Abstract
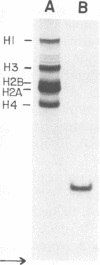
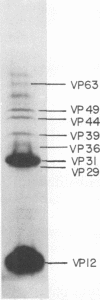
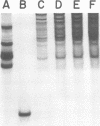
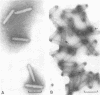
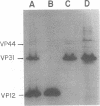
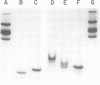
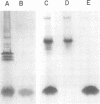
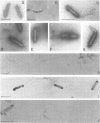
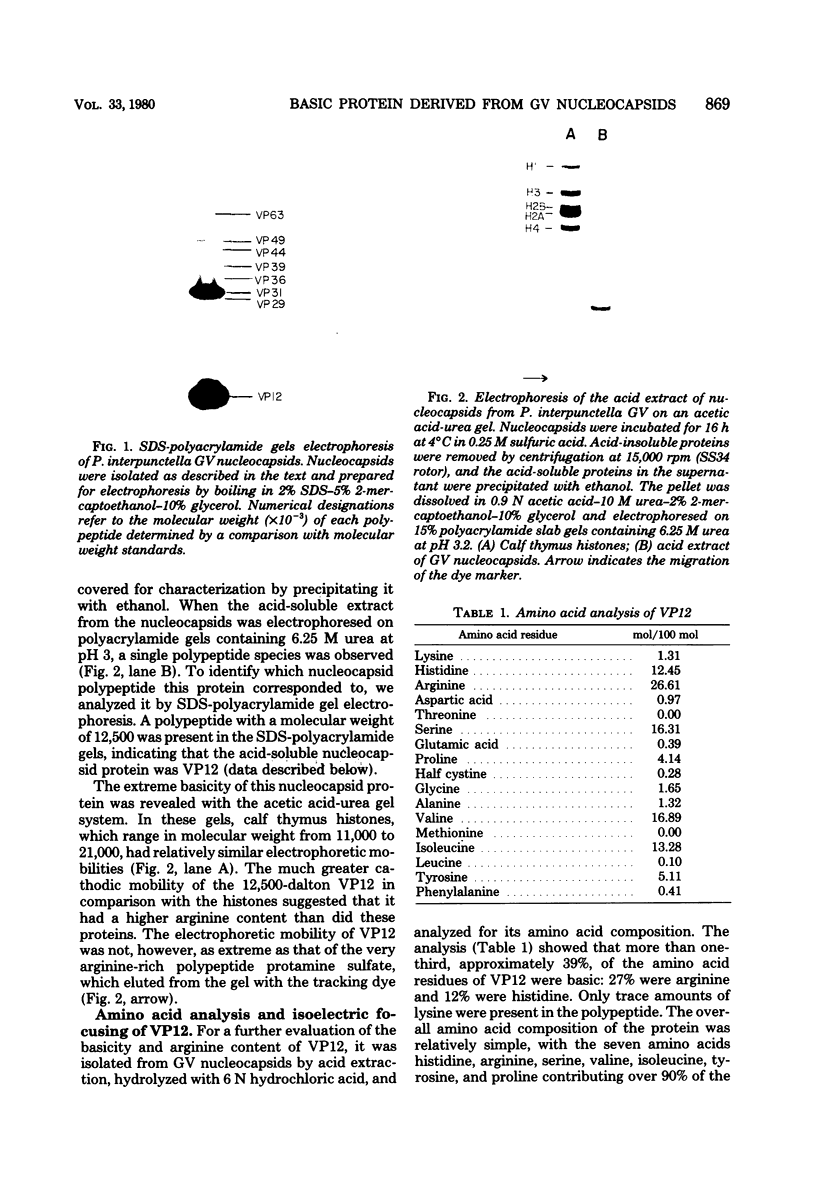
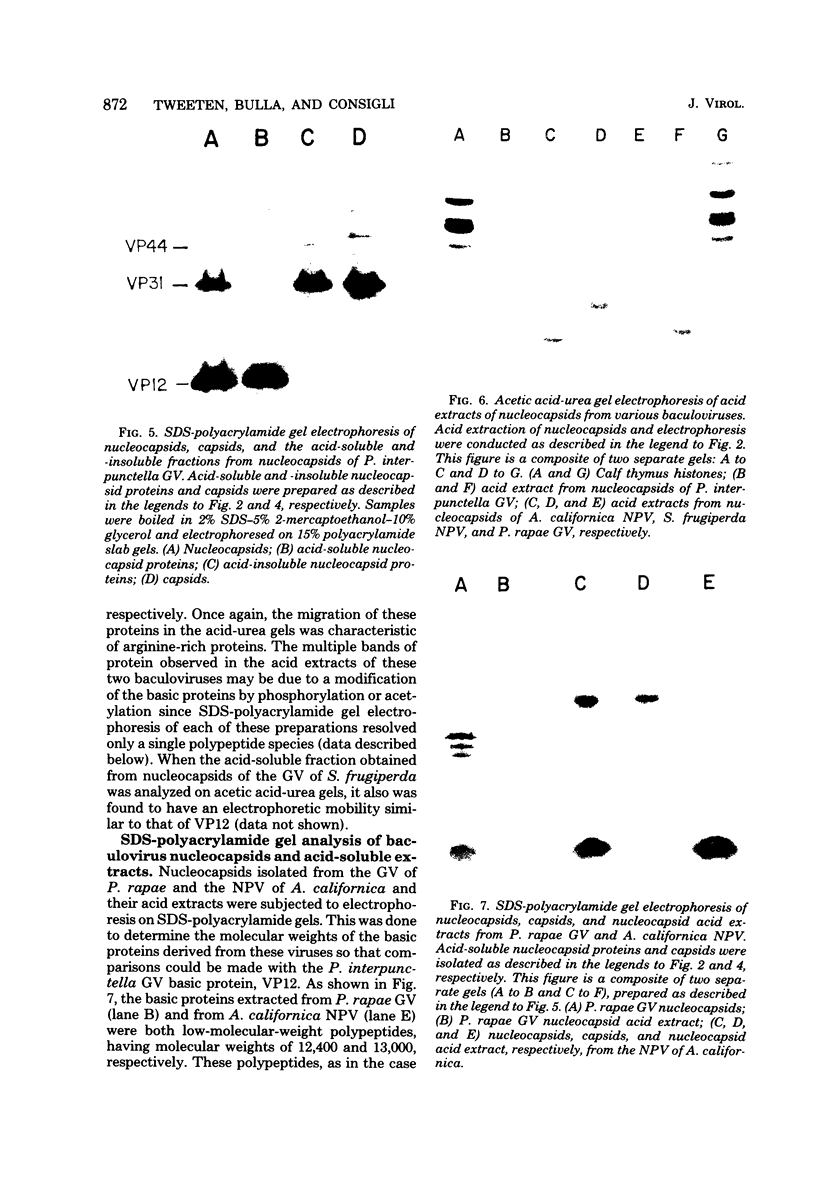
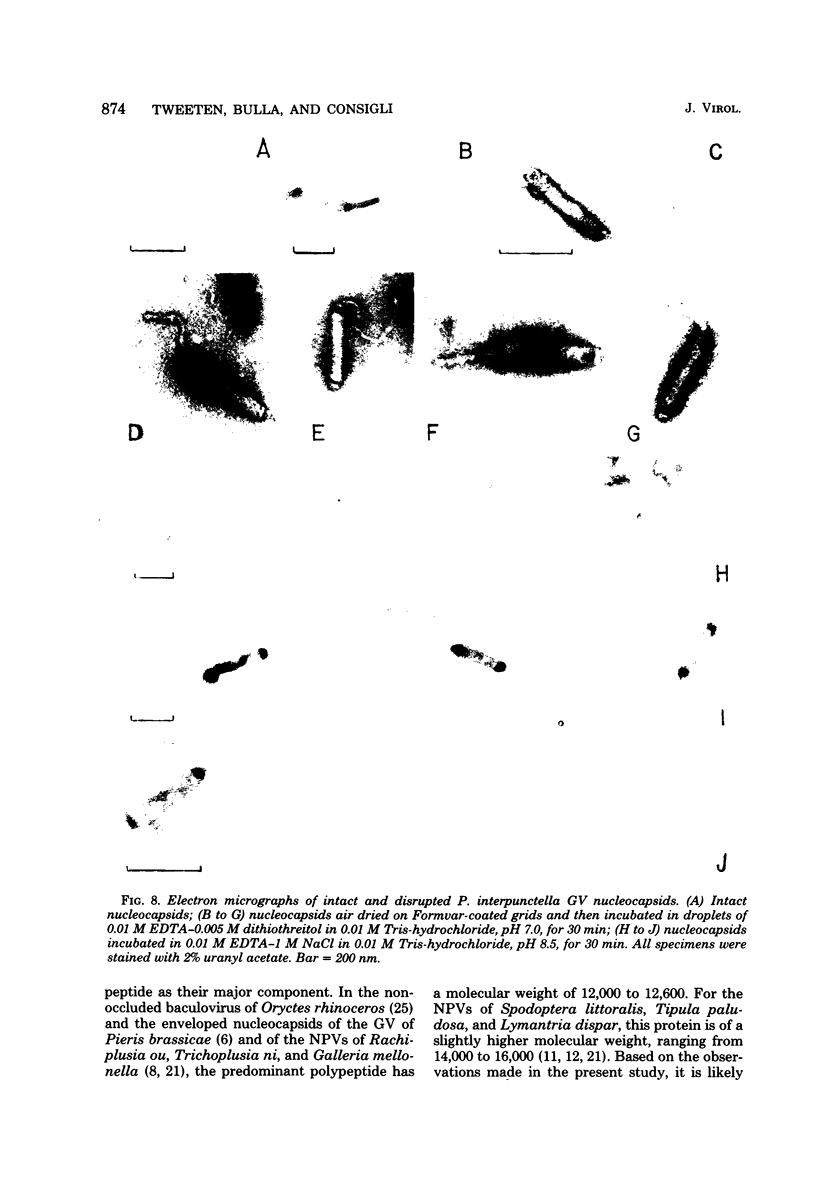
Nucleocapsids were isolated from purified enveloped nucleocapsids of Plodia interpunctella granulosis virus by treatment with Nonidet P-40. When analyzed on sodium dodecyl sulfate-polyacrylamide gels, the nucleocapsids consisted of eight polypeptides. One of these, a major component with a molecular weight of 12,500 (VP12), was selectively extracted from the nucleocapsids with 0.25 M sulfuric acid. Its electrophoretic mobility on acetic acid-urea gels was intermediate to that of cellular histones and protamine. Amino acid analysis showed that 39% of the amino acid residues of VP12 were basic: 27% were arginine and 12% were histidine. The remaining residues consisted primarily of serine, valine, and isoleucine. Proteins of similar arginine content also were extracted from the granulosis virus of Pieris rapae and from the nuclear polyhedrosis viruses of Spodoptera frugiperda and Autographa californica. The basic polypeptide appeared to be virus specific because it was found in nucleocapsids and virus-infected cells but not in uninfected cells. VP12 was not present in polypeptide profiles of granulosis virus capsids, indicating that it was an internal or core protein of the nucleocapsids. Electron microscopic observations suggested that the basic protein was associated with the viral DNA in the form of a DNA-protein complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaton C. D., Filshie B. K. Comparative ultrastructural studies of insect granulosis and nuclear polyhedrosis viruses. J Gen Virol. 1976 May;31(2):151–161. doi: 10.1099/0022-1317-31-2-151. [DOI] [PubMed] [Google Scholar]
- Bina-Stein M., Simpson R. T. Specific folding and contraction of DNA by histones H3 and H4. Cell. 1977 Jul;11(3):609–618. doi: 10.1016/0092-8674(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Bloch D. P. A catalog of sperm histones. Genetics. 1969;61(1 Suppl):93–111. [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Bud H. M., Kelly D. C. Biophysical properties of the structural components of a granulosis virus isolated from the cabbage white butterfly (Pieris brassicae). Virology. 1977 Sep;81(2):317–327. doi: 10.1016/0042-6822(77)90148-9. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelpa B., Bergoin M., Croizier G. La protéine d'inclusion et les protéines du virion du baculovirus du diptére Tipula paludosa (Meigen) C R Acad Sci Hebd Seances Acad Sci D. 1977 Feb 28;284(9):779–782. [PubMed] [Google Scholar]
- Harrap K. A., Payne C. C., Robertson J. S. The properties of three baculoviruses from closely related hosts. Virology. 1977 Jun 1;79(1):14–31. doi: 10.1016/0042-6822(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Sung M. T. Isolation and characterization of an extremely basic protein from adenovirus type 5. J Virol. 1976 Mar;17(3):924–934. doi: 10.1128/jvi.17.3.924-934.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975 May;65(2):258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsarrat P., Revet B., Gourevitch I. Mise en évidence, stabilisation et purification d'une structure nucléoprotéique intracapsidaire chez le Baculovirus. C R Acad Sci Hebd Seances Acad Sci D. 1975 Nov 10;281(19):1439–1442. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Payne C. C., Compson D., de looze S. M. Properties of the nucleocapsids of a virus isolated from Oryctes rhinoceros. Virology. 1977 Mar;77(1):269–280. doi: 10.1016/0042-6822(77)90424-x. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of nuclear polyhedrosis virus DNAs. J Virol. 1973 Dec;12(6):1336–1346. doi: 10.1128/jvi.12.6.1336-1346.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Baculovirus structural polypeptides. Virology. 1978 Feb;84(2):390–402. doi: 10.1016/0042-6822(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Consigli R. A. Structural Polypeptides of the Granulosis Virus of Plodia interpunctella. J Virol. 1980 Feb;33(2):877–886. doi: 10.1128/jvi.33.2.877-886.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Jr, Consigli R. A. Characterization of an alkaline protease associated with a granulosis virus of Plodia interpunctella. J Virol. 1978 Jun;26(3):703–711. [PMC free article] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Jr, Consigli R. A. Isolation and purification of a granulosis virus from infected larvae of the Indian meal moth, Plodia interpunctella. Appl Environ Microbiol. 1977 Sep;34(3):320–327. doi: 10.1128/aem.34.3.320-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Jr, Consigli R. A. Supercoiled circular DNA of an insect granulosis virus. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3574–3578. doi: 10.1073/pnas.74.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weihe A., von Mickwitz C. U., Grade K., Lindigkeit R. Complexes of DNA with arginine-rich and slightly lysine-rich histones. Transcription and electron microscopy. Biochim Biophys Acta. 1978 Mar 29;518(1):172–176. doi: 10.1016/0005-2787(78)90126-0. [DOI] [PubMed] [Google Scholar]