Abstract
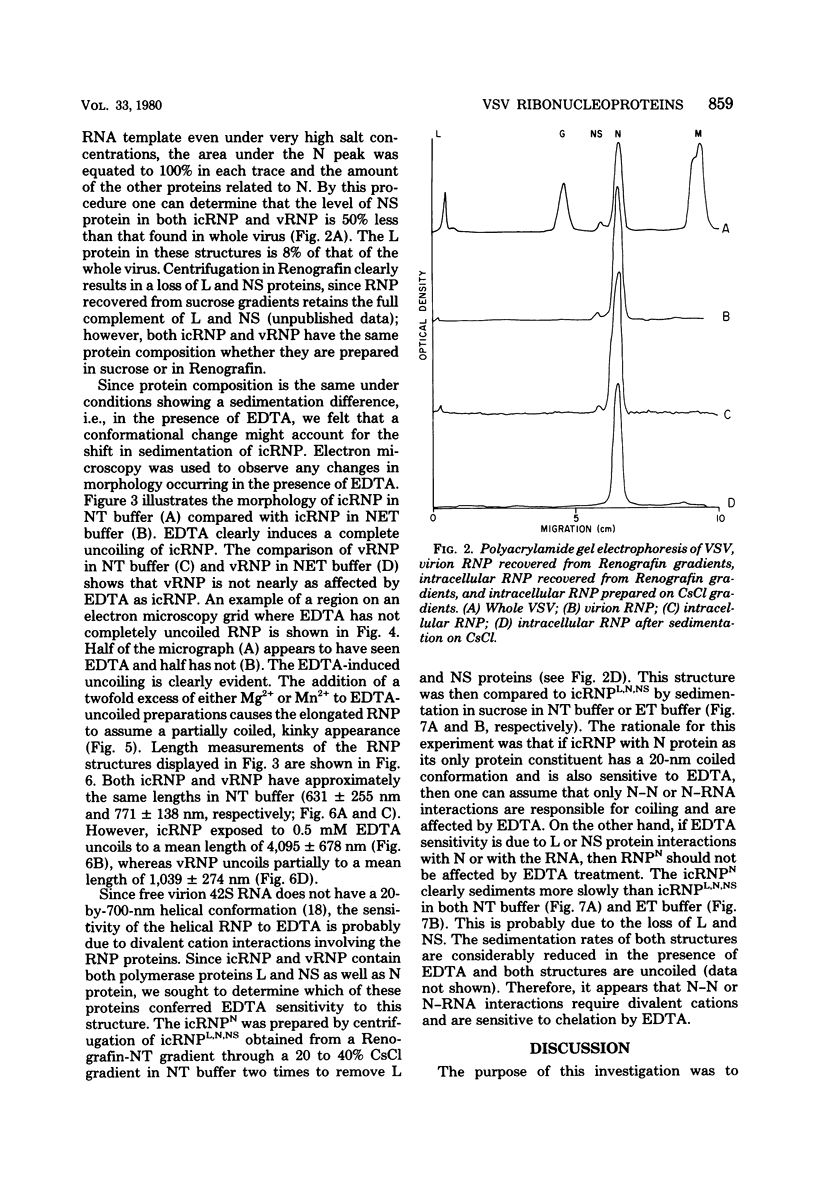
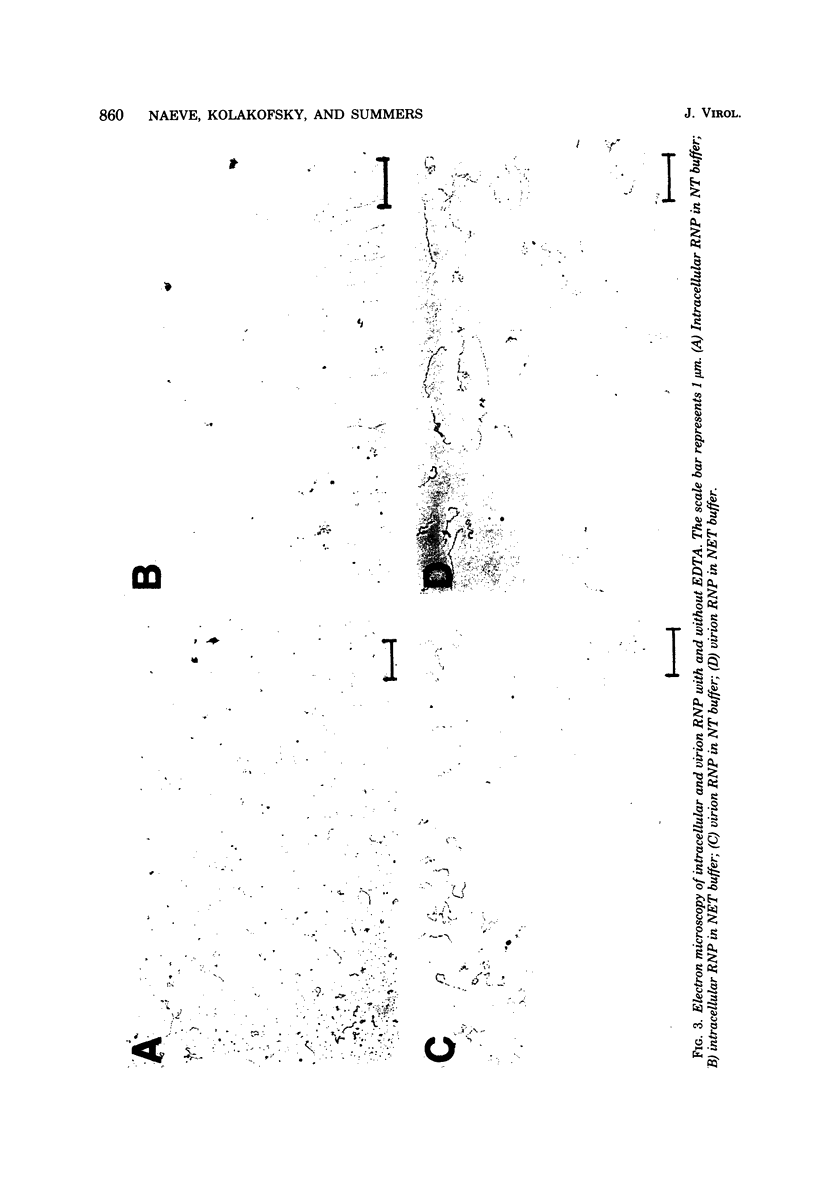
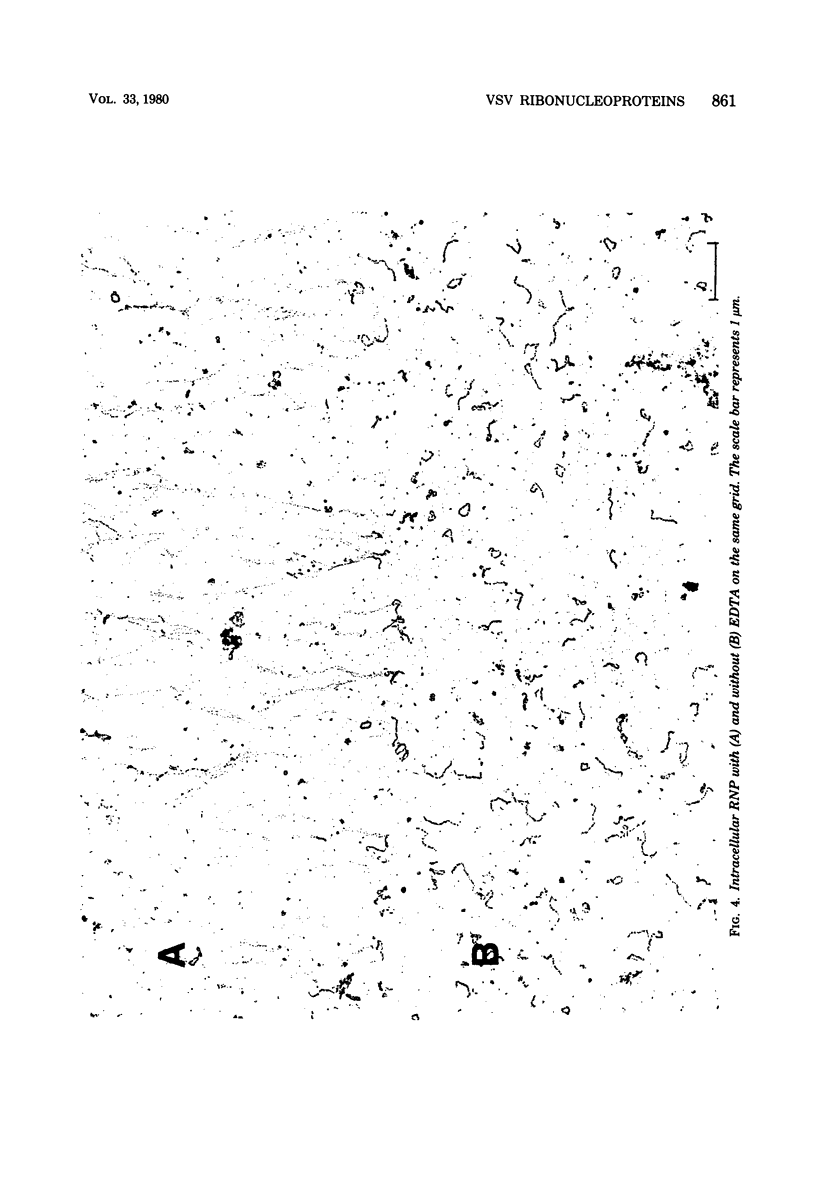
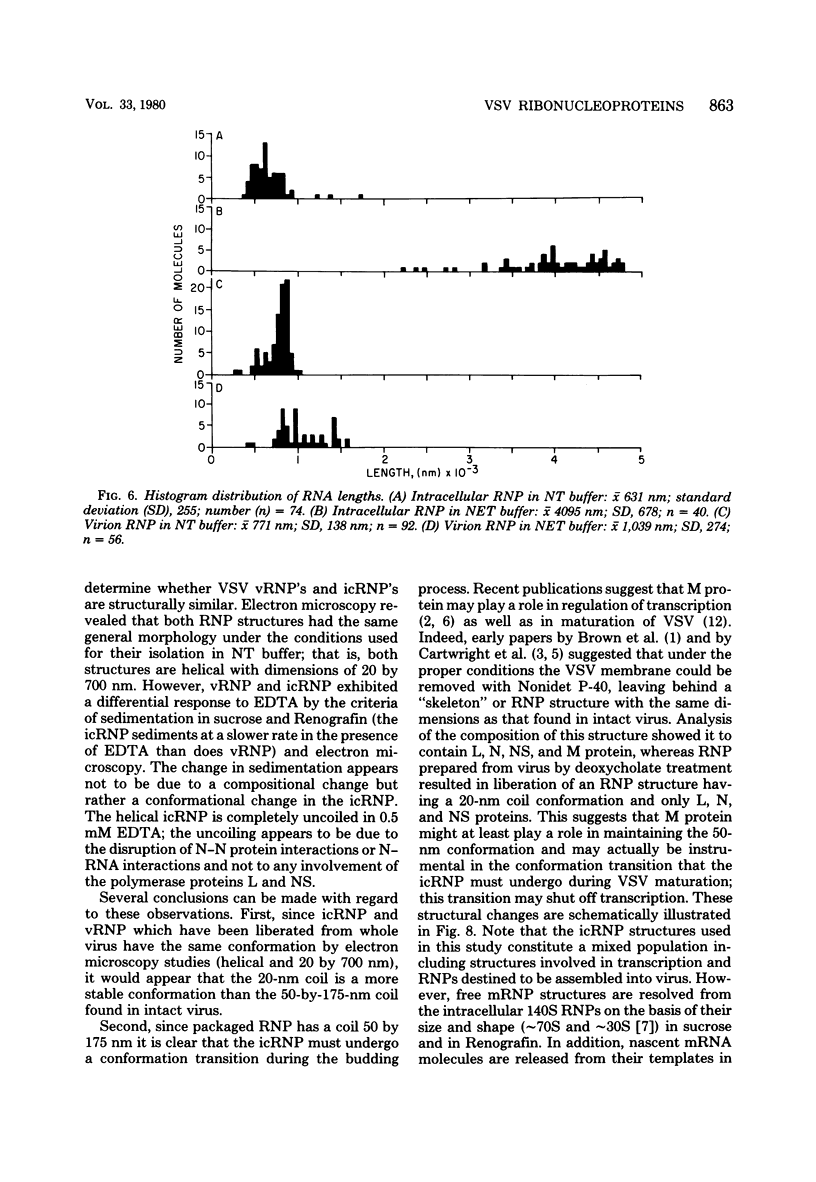
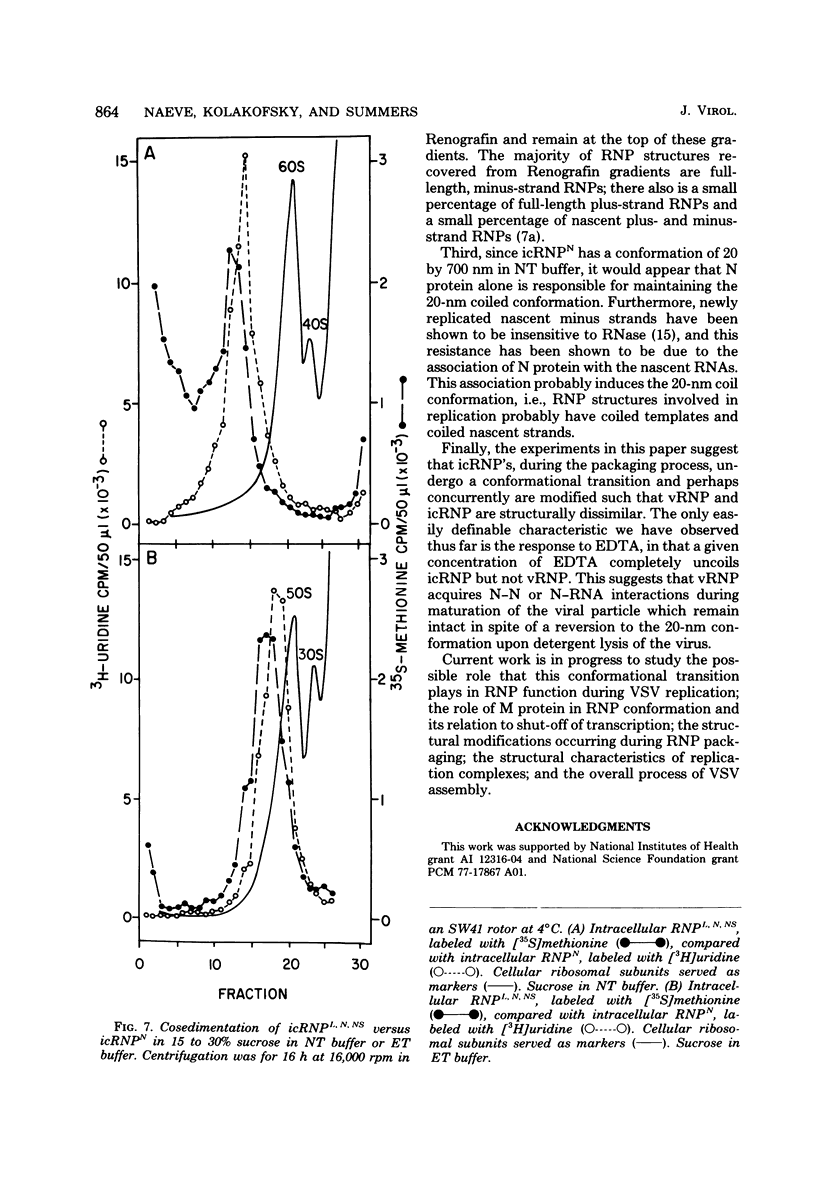
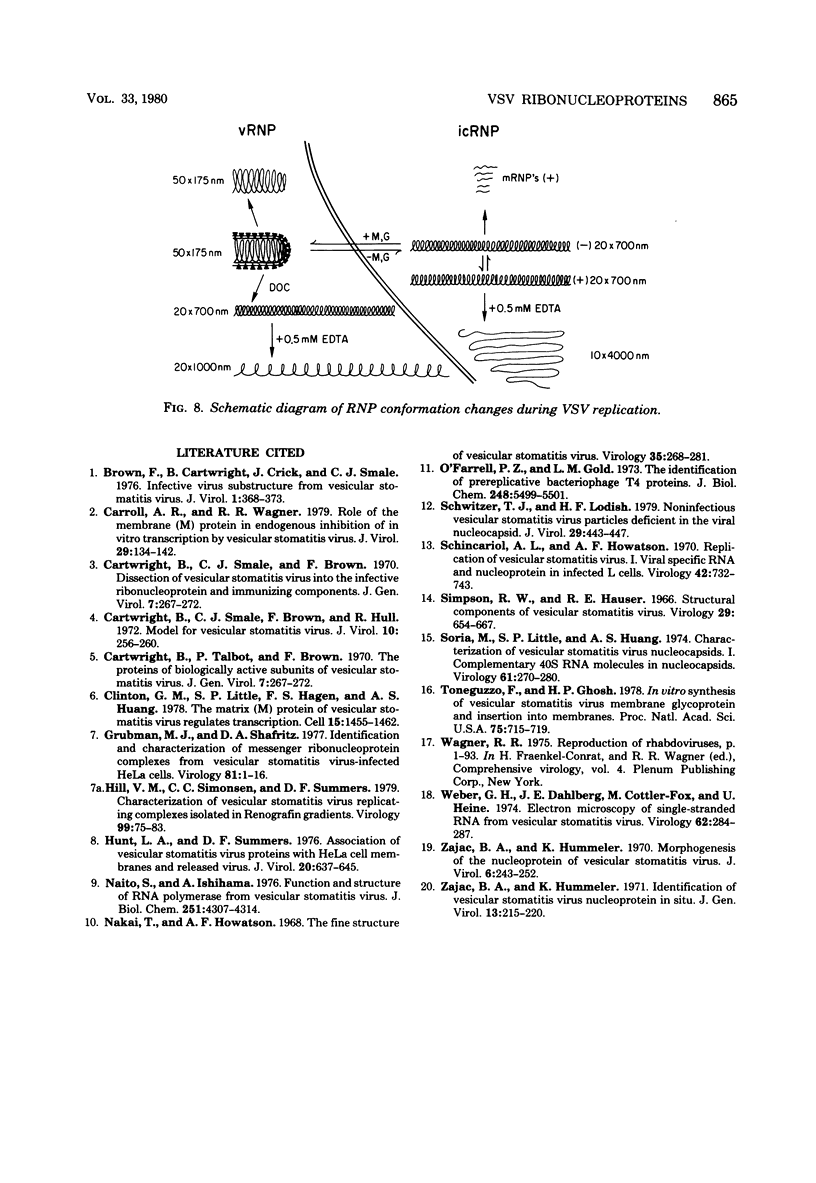
Vesicular stomatitis virus ribonucleoproteins (RNP) obtained by a detergent treatment of purified virus (vRNP) or from infected HeLa cell cytoplasm (icRNP) were examined by sedimentation in sucrose or Renografin gradients in the presence or absence of EDTA. It was shown that vRNP and icRNP sediment at the same rate in sucrose and Renografin in the absence of EDTA; however, icRNP sedimented more slowly in the presence of EDTA than did vRNP. Polyacrylamide gel electrophoresis of the proteins of vRNA and icRNP recovered from EDTA-containing gradients demonstrated that both RNP structures contained L, N, and NS proteins in the same proportion. Electron microscopy of both RNP structures, in the absence of EDTA, demonstrated that both exist as helical structures ∼20 by 700 nm. However, in the presence of EDTA the icRNP was completely uncoiled with a mean length of 4,095 nm, whereas vRNP was hardly affected. The addition of excess Mg2+ or Mn2+ to uncoiled icRNP preparations partially restored the coiled configuration. These observations suggest that the change in sedimentation of icRNP in the presence of EDTA is due to a change from a coiled to an uncoiled conformation, that icRNP and vRNP are not structurally identical, and that icRNP must undergo a conformational change during maturation of VSV from the 20-by-700-nm intracellular form to the 50-by-175-nm form found in intact virus. The icRNP containing L, N, and NS proteins (icRNPL,N,NS) and icRNP containing only N protein (icRNPN), prepared by centrifugation of icRNPL,N,NS in CsCl to remove L and NS, were compared by cosedimentation in sucrose gradients. There was a decrease in sedimentation rate of icRNPN due to loss of L and NS. This sedimentation difference was also apparent in the presence of EDTA; however, both icRNPL,N,NS and icRNPN sedimented at a much slower rate in the presence of EDTA, and by electron microscopy both were completely uncoiled. These observations suggest that N protein alone is responsible for the 20-by-700-nm coiled structure and that the divalent cation interactions disrupted by EDTA are N-N or N-RNA interactions. These results are discussed with regard to vesicular stomatitis virus maturation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F., Cartwright B., Crick J., Smale C. J. Infective virus substructure from vesicular stomatitis virus. J Virol. 1967 Apr;1(2):368–373. doi: 10.1128/jvi.1.2.368-373.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Shafritz D. A. Identification and characterization of messenger ribonucleoprotein complexes from vesicular stomatitis virus-infected HeLa cells. Virology. 1977 Aug;81(1):1–16. doi: 10.1016/0042-6822(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Simonsen C. C., Summers D. F. Characterization of vesicular stomatitis virus replicating complexes isolated in renografin gradients. Virology. 1979 Nov;99(1):75–83. doi: 10.1016/0042-6822(79)90038-2. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Association of vesicular stomatitis virus proteins with HeLa cell membranes and released virus. J Virol. 1976 Dec;20(3):637–645. doi: 10.1128/jvi.20.3.637-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. I. Viral specific RNA and nucleoprotein in infected L cells. Virology. 1970 Nov;42(3):732–743. doi: 10.1016/0042-6822(70)90319-3. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Lodish H. F. Noninfectious vesicular stomatitis virus particles deficient in the viral nucleocapsid. J Virol. 1979 Feb;29(2):443–447. doi: 10.1128/jvi.29.2.443-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. In vitro synthesis of vesicular stomatitis virus membrane glycoprotein and insertion into membranes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):715–719. doi: 10.1073/pnas.75.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. H., Dahlberg J. E., Cottler-Fox M., Heine U. Electron microscopy of single-stranded RNA from vesicular stomatitis virus. Virology. 1974 Nov;62(1):284–287. doi: 10.1016/0042-6822(74)90324-9. [DOI] [PubMed] [Google Scholar]
- Zajac B. A., Hummeler K. Identification of vesicular stomatitis virus nucleoprotein in situ. J Gen Virol. 1971 Nov;13(2):215–220. doi: 10.1099/0022-1317-13-2-215. [DOI] [PubMed] [Google Scholar]
- Zajac B. A., Hummeler K. Morphogenesis of the nucleoprotein of vesicular stomatitis virus. J Virol. 1970 Aug;6(2):243–252. doi: 10.1128/jvi.6.2.243-252.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]






