D. kutejensis in Brunei Darussalam. Australasian Plant Pathology 27: 274–277. [Google Scholar]
- Sobers EK, Littrell RH (1974). Pathogenicity of three species of Cylindrocladium to select hosts. Plant Disease Reporter 58: 1017–1019. [Google Scholar]
- Sobers EK, Seymour CP (1967). Cylindrocladium floridanum sp. nov. associated with decline of peach trees in Florida. Phytopathology 57: 389–393. [Google Scholar]
- Stevens C, Palmer MA, McRoberts RE (1990). Use of aminopeptidase substrate specificities to identify species of Cylindrocladium in Wisconsin nurseries. Mycologia 82: 436–443. [Google Scholar]
- Taniguchi T, Tanaka C, Tamai S, Yamanaka N, Futai K (2008). Identification of Cylindrocladium sp. causing damping-off disease of Japanese black pine (Pinus thunbergii) and factor affecting the disease severity in a black locust (Robinia pseudoacacia)-dominated area. Journal of Forest Research 13: 233–240. [Google Scholar]
- Taylor JW, Jacobson DJ, Fisher MC (1999). The evolution of asexual fungi: Reproduction, speciation and classification. Annual Review of Phytopathology 37: 197–246. [DOI] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken SM, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Terashita T (1968). A new species of Calonectria and its conidial state. Transactions of the Mycological Society of Japan 8: 124–129. [Google Scholar]
- Terashita T, Itô K (1956). Some notes on Cylindrocladium scoparium in Japan. Bulletin of the Forestry Experiment Station Tokyo 87: 33–47. [Google Scholar]
- Tubaki K (1958). Studies on Japanese Hyphomycetes. 5. Leaf & stem group with a discussion of the classification of Hyphomycetes and their perfect stages. Journal of Hattori Botanical Laboratory 20: 142–144. [Google Scholar]
- Uchida JY, Kadooka CY (1997). Blight of leatherfern caused by Calonectria theae, and Cylindrocladium spp. Phytopathology 87: S98–S99. [Google Scholar]
- Vaidya P, Rao VG (1992). Three undescribed post-harvest diseases of fruits from Maharashtra. Journal of Economic and Taxonomic Botany 16: 241–244. [Google Scholar]
- Varon AF de (1991). Cylindrocladium scoparium associated with drying up and early death of soybean plants. Fitopatologia Colombiana 15: 2–7. [Google Scholar]
- Victor D, Crous PW, Janse BJH, Wingfield MJ (1997). Genetic variation in Cylindrocladium floridanum and other morphologically similar Cylindrocladium species. Systemic and Applied Microbiology 20: 268–285. [Google Scholar]
- Vitale A, Aiello D, Castello I, Dimartino MA, Parlavecchio G, Polizzi G (2009). Severe outbreak of crown rot and root rot caused by Cylindrocladium pauciramosum on strawberry tree in Italy. Plant Disease 93: 842. [DOI] [PubMed] [Google Scholar]
- Vitale A, Aiello D, Castello I, Parlavecchio G, Polizzi G (2008). First report of crown rot and root rot caused by Cylindrocladium pauciramosum on Feijoa (Feijoa settowiana) in Italy. Plant Disease 92: 1590. [DOI] [PubMed] [Google Scholar]
- Vitale A, Polizzi G (2007). First record of the perfect stage Calonectria pauciramosa on mastic tree in Italy. Plant Disease 91: 328. [DOI] [PubMed] [Google Scholar]
- Vitale A, Polizzi G (2008). First record of leaf spots and stem lesions on Pistacia lentiscus caused by Cylindrocladium pauciramosum and C. scoparium in Italy. Plant Pathology 57: 384. [Google Scholar]
- Wiapara NW, Di Menna ME, Cole ALJ, Skipp RA (1996). Pathogenicity of Cylindrocladium scoparium to pasture clover and grass species. Australasian Plant Pathology 25: 205–211. [Google Scholar]
- Wolf FA (1926). Brown leaf spot of leather fern. Journal of the Elisha Mitchell Scientific Society 42: 55–62. [Google Scholar]
- Wormald H (1944). A Cylindrocladium as the cause of a shoot wilt of varieties of plum and cherry used for rootstocks. Transactions of the British Mycological Society 27: 71–80. [Google Scholar]
- Wright LP, Wingfield BD, Crous PW, Brenneman T, Wingfield MJ (2006). Isolation and characterization of microsatellite loci in Cylindrocladium parasiticum. Molecular Ecology Notes 6: 110–112. [Google Scholar]
- Wright LP, Wingfield BD, Crous PW, Wingfield MJ (2007). Isolation and characterization of microsatellite loci in Cylindrocladium pauciramosum. Molecular Ecology Notes 7: 343–345. [Google Scholar]
- Yoder OC, Turgeon BG (2001). Fungal genomics and pathogenicity. Current Opinion in Plant Biology 4: 315–321. [DOI] [PubMed] [Google Scholar]