Abstract
Background
In order to maintain genome information accurately and relevantly, original genome annotations need to be updated and evaluated regularly. Manual reannotation of genomes is important as it can significantly reduce the propagation of errors and consequently diminishes the time spent on mistaken research. For this reason, after five years from the initial submission of the Entamoeba histolytica draft genome publication, we have re-examined the original 23 Mb assembly and the annotation of the predicted genes.
Principal Findings
The evaluation of the genomic sequence led to the identification of more than one hundred artifactual tandem duplications that were eliminated by re-assembling the genome. The reannotation was done using a combination of manual and automated genome analysis. The new 20 Mb assembly contains 1,496 scaffolds and 8,201 predicted genes, of which 60% are identical to the initial annotation and the remaining 40% underwent structural changes. Functional classification of 60% of the genes was modified based on recent sequence comparisons and new experimental data. We have assigned putative function to 3,788 proteins (46% of the predicted proteome) based on the annotation of predicted gene families, and have identified 58 protein families of five or more members that share no homology with known proteins and thus could be entamoeba specific. Genome analysis also revealed new features such as the presence of segmental duplications of up to 16 kb flanked by inverted repeats, and the tight association of some gene families with transposable elements.
Significance
This new genome annotation and analysis represents a more refined and accurate blueprint of the pathogen genome, and provides an upgraded tool as reference for the study of many important aspects of E. histolytica biology, such as genome evolution and pathogenesis.
Author Summary
Entamoeba histolytica is an anaerobic parasitic protozoan that causes amoebic dysentery. The parasites colonize the large intestine, but under some circumstances may invade the intestinal mucosa, enter the bloodstream and lead to the formation of abscesses such amoebic liver abscesses. The draft genome of E. histolytica, published in 2005, provided the scientific community with the first comprehensive view of the gene set for this parasite and important tools for elucidating the genetic basis of Entamoeba pathogenicity. Because complete genetic knowledge is critical for drug discovery and potential vaccine development for amoebiases, we have re-examined the original draft genome for E. histolytica. We have corrected the sequence assembly, improved the gene predictions and refreshed the functional gene assignments. As a result, this effort has led to a more accurate gene annotation, and the discovery of novel features, such as the presence of genome segmental duplications and the close association of some gene families with transposable elements. We believe that continuing efforts to improve genomic data will undoubtedly help to identify and characterize potential targets for amoebiasis control, as well as to contribute to a better understanding of genome evolution and pathogenesis for this parasite.
Introduction
Although many infectious diseases receive little attention in today's world, the pathogenic intestinal parasite E. histolytica occupies a major place in the list of ignored illnesses. The parasite is the causative agent of amoebiasis, causes a significant level of morbidity and mortality in developing countries, and affects at least 50 million people every year, causing over 100,000 deaths [1]. Yet, a lot is there to be learned about this important protozoan. Genome information allows for better understanding of pathogenic processes and consequently helps improve the prevention, diagnosis, and treatment of the disease. Therefore, accurate and up to date data is fundamental to generate a reliable tool for both research and medical use. The E. histolytica genome was automatically annotated and published in 2005 [2]. This draft genome provided the scientific community with the first blueprint of this pathogen, its gene organization and content. However, genome annotation was performed in an automated way, leading to a very raw dataset to work with. Here, in an effort to improve the structural and functional annotation for this organism, we have reviewed, re-assembled and re-annotated the E. histolytica genome. The ultimate goal was to generate a high-quality annotation dataset to be used as gold standard by the scientific community and to carry on comparative analysis with the closely related species Entamoeba dispar and Entamoeba invadens. Using a combination of manual and automated methods we significantly improved the E. histolytica assembly. In addition, we generated a new training set of genes for training gene finders, created new gene models and reevaluated and refined previous gene structures based on up to date information, reassessed gene functions, and mapped transposable elements to remove overlapping predicted genes. Here we present an overview of the methods employed for this task and protocols followed, summarizing the contents of the latest data release, with special emphasis on our final assembly and annotation release.
Methods
Genome reassembly
Reads were obtained directly from the Sanger Institute and JCVI databases. Reads were filtered based on similarity to an E. histolytica plasmid sequence [3] or to tRNA models [4]. Reads were assembled with UMD Overlapper [5] and Celera Assembler [6]. See Text S1 for assembly details. The re-assembled sequence was deposited at the National Center for Biotechnology Information (NCBI) with the accession number AAFB02000000.
New gene predictions and improvement of gene structures
A set of 20,192 ESTs and 71 full-length cDNAs were downloaded form GenBank. ESTs were assembled and aligned to the newly assembled genome using PASA [7]. A training set consisting of 300 genes supported by 60 full length cDNAs and 240 assembled ESTs was created to train the following gene finders: Genezilla [8], and GlimmerHMM [8]. EVidenceModeler (EVM) [9] was used to generate the new gene dataset, as a weighted consensus of all available evidence, including proteins and conserved protein-domains alignments, cDNAs/ESTs and gene finder output predictions. The new datas[6]et was manually inspected in areas covered by transposable elements (see below). Coding regions shorter than 300 bp supported by no evidence other than Gene Finders were eliminated from the gene dataset. To generate more accurate gene structures in our new dataset, we focused on structural reannotation by improving the accuracy of existing gene models, validating intron/exon boundaries, incorporation of UTRs when available (using PASA), identifying pseudogenes and eliminating spurious genes.
Repeat finding
First, we created a comprehensive custom database containing all reported E. histolytica repetitive elements: LINEs, SINEs, EhERE1 and EhERE2 [10]. Then, we ran RepeatMasker (http://www.repeatmasker.org/) on the current assembly to map and quantify the elements. Regions of the genome that match the repeats were masked to avoid gene prediction on these regions. Any gene predicted on masked regions was removed from the annotation.
Comparison between original gene annotation (OGA) and new gene annotation (NGA) set
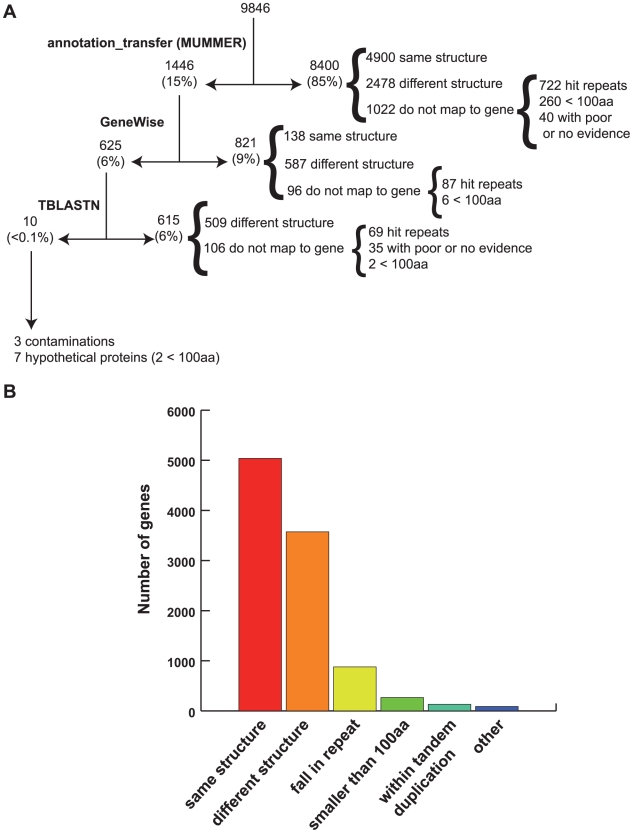
Predicted gene models from the previous assembly were mapped to the new assembly using a combination of methods (Fig. 1). First we identified the correspondence between the scaffolds in the first assembly and the new assembly. Once this correspondence was identified, gene models from the old annotation were mapped onto the new assembly in a multistep fashion. During the first mapping iteration performed with an in-house tool, annotation_transfer, based on the software Mummer [11], not all models were transferred as expected due to small sequence variation resulting from a new, independent assembly. In a second mapping round, unmapped genes were aligned to the new assembly using GeneWise [12] an algorithm that combines protein alignment and gene prediction into a single statistical model as a paired Hidden Markov Model (HMM) and provides a gene prediction based on protein homology. Then, genes that failed to map by the previous methods were positioned on the new assembly by tblastn, using a coverage of at least 80% identity, 80% coverage, and an e-value <1×10−20. Finally, structural changes between OGA and NGA predictions was assessed using GSAC (Gene Structure Annotation Comparison, unpublished), a JCVI in-house tool that evaluates coordinate differences between two gff3 (generic feature format version 3) files (http://www.sequenceontology.org/gff3.shtml).
Figure 1. Re-mapping strategy to transfer old annotation .
A) Steps followed to achieve the full mapping of OGA (9,846 gene models) into the new E. histolytica assembly, resulting in NGA (8,201 gene models). B) Mapping of the OGA gene models fell into different categories: genes with perfect map to new assembly (same structure), genes that map to a location but have to be modified (different structure), genes that mapped to a repeat (discarded), genes smaller than 100 amino acids (discarded if they had no evidence), genes that fell within tandem duplications (discarded), and other smaller categories (pseudogenes, truncated genes).
Evaluation of annotation improvement
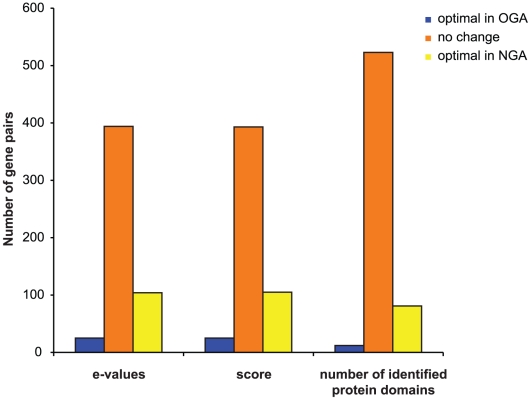
To evaluate the structural improvement of gene models in the new annotation we selected a dataset of 1024 pairs of genes. Each pair was composed of an OGA and a NGA gene that only map to each other (i.e. they represent the same gene in each annotation) but are structurally different. This dataset was used to perform two types of analyses. First we ran HMM-searches on each pair against the Pfam HMM database and then, we evaluated NGA HMM-searches statistic (e-value, score or number of a particular Pfam domain) compared to their OGA counterparts.
In addition, we performed local blastp searches against our internal non-redundant protein database, PANDA.db (ftp.jcvi.org/pub/data/panda) and identified pairs that shared the same top-hit to run stretcher, a global pairwise alignment tool (bioweb2.pasteur.fr/docs/EMBOSS/stretcher.html), between each gene and its corresponding top-hit. Pairs having hits with percent identity below 30% were removed from the results to eliminate false positive hits and results for each pair were analyzed according to their alignment statistics (score, percent identity, percent similarity and percent of gaps) to determine the level of improvement between the annotations. For measuring functional annotation improvement, we estimated the number of genes in the NGA that acquired a descriptive name or an improved name with respect to the OGA only for those genes that did not undergo structural changes to discard functional improvements associated with drastic structural changes, such as incorporation of new exons and changes in coding frame.
Functional annotation assignments
Gene level searches were performed against protein, domain and profile databases including JCVI in-house non-redundant protein database Panda-AllGroup.niaa, Pfam [13] and TIGRfam [14] HMMs, Prosite [15], and InterPro [16]. In addition, programs to predict membrane localization such as SignalP [17], TMHMM and TargetP [17] were run. After the working gene set had been assigned function, predicted proteins were organized into protein families as previously described [7] with the purpose of refining the annotation in the context of related genes in the genome. Predicted genes were assigned informative names and classified using Gene Ontology (GO) [18]. GO assignments were attributed automatically, based on other assignments from closely related organisms using Pfam2GO, a tool that allows automatic mapping of Pfam hits to GO assignments as well as manually by expert annotators. All assignments were reviewed manually for consistency, on a family based approach, via Manatee, a web-based genome annotation tool that can view, modify, and store annotation for prokaryotic and eukaryotic genomes. Names between OGA and NGA were compared by simple query for corresponding genes to determine the level of change and improvement. Annotation of transporter proteins was performed using TransportDB (http://www.membranetransport.org/) [19].
Identification of genome duplications
Segmental genome duplications along the E. histolytica genome were identified with DAGchainner [20], a program that looks for chains of syntenic genes within complete genome sequences, using default parameters. Briefly, we performed all-vs-all blastp searches using the E. histolytica proteome. The blastp output was then filtered out to remove repetitive matches that could potentially contribute noise to the data. Finally, all segmental genome duplications containing five or more duplicated set of genes were further analyzed.
Results and Discussion
Characteristic of the new annotation: Improvements to the genome assembly, gene structures and functional assignments
Close examination of the initial assembly of E. histolytica strain HM-1:IMSS revealed multiple problems. Sequence analysis using intra-scaffold dot-plots exposed 161 artifactual tandem duplications (Figure S1, panel A) located at the boundaries between neighboring contigs (a contiguous assembled sequence ordered together to form a scaffold). Tandem duplications spanned 364,707 bp of genomic sequence with a median length of 892 bp. In the previous assembly, genes predicted on these regions and on unmasked repetitive regions caused an over-estimation of genes by approximately 18%. Indeed, of the 399 genes located in those regions, 61 hit transposable elements (TEs) or were likely pseudogenes, while most of the remaining 338 coding sequences were artifactually duplicated and so collapsed into 206 individual genes in the new annotation (Figure S1, panel B). A comparative description of the features of the original and the new E. histolytica assemblies is summarized in Table 1A.
Table 1. Genome statistics and annotation comparison.
| A | ||
| Genome | New E. histolytica assembly | Old E. histolytica assembly |
| Size (bp) | 20799072 | 23361983 |
| GC Content (%) | 24.2 | 24.1 |
| Number of Genes | 8201 | 9985 |
| Mean Gene Length (bp) | 1260.9 | 1170.7 |
| Number of Genes/10 Kbp | 3.9 | 4.3 |
| Longest Gene (bp) | 15,210 | 15,210 |
| Shortest Gene (bp) | 147 | 96 |
| Percent Coding (%) | 49.7 | 50 |
| Percent Genes with Introns (%) | 24.4 | 24.9 |
| Exons | New E. histolytica assembly | Old E. histolytica assembly |
| Number | 10,754 | 13,176 |
| Mean number per Gene | 1.3 | 1.3 |
| GC Content (%) | 28 | 28.1 |
| Mean Length (bp) | 962 | 886.1 |
| Total Length (bp) | 10,340,284 | 11,675,669 |
| Introns | New E. histolytica assembly | Old E. histolytica assembly |
| Number | 2,553 | 3191 |
| GC Content (%) | 19.3 | 21.7 |
| Mean Length (bp) | 74.1 | 100 |
| Total Length (bp) | 189,260 | 319,223 |
| Intergenic Regions | New E. histolytica assembly | Old E. histolytica assembly |
| GC Content (%) | 20.5 | 20 |
| Mean Length (bp) | 708.7 | 823.5 |
A) Comparative genome statistics between the old and current E. histolytica genome assemblies. B) Comparative view of EC number, GO terms and domain identification between the old and the new E. histolytica annotations. OGA: original genome annotation, NGA: new genome annotation.
*functional annotation was manually reviewed.
The new genome assembly consists of ∼20 Mb of sequence organized into 1,496 scaffolds. To generate a “core” assembly for functional annotation, scaffolds lacking predicted genes were not considered. The resulting core assembly consisted of 818 non-redundant scaffolds with a total of 19,220,345 bp. All scaffolds that were excluded from the core assembly as well as degenerate contigs and singleton reads, although not annotated, were considered to survey the presence or absence of genes when necessary, and all sequences were deposited in GenBank (see Methods).
The results of the new assembly show higher fragmentation and a reduction in genome size with respect of the published assembly. However, our comparative analysis between the two annotations shows that there is no loss of coding information from one assembly to the other.
The new assembly contains 8,201 de novo predicted protein coding genes, 1,784 fewer than previously reported for this genome (Table 1) [2]. To determine the origin of these differences and to evaluate changes in gene structure between the original (OGA) and new (NGA) annotation, genes from OGA were mapped onto the new assembly and structural differences were estimated using GSAC (see Methods and Fig. 1A). Mapping results indicated that the main reason for gene number reduction is the elimination of genes within repetitive regions and artifactual tandem duplications, and the removal of genes smaller than 300 bp without any supporting evidence (Fig. 1B). Noteworthy, less than 0.2% of the genes from the original annotation do not map onto the new assembly, despite the fact that the assembly is 2,562,911 bp smaller than the published one. These missing OGA genes contained no supporting evidence and were originally annotated as hypothetical protein coding genes. This analysis also showed that 51% of the OGA genes keep the same structure in the new annotation (same isoform in Fig. 1B), while 36% underwent structural change (different isoform in Fig. 1B).
As part of the curation process, the structure of 740 genes was manually reviewed and curated based on supporting evidence such as ESTs. An important hallmark of this work is the concerted effort from scientists of the Entamoeba community that contributed to the curation of the genome by direct communication with the authors as well as participation via specific workshops held at JCVI.
To evaluate whether structural changes in the new annotation reflect an overall improvement of gene structures we selected a group of 1,024 OGA-NGA pairs of genes that map to each other but are structurally different. Then, we ran HMM-searches and global pairwise alignments on each pair of proteins against Pfam HMMs and our PANDA database (see Methods). Finally, we compared the resulting statistics between OGA and NGA peptides from each pair (Fig. 2). These analyses showed that translated products from NGA genes consistently give better hits against Pfam and PANDA databases when compared to OGA genes, demonstrating an overall improvement in gene structures for the new annotation. In those cases where NGA genes gave worse hits compared to their OGA counterparts, we manually inspected and corrected gene structures in the new annotation.
Figure 2. Structural annotation improvement in the new E. histolytica assembly.
Comparative analysis of Pfam HMM searches statistics between equivalent genes in the old and new annotation. Blue bars, genes that have better statistics/hits in the old annotation compared to the new annotation; orange bars, old and new annotation genes give exactly the same result; yellow bars, number of genes from the new annotation with better statistics/hits compared to their counterparts in the old annotation.
Structural improvements in the new annotation were also reflected by (1) the appearance of new Pfam/TIGRfam domain hits not present in the original protein dataset and (2) the identification of genes coding for additional members of different protein families. Noteworthy, among novel protein domains are a domain typically found in some subunits of several DNA polymerases (PF04042), a domain found in phospholipid methyltransferases (PF04191) and another present in panthotenate kinase proteins (PF03630, see section below). On the other hand, point (2) is very well exemplified by the subunits of the Gal/GalNAc lectins. In E. histolytica these lectins are composed of three different subunits: a 170 kDa heavy subunit (Hgl), a 150 kDa intermediate subunit (Igl) and a 31–35 kDa light subunit (Lgl) [21], [22]. In agreement with the current number of Hgl and Lgl genes in the new annotation, studies of pulse-field gel electrophoresis have shown that there are five hgl and six lgl genes in the genome [22]. However, only four Hgl genes, one of them truncated, and four Lgl genes are part of the old dataset.
Particular effort was directed towards the improvement of functional annotation (summarized in Table 1B) by the incorporation of additional 974 enzyme commission (EC) numbers and 531 Pfam/TIGRfam domains. Gene ontology (GO) terms were automatically assigned from Pfam HMM searches refreshing and updating the assignments from InterPro evidence used in the old annotation. The advantage of using hits from Pfam HMM searches is that results can then be filtered not just by e-value but also by trusted cutoff scores, giving a more accurate estimation than InterPro searches and therefore, a more confident GO assignment. In addition to automatic EC number and GO term assignments, functional annotation has been manually curated for 2,130 genes. A total of 3,468 genes have been assigned GO terms, of which 3,216 have a molecular function term. We have distributed the specific terms in a total of 30 molecular function GO-Slim categories (Table S1). No difference was observed in the representation of GO categories in the protein families with respect to that of singletons.
Classification and function of protein families in Entamoeba histolytica
E. histolytica predicted proteins were organized into protein families to facilitate the review of their functional annotation, visualizing relationships between proteins and allowing annotators to examine related genes as a group. Our family clustering method produces groups of proteins sharing protein domains conserved across the proteome, and consequently, related biochemical function, as described in Methods [23], [24]. For example, based on our clustering criteria, all proteins containing a single RhoGAP domain (PF00620) fall within the same family irrespectively of their length.
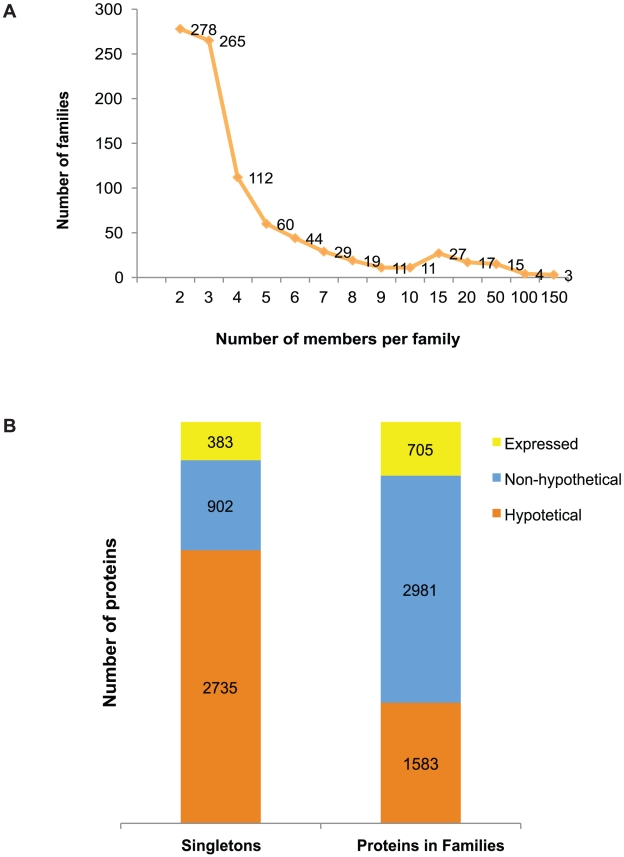
A total of 897 protein families containing 4,564 proteins (56% of the proteome) were identified from the 8,201 predicted polypeptides in the new annotation, leaving 3,637 “orphan” proteins. Among the families, 247 clusters (479 proteins) have no homology to any known Pfam or TIGRfam domain, and harbor potentially novel domains (91 of these families contain five members or more). On average, E. histolytica families contain five proteins, ranging from two to 149 members (Fig. 3A). We identified seven families with more than 50 members encoding proteins such as small GTP binding proteins, BspA-like leucine-rich repeat proteins, kinase domain-containing proteins, WD domain-containing proteins, a large family of uncharacterized hypothetical proteins, a RNA recognition motif domain-containing protein family and a RhoGAP domain-containing protein family (see Table S2 for the complete list of families).
Figure 3. E. histolytica protein families.
A) Size distribution of protein families. B) Functional assignments in Singletons (proteins not assigned to families) versus Proteins within Families. Hypothetical: predicted hypothetical proteins; Non-hypothetical: predicted proteins with functional assignments; Expressed: predicted proteins with EST (expressed sequence tag) support.
Interestingly, a number of protein families appear to be physically linked to transposable elements. Table 2 shows the top 27 families that present this type of association (for the entire repertoire of genes see Table S3). For example, a cluster of 31 members of the Hsp70 protein family appears associated 30% of the time with TEs within 1 kb of the gene context. Hsp70 proteins are molecular chaperones that assist a large variety of protein folding processes in the cell by the transient association between their substrate-binding domain and the short hydrophobic peptide segments present in their target proteins. Hsp70 s are highly conserved and are known to be induced by a variety of stresses [25]. It has been previously reported that multiple natural TE insertions in Drosophila reduce the level of expression of hsp70 genes by insertion nearby gene promoter regions [26]. The characteristics of the hsp70 promoter in the fly may make it a suitable target for transposition leading to the generation of novel alleles. In this sense, TEs could be playing an adaptive role in microevolution by gene amplification and also manipulating the expression of genes critical for the parasite fitness [27].
Table 2. Entamoeba histolytica protein families showing high association with repetitive elements.
| Family ID* | Protein family name | Number of associated elements | Number of genes in Family | Percentage of Association |
| 238 | hypothetical protein | 5 | 5 | 100% |
| 133 | hypothetical protein | 7 | 7 | 100% |
| 64 | hypothetical protein | 10 | 10 | 100% |
| 145 | hypothetical protein | 5 | 6 | 83% |
| 52 | hypothetical protein | 4 | 5 | 80% |
| 236 | cystein protease family | 4 | 5 | 80% |
| 66 | hypothetical protein | 6 | 8 | 75% |
| 42 | hypothetical protein, conserved | 11 | 15 | 73% |
| 157 | Gal/Gal/Nac lectin complex family | 4 | 6 | 66% |
| 87/29/274 | AIG1 family protein | 18 | 29 | 62% |
| 77 | regulator of nonsense transcripts family | 6 | 10 | 60% |
| 93 | hypothetical protein | 5 | 9 | 55% |
| 111 | hypothetical protein | 4 | 8 | 50% |
| 15 | hypothetical protein | 12 | 29 | 41% |
| 67 | hypothetical protein | 4 | 11 | 36% |
| 2 | BspA-like family protein | 41 | 114 | 35% |
| 12 | HSP 70 family | 11 | 31 | 35% |
| 63 | peroxiredoxin family protein | 4 | 12 | 33% |
| 54 | hypothetical protein | 4 | 13 | 30% |
| 41 | cystein protease family | 4 | 14 | 28% |
| 32 | DEAD/DEAH-box helicase family protein | 5 | 18 | 27% |
| 9 | kinase family protein | 9 | 39 | 23% |
| 19 | zinc-finger domain containing protein | 6 | 26 | 23% |
| 8 | hypothetical protein | 9 | 38 | 23% |
| 5 | hypothetical protein, conserved | 13 | 61 | 21% |
| 24 | kinase family protein | 4 | 20 | 20% |
| 13 | LRR repeat containing protein | 5 | 29 | 17% |
*Only families with at least five proteins and showing more than 15% association are shown.
Another family showing a high correlation with transposable elements is the large BspA-like surface protein family [28], [29]. Initially, Davis et al. identified 89 genes coding for BspA-like proteins in the genome of E. histolytica, containing a leucine-rich repeat motif (LRRs). LRRs serve as recognition motifs for surface proteins in bacteria and other eukaryotes [30] and have been shown to be involved in binding to fibronectin. E. histolytica BspA-like proteins have unique LRR-like repeats that resemble, to certain extent, to the Treponema pallidum LLRs (LrrA proteins) [28], that appear to have a role in attachment and penetration to host tissues [31], suggesting they may be involved in attachment to the host cells. Our analysis identified 116 BspA-like genes in the genome, 41 of them associated with transposable elements. The core domain of the BspA-like proteins contains 23 amino acids with the consensus P[T/S][T/S][V/I/L]xx[I/L]GxxCFxxCxxLxx[I/L]x[I/L], and these units form tandem blocks that can contain two or more core motifs represented from 1 to 21 times in a single molecule, leading to a great variability in the protein length in the family. Most of the proteins in the family contain a novel 50 amino acids N-terminal domain that is preserved in 85 members of this cluster. A closer examination of those genes encoding proteins lacking the N-terminal domain showed they are probably truncated by the insertion of transposable elements, primarily SINE and LINE elements at their 5′ end. BspA-like proteins are located on the surface of E. histolytica [28] however no classic membrane-targeting signal is present in the proteins. Therefore, it is tempting to speculate that the conserved N-terminal domain of these proteins might function as either an export signal or serve as a membrane-anchor domain or that export involves a non-classical transport mechanism, independent of the ER–Golgi pathway, similar to those that have been detected in yeast and mammalian cells [32]. Details on the motifs and domain structure are shown in Figure S2.
A third worthy of note family associated with TEs is the AIG family of proteins, comprising 29 members distributed in 3 clusters, of which 18 genes are in close proximity to repetitive elements (Table 2). AIG1 proteins are associated with resistance to bacteria [33]. Interestingly, comparative gene expression studies have shown that AIG1 proteins as well as heat shock proteins have significantly reduced expression levels in E. dispar [34], when compared to E. histolytica. This observation leads us to speculate that transposable elements inserted in the neighborhood of these genes could lead to the enhanced expression of these genes and ultimately could be related to the increased virulence. Indeed we have previously shown that LINEs and SINEs are involved in genome rearrangements driving in consequence genomic evolution [10]. It is tempting to speculate that the amplification of the AIG family was mediated by the close association of TEs, but the observation that non-virulent E. dispar contains the same number of genes without the TE association seems to indicate that this is not the case. We are currently analyzing all gene family/transposable element associations in the context of comparative genomics with other Entamoeba species (manuscript in preparation).
Close examination of the functional annotation of protein families and singleton proteins revealed that a total of 2,981 (65%) genes within the families were annotated as encoding proteins with putative functions and 1,583 genes are hypothetical proteins (34%, Fig. 3B). Of a total of 1,088 genes that have EST support in the whole genome, 705 are genes within protein families. In contrast, singletons had a larger proportion of hypothetical genes (76%) and a smaller portion of genes with a known or putative function (24%), and half the number of genes supported by EST evidence (383).
Segmental genome duplications
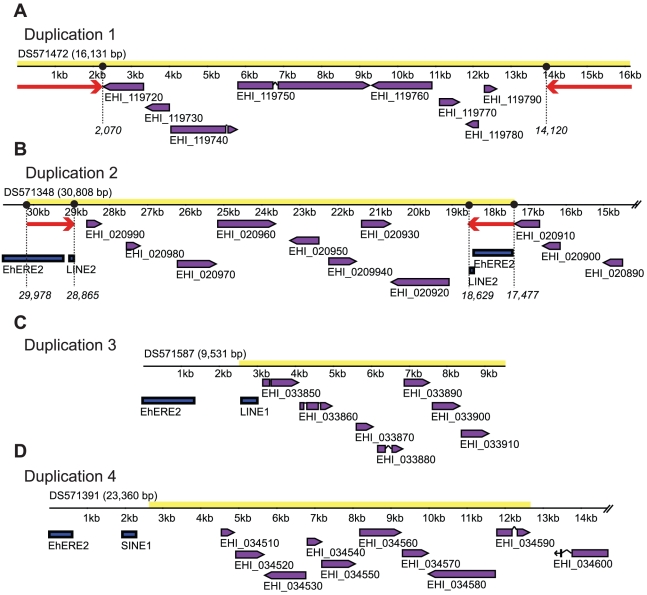
As mentioned above, about 20% of the E. histolytica genome consists of transposable elements. These repeats show a tendency to insert close to each other forming large TE clusters. We have previously shown that these repeat clusters are frequently found at syntenic breakpoints between E. histolytica and E. dispar suggesting that they could contribute to parasite genome instability and, consequently, to the evolution of these species [10]. It is also possible that the highly repetitive nature of this genome led to genome duplications. In order to evaluate this possibility we analyzed the presence of additional rearrangements within the genome by searching for segmental duplications using DAGchainer as explained in Methods [20]. We observed the presence of four different types of segmental duplications, named D1-D4, spanning seven to ten genes each (Fig. 4).
Figure 4. Entamoeba histolytica segmental genome duplications.
A) D1-type duplications flanked by unique 2.3 kb inverted repeats (IR), B) D2-type duplications flanked by EhERE1/EhLINE2- derived 1.2 kb IRs, C) D3-type duplications usually associated to EhLINE1, but lacking IRs, and D) D4-type duplications present in the vicinity of TE elements, and lacking IRs. Inverted red arrows: IRs; purple boxes: open reading frames; blue boxes: repetitive elements; DS identifiers correspond to GenBank accession numbers for the corresponding scaffolds.
The first duplication (D1, Fig. 4A) spans a 16.6 kb region containing up to 8 hypothetical protein coding genes. These duplications are approximately 94% identical at the nucleotide level. All D1-type duplications are flanked by 2.3 kb inverted repeats (IR) not found in the rest of the genome. Nucleotide composition analysis revealed that D1-IRs are highly AT-rich (84.3%) compared to the average content of those regions 71.4% and they are 95% identical at the nucleotide level. A genome wide survey of D1-duplications led to the identification of four complete and two partial copies of this element in the genome. It is interesting to mention that all the scaffolds containing the four complete duplications have similar sizes (16.6 kb on average) and are spanned almost in their entire length by their respective segmental duplications. The two partial D1-duplications are located in shorter scaffolds of 14.4 kb and 6.6 kb, respectively.
The second duplication (D2, Fig. 4B) is 12.5 kb long and contains up to eight duplicated hypothetical protein coding genes depending on the duplication. Comparative analysis showed that these duplications are more than 80% identical at the nucleotide level with an average of 92%. Similar to D1-type duplications, D2 are frequently flanked by 1.2 kb IRs, composed of two fragments derived from the TEs EhERE1 and EhLINE2. D2-IRs share 92.6% identity at the nucleotide level and are also very AT-rich (85%AT). The organization of the duplications is not conserved in all copies across the genome, with some copies flanked by IRs composed of either EhERE1 or EhLINE2 fragments, while in others we could not identify any IR.
D3-type duplications are 7.4 kb long and 83% identical at the nucleotide level. Although frequently found nearby TEs (mostly EhLINE1), none of the eight identified genome duplications are flanked by IRs as D1- and D2-type duplications. D3 presents a very unique gene content that suggest that the segment could present a unique functionality, represented in Fig. 4C. A total of seven protein coding genes are arranged in the same orientation, and include a putative serine-threonine kinase similar to ARK1, a human protein that participates in cell cycle regulation; an endonuclease V domain-containing protein coding gene potentially involved in DNA repair; a putative secreted hypothetical protein coding gene; a tandem duplicated gene coding for a putative protein containing a type-1 glutamine amido transferase-like domain and a GDSL-like lipase/acylhydrolase domain-containing protein coding gene. Interestingly, D3-type duplications are found at or in close proximity to the end of scaffolds, and therefore, they could potentially be located at subtelomeric regions. However, in spite of a thorough analysis we could not identify any repetitive telomeric/subtelomeric motif in these regions.
Lastly, the 10 kb long D4 (Fig. 4D) shares more than 85% identity at the nucleotide level and spans up to 9 hypothetical protein coding and one putative dUTP hydrolase-coding genes. Most D4-type duplications have TEs inserted nearby, but no flanking IRs were identified.
The presence of these duplications is not likely to be an artifact of the assembly due to the fact that they are also appear duplicated in E. dispar. It is possible that some of these duplications, that in some cases span full scaffolds represent different copies of one of the several extrachromosomal elements known to exist in Entamoeba species, as described by Dhar et al [35].
New features
Our work has led to the identification of 460 novel putative protein coding genes not present in the OGA, 16% of which have some functional annotation. One of these genes codes for a putative pantothenate kinase (EHI_183060) the first enzyme in the biosynthesis of coenzyme A from pantothenate. Although the coding genomic region was present in the original assembly, the gene had not been predicted and therefore, it was missing from the previous annotation. Only the enzymes phophopantothenoyl-cysteine decarboxilase (EC 4.1.1.36), phosphopantothenoyl-cysteine synthase (EC 6.3.2.5), and dephospho-CoA kinase (EC 2.7.1.24), responsible for the second, third and last of the five consecutive enzymatic reactions, had been previously identified in the OGA (EHI_164490, EHI_092330, EHI_040840). However, the lack of candidate enzymes for the remaining two biochemical reactions of this pathway raised the question whether E. histolytica can synthesize coenzyme A from pantothenate [36]. Our de novo gene prediction for a putative pantothenate kinase plus the identification of a candidate gene for the forth step of this pathway, a putative pantetheine-phosphate adenylyltransferase (EC 2.7.7.3), indicates that the whole set of metabolic reactions required to synthesize coenzyme A from pantothenate is present in this amoeba. Interestingly, the enzymes that participate in this pathway resemble those from eubacteria but not higher eukaryotes. Indeed, the second and third sets of reactions are catalyzed by a single enzyme present in two copies (EHI_164490, EHI_092330), while the fourth and fifth steps are carried out by independent enzymes, EHI_006680 and EHI_040840, respectively. In higher eukaryotes the last two reactions are carried out by the same enzyme [37].
Another gene not present in the OGA (EHI_141410) codes for a protein with a predicted molecular weight of 44.6 kDa similar to subunit p50 of the DNA polymerase delta, a key enzyme for chromosomal DNA replication in higher eukaryotes. In mammals, it has been shown that p50 is tightly associated with p125, the catalytic subunit of these types of DNA polymerases. Accordingly, a gene coding for a putative 124.4 kDa catalytic subunit of the DNA polymerase delta (EHI_006690), is also present in the NGA. These results are in agreement with a previous work showing that the sensitivity to different inhibitors of the DNA polymerase activity of E. histolytica resembles that of mammalian DNA alpha, delta and epsilon polymerases [38].
In addition, a gene coding for a protein containing a Yos1-like Pfam domain is also absent from OGA (EHI_178640). This putative protein has similarity to other members of the Yos1 family, involved in protein transport between the endoplasmic reticulum and the Golgi apparatus [39].
Comparative analysis between the two annotation datasets also allowed us to identify genes present in their complete form in NGA but truncated in OGA. Example of these genes are two copies of a gene coding for a putative pyridine nucleotide transhydrogenase, EHI_055400 and EHI_014030, the latter identical to a gene previously cloned by Clark et al. [40], which exists as a single truncated copy in the OGA. Another example is a 605 bp gene coding for a putative phospholipid methyltransferase protein (EHI_153710) similar to Schizosaccharomyces pombe cho1 (35% identity; e-value = 4×10−21), an enzyme that participates in the synthesis of phosphatidylcholine via the methylation of phosphatidylethanolamine. A coding sequence containing only the last 222 bp of this gene is present in the OGA.
Final remarks
Our reannotation effort has focused mostly on the improvement of the assembly and the gene content and structure of the E. histolytica genome. The new assembly, annotation and analysis of the genome has incorporated many updates and enhancements to the structural and functional assignments of the original gene predictions, including an improved assembly, removal of spurious genes, improved gene structures and functional assignments, and generation of gene families.
Regardless of the advancement of the computational methods and of the exponentially growing amount of data that could be used for automated genome annotation, only experimental evidence from expression data will conclusively validate the accuracy of computationally assigned functions done at the genome-wide level. Nevertheless, in order to provide a sound bases to drive research, genome annotations have to be maintained and revised, either by expert annotators in the field and/or community involvement. Additional sequence information will allow the further refinement of gene structures and a deeper understanding of the genome architecture, while the functional annotation will be enriched both by the availability of new experimental data and from expression and other kinds of analyses to characterize each gene and its function fully.
This reannotation effort will be the base for the future analysis and annotation of new E. histolytica genomes from patient isolates, a project recently approved under the NIAID supported program Genome Sequence Centers for Infectious Disease, GSCID (http://gsc.jcvi.org/).
Supporting Information
Example of artifactual tandem duplications identified in the old Entamoeba histolytica assembly. A) Dot-plot analysis of scaffold Scaffold_00115 reveals the presence of two tandem duplications spanning contig junctions. Numbers on top and on the left of the dot-plot indicate positions in base pairs along the scaffold. Green lines represent contigs and tandem duplications 1 and 2 within contigs are denoted by yellow and light-blue boxes, respectively. Contig GenbBank accession numbers are shown on the left. B) Schematic representation of the resolution of the artifactual tandem duplication 1 in the new assembly. Old (Scaffold_00115) and new (DS571349) scaffolds are represented as black horizontal lines. Colored boxes above or below the scaffold represent genes on the forward or reverse strand, respectively. Grey areas represent tandem duplicated regions and how they are resolved on the new assembly. Numbers indicate positions in base pairs within scaffolds. Scaffold sequence truncations are represented by double back-slashes. Old and new locus tags are depicted above and below genes, respectively.
(0.43 MB EPS)
Domain composition of BspA-like family proteins. A) LOGO for consensus N-terminal conserved domain; B) LOGO for consensus LRR-like repetitive domain; C) schematic representation of typical BspA-like family proteins showing different number of domains; D) size distribution chart showing the great variation in size in the family members due to the different number of domain units. Domain logos were created using WebLogo.
(1.44 MB EPS)
Distribution of Entamoeba histolytica genes into GO slim categories. The table shows the number of E. histolytica genes associated to each GO slim category we selected for this genome. Column 1, GO identifier; column 2, number of genes that share that GO slim; column 3, GO identifier definition.
(0.05 MB DOC)
Entamoeba histolytica-specific families. Table S2 lists families of Entamoeba histolytica proteins that do not share homology with any other organisms but E. histolytica. Some of the families may share a degree of homology to other closely related Entamoeba species. Column 1, Family ID corresponds to the specific identifier for each group; column 2, product name assigned to each gene; column 3, public locus name that represents the stable identifier, searchable in GenBank.
(0.76 MB DOC)
Entamoeba histolytica genes associated to Repetitive elements. Table S3 provides the complete list of E. histolytica protein families that show a close association (within 1 kb upstream or downstream) with transposable elements.
(0.54 MB DOC)
Assembly supplement.
(0.05 MB DOC)
Acknowledgments
The authors would like to thank the contributions of Ibne Ali, Elisa Azuara, Gretchen Ehrenkavfer, Carol Gilchrist, Rashidul Haque, SriTulasi Karri, Ozgur Koru, Jose de Jesus Olivares Tejo, Girija Ramakrishnan, Shahram Solaymani for their contribution towards the annotation through their participation in the Pathema Entamoeba Workshop, 2007. The authors thank Michael Schatz, Art Delcher, and Granger Sutton for assembly analysis. The authors would like to also thank members of the JCVI informatics and annotation team Tanja Davidsen, Scott Durkin, Bob Dodson, Sean Daugherty, Derek Harkins, Susmita Shrivastava, Ramana Madupu, Mathangi Thiagarajan, Vinita Joardar, Shelby Bidwell and Linda Hannick for their contribution to the curation of functional annotation, and Jason Inman for submission to GenBank. Lastly, the authors thank Dr. Graham Clark and John Ackers for their valuable advice and mentorship.
Footnotes
The authors have declared that no competing interests exist.
This project has been funded in whole or part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under contract numbers N01-AI-33071 and HHSN266200400038C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ximenez C, Moran P, Rojas L, Valadez A, Gomez A. Reassessment of the epidemiology of amebiasis: state of the art. Infect Genet Evol. 2009;9:1023–1032. doi: 10.1016/j.meegid.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Loftus B, Anderson I, Davies R, Alsmark UC, Samuelson J, et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal D, Mittal V, Ramachandran S, Dhar SK, Bhattacharya A, et al. Nucleotide sequence organisation and analysis of the nuclear ribosomal DNA circle of the protozoan parasite Entamoeba histolytica. Mol Biochem Parasitol. 1994;67:205–214. doi: 10.1016/0166-6851(94)00129-4. [DOI] [PubMed] [Google Scholar]
- 4.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts M, Hunt BR, Yorke JA, Bolanos RA, Delcher AL. A preprocessor for shotgun assembly of large genomes. J Comput Biol. 2004;11:734–752. doi: 10.1089/cmb.2004.11.734. [DOI] [PubMed] [Google Scholar]
- 6.Myers EW, Sutton GG, Delcher AL, Dew IM, Fasulo DP, et al. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- 7.Haas BJ, Delcher AL, Mount SM, Wortman JR, Smith RK, Jr, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31:5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JE, Majoros WH, Pertea M, Salzberg SL. JIGSAW, GeneZilla, and GlimmerHMM: puzzling out the features of human genes in the ENCODE regions. Genome Biol. 2006;7(Suppl 1):S9 1–13. doi: 10.1186/gb-2006-7-s1-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzi H, Thiagarajan M, Haas B, Wortman J, Hall N, et al. Genome wide survey, discovery and evolution of repetitive elements in three Entamoeba species. BMC Genomics. 2008;9:595. doi: 10.1186/1471-2164-9-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delcher AL, Salzberg SL, Phillippy AM. Using MUMmer to identify similar regions in large sequence sets. Curr Protoc Bioinformatics. 2003;Chapter 10:Unit 10 13. doi: 10.1002/0471250953.bi1003s00. [DOI] [PubMed] [Google Scholar]
- 12.Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnhammer EL, Eddy SR, Durbin R. Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins. 1997;28:405–420. doi: 10.1002/(sici)1097-0134(199707)28:3<405::aid-prot10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Haft DH, Selengut JD, White O. The TIGRFAMs database of protein families. Nucleic Acids Res. 2003;31:371–373. doi: 10.1093/nar/gkg128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulo N, Bairoch A, Bulliard V, Cerutti L, Cuche BA, et al. The 20 years of PROSITE. Nucleic Acids Res. 2008;36:D245–249. doi: 10.1093/nar/gkm977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulder N, Apweiler R. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol Biol. 2007;396:59–70. doi: 10.1007/978-1-59745-515-2_5. [DOI] [PubMed] [Google Scholar]
- 17.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 18.Camon E, Magrane M, Barrell D, Lee V, Dimmer E, et al. The Gene Ontology Annotation (GOA) Database: sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 2004;32:D262–266. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren Q, Kang KH, Paulsen IT. TransportDB: a relational database of cellular membrane transport systems. Nucleic Acids Res. 2004;32:D284–288. doi: 10.1093/nar/gkh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas BJ, Delcher AL, Wortman JR, Salzberg SL. DAGchainer: a tool for mining segmental genome duplications and synteny. Bioinformatics. 2004;20:3643–3646. doi: 10.1093/bioinformatics/bth397. [DOI] [PubMed] [Google Scholar]
- 21.Cheng XJ, Hughes MA, Huston CD, Loftus B, Gilchrist CA, et al. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect Immun. 2001;69:5892–5898. doi: 10.1128/IAI.69.9.5892-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan G, Ragland BD, Purdy JE, Mann BJ. Physical mapping and expression of gene families encoding the N-acetyl D-galactosamine adherence lectin of Entamoeba histolytica. Mol Microbiol. 1996;19:91–100. doi: 10.1046/j.1365-2958.1996.356885.x. [DOI] [PubMed] [Google Scholar]
- 23.Sankoff D. Gene and genome duplication. Curr Opin Genet Dev. 2001;11:681–684. doi: 10.1016/s0959-437x(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 24.Doolittle RF. Similar amino acid sequences: chance or common ancestry? Science. 1981;214:149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- 25.Kazmierczuk A, Kilianska ZM. [The pleiotropic activity of heat-shock proteins]. Postepy Hig Med Dosw (Online) 2009;63:502–521. [PubMed] [Google Scholar]
- 26.Shilova VY, Garbuz DG, Myasyankina EN, Chen B, Evgen'ev MB, et al. Remarkable site specificity of local transposition into the Hsp70 promoter of Drosophila melanogaster. Genetics. 2006;173:809–820. doi: 10.1534/genetics.105.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zatsepina OG, Velikodvorskaia VV, Molodtsov VB, Garbuz D, Lerman DN, et al. A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased Hsp70 expression. J Exp Biol. 2001;204:1869–1881. doi: 10.1242/jeb.204.11.1869. [DOI] [PubMed] [Google Scholar]
- 28.Davis PH, Zhang Z, Chen M, Zhang X, Chakraborty S, et al. Identification of a family of BspA like surface proteins of Entamoeba histolytica with novel leucine rich repeats. Mol Biochem Parasitol. 2006;145:111–116. doi: 10.1016/j.molbiopara.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Samuelson J, Clark CG, Eichinger D, Paul J, et al. Gene discovery in the Entamoeba invadens genome. Mol Biochem Parasitol. 2003;129:23–31. doi: 10.1016/s0166-6851(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 30.Loimaranta V, Hytonen J, Pulliainen AT, Sharma A, Tenovuo J, et al. Leucine-rich repeats of bacterial surface proteins serve as common pattern recognition motifs of human scavenger receptor gp340. J Biol Chem. 2009;284:18614–18623. doi: 10.1074/jbc.M900581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikegami A, Honma K, Sharma A, Kuramitsu HK. Multiple functions of the leucine-rich repeat protein LrrA of Treponema denticola. Infect Immun. 2004;72:4619–4627. doi: 10.1128/IAI.72.8.4619-4627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, et al. Secretion without Golgi. J Cell Biochem. 2008;103:1327–1343. doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilchrist CA, Houpt E, Trapaidze N, Fei Z, Crasta O, et al. Impact of intestinal colonization and invasion on the Entamoeba histolytica transcriptome. Mol Biochem Parasitol. 2006;147:163–176. doi: 10.1016/j.molbiopara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.MacFarlane RC, Singh U. Identification of differentially expressed genes in virulent and nonvirulent Entamoeba species: potential implications for amebic pathogenesis. Infect Immun. 2006;74:340–351. doi: 10.1128/IAI.74.1.340-351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar SK, Choudhury NR, Bhattacharaya A, Bhattacharya S. A multitude of circular DNAs exist in the nucleus of Entamoeba histolytica. Mol Biochem Parasitol. 1995;70:203–206. doi: 10.1016/0166-6851(95)00008-o. [DOI] [PubMed] [Google Scholar]
- 36.Clark CG, Alsmark UC, Tazreiter M, Saito-Nakano Y, Ali V, et al. Structure and content of the Entamoeba histolytica genome. Adv Parasitol. 2007;65:51–190. doi: 10.1016/S0065-308X(07)65002-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhyvoloup A, Nemazanyy I, Babich A, Panasyuk G, Pobigailo N, et al. Molecular cloning of CoA Synthase. The missing link in CoA biosynthesis. J Biol Chem. 2002;277:22107–22110. doi: 10.1074/jbc.C200195200. [DOI] [PubMed] [Google Scholar]
- 38.Makioka A, Kobayashi S, Takeuchi T. Detection and characterization of DNA polymerase activity in Entamoeba histolytica. Parasitol Res. 1996;82:87–89. doi: 10.1007/s004360050074. [DOI] [PubMed] [Google Scholar]
- 39.Heidtman M, Chen CZ, Collins RN, Barlowe C. Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex. Mol Biol Cell. 2005;16:1673–1683. doi: 10.1091/mbc.E04-10-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark CG, Roger AJ. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proc Natl Acad Sci U S A. 1995;92:6518–6521. doi: 10.1073/pnas.92.14.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example of artifactual tandem duplications identified in the old Entamoeba histolytica assembly. A) Dot-plot analysis of scaffold Scaffold_00115 reveals the presence of two tandem duplications spanning contig junctions. Numbers on top and on the left of the dot-plot indicate positions in base pairs along the scaffold. Green lines represent contigs and tandem duplications 1 and 2 within contigs are denoted by yellow and light-blue boxes, respectively. Contig GenbBank accession numbers are shown on the left. B) Schematic representation of the resolution of the artifactual tandem duplication 1 in the new assembly. Old (Scaffold_00115) and new (DS571349) scaffolds are represented as black horizontal lines. Colored boxes above or below the scaffold represent genes on the forward or reverse strand, respectively. Grey areas represent tandem duplicated regions and how they are resolved on the new assembly. Numbers indicate positions in base pairs within scaffolds. Scaffold sequence truncations are represented by double back-slashes. Old and new locus tags are depicted above and below genes, respectively.
(0.43 MB EPS)
Domain composition of BspA-like family proteins. A) LOGO for consensus N-terminal conserved domain; B) LOGO for consensus LRR-like repetitive domain; C) schematic representation of typical BspA-like family proteins showing different number of domains; D) size distribution chart showing the great variation in size in the family members due to the different number of domain units. Domain logos were created using WebLogo.
(1.44 MB EPS)
Distribution of Entamoeba histolytica genes into GO slim categories. The table shows the number of E. histolytica genes associated to each GO slim category we selected for this genome. Column 1, GO identifier; column 2, number of genes that share that GO slim; column 3, GO identifier definition.
(0.05 MB DOC)
Entamoeba histolytica-specific families. Table S2 lists families of Entamoeba histolytica proteins that do not share homology with any other organisms but E. histolytica. Some of the families may share a degree of homology to other closely related Entamoeba species. Column 1, Family ID corresponds to the specific identifier for each group; column 2, product name assigned to each gene; column 3, public locus name that represents the stable identifier, searchable in GenBank.
(0.76 MB DOC)
Entamoeba histolytica genes associated to Repetitive elements. Table S3 provides the complete list of E. histolytica protein families that show a close association (within 1 kb upstream or downstream) with transposable elements.
(0.54 MB DOC)
Assembly supplement.
(0.05 MB DOC)