Abstract
Anti-nuclear antibodies constitute the hallmark of lupus. The NZM2410-derived Sle1 lupus susceptibility interval on murine chromosome 1 breaches tolerance, leading to the emergence of anti-nuclear autoantibodies targeting nucleosomes. However, little is known about the molecular structure of the anti-nucleosome autoantibodies from this genetically simplified mouse model of lupus. In this study, the immunoglobulin heavy chain and light chain sequences of 50 anti-nuclear monoclonal antibodies derived from five B6.Sle1z mice were compared to non-nuclear antibody controls. Compared to 2 different sets of non-nuclear antibodies, anti-nucleosome antibodies derived from B6.Sle1z congenic mice exhibited a high degree of clonal expansion and 3 distinct sequence motifs in their heavy chains–cationic CDR3 stretches, non-anionic CDR2 regions, and an increased frequency of aspartate residues at H50, which together increased the likelihood of an antibody being chromatin-reactive by ~4-fold.
Keywords: Autoimmunity, B-cells, autoantibodies, genetics, immunoglobulin repertoire
1. Introduction
Antinuclear antibodies (ANAs) constitute an important hallmark of systemic lupus erythematosus, as extensively reviewed (Hahn, 1998; Kotzin, 1996; Pisetsky, 2000), though autoantibody-independent mechanisms leading to lupus nephritis have also been described (Chan et al., 1999; Lefkowith and Gilkeson, 1996; Liang et al., 2004; Shi et al., 2007; Waters et al., 2004). Comparative studies of ANAs with non-nuclear antigen reactive Abs have highlighted several interesting molecular features, particularly in the immunoglobulin (Ig) heavy chains (HC), including the prominence of “R” residues in the CDR3 regions (Eilat and Anderson, 1994; Liang et al., 2004; Marion et al., 1992; Radic and Weigert, 1994); (Chen et al., 2002), whose importance in facilitating DNA-reactivity has been unequivocally demonstrated through site-directed mutagenesis (Martin et al., 1994; Radic et al., 1993; Radic and Seal, 1997; Wloch et al., 1997). The evidence for distinct molecular signatures that distinguish the CDR2 regions of ANA HCs from those of non-ANAs has been less convincing. Nevertheless, sequence comparison studies and site-directed mutagenesis has helped demonstrate the potential importance of polarity at selected CDR2 positions in conferring or enhancing DNA-reactivity (Chen et al., 2002; Katz et al., 1994; Radic et al., 1993; Radic and Seal, 1997). In contrast to the HC, the light chains (LCs) of ANAs possess few molecular “signatures” that consistently light up across different data sets (Liang et al., 2003; Marion et al., 1992). This is in line with the prevailing notion that the HC may play the “dominant” role in dictating nuclear antigen reactivity, while the LC may serve to modulate, or even veto this reactivity in the context of certain HC partners (Fitzsimons et al., 2000; Ibrahim et al., 1995; Li et al., 2001; Spatz et al., 1997).
As reviewed above, several previous studies have documented the sequence differences between anti-nuclear Abs and non-ANA controls. However, caution should be exercised in interpreting these data, for 2 important reasons. First, in most documented murine and human Ig repertoire studies, the lupus-afflicted subjects (or mice) and normal controls have had very different genetic backgrounds. Second, in both species, since lupus is polygenic in origin, one cannot attribute the observed repertoire differences to any single genetic event. To circumvent these two limitations, we elected to study the antibody repertoire in lupus using a genetically simplified mouse model-B6 mice rendered congenic for the NZM2410-derived lupus susceptibility interval, Sle1z (Mohan et al., 1998; Morel et al., 1997).
Whereas B6 mice do not exhibit anti-nuclear autoantibodies, B6.Sle1z congenics (which are on the same B6 genetic background) exhibit high titers of anti-nuclear autoantibodies, with preferential binding to nucleosomes and DNA/histone complexes (Mohan et al., 1998; Morel et al., 1997). A panel of anti-chromatin mAbs were generated from this strain and examined for antigen specificity and sequence structure. In contrast to their LC sequences, ANA HC sequences from B6.Sle1z-derived mAbs exhibited three distinct sequence motifs–cationic CDR3 stretches, non-anionic CDR2 regions, and an increased frequency of aspartate residues at H50. Together, these 3 motifs increased the likelihood of an antibody being chromatin-reactive by ~4-fold.
2. Materials and Methods
2.1 Mice
B6.Sle1z are C57BL/6 (B6) mice rendered congenic homozygotes for NZM2410-derived Sle1z, a 37 centimorgan lupus susceptibility interval on chromosome 1, with termini at D1MIT101 and D1MIT155 (Morel et al., 1997). These mice are strongly seropositive for anti-chromatin and anti-histone/DNA Abs, but weakly positive for anti-dsDNA Abs (Mohan et al., 1998), while the B6 controls were seronegative for these specificities. Mice used for studies were 6–9 mo old females, housed in a specific pathogen free colony at UT Southwestern Medical Center Department of Animal Resources.
2.2 Hybridoma Studies
Spleens removed aseptically from 6–9 mo old, anti-chromatin seropositive B6.Sle1z mice were fused to the SP2/0 fusion partner and plated as described (Liang et al., 2004). Single-colony wells that were secreting antibodies (IgM or IgG) were subcloned twice, to ensure clonality, as described (Liang et al., 2004). Hybridoma supernatants were purified using ammonium sulfate precipitation and Protein A chromatography, quantitated using a Coomassie PLUS assay kit (Pierce, Rockford, IL), isotyped using ELISA, adjusted to a concentration of 1–10 ug/ml and tested for anti-nuclear reactivity by ELISA, as described (Liang et al., 2004; Mohan et al., 1998). For the binding strengths shown in Table 1, ODs in the respective antigen-specific ELISAs were mapped onto a semi-quantitative scale, by normalizing against the total Ig level in each sample, as described (Liang et al., 2004). On this scale, “+”, and “++” indicate that the antigen specific OD values registered by the respective mAbs were 0.2 to 0.5, or > 0.5 fold higher, respectively, relative to the corresponding OD values recorded for “total Ig”, assayed in parallel.
Table 1.
Monoclonal antinuclear antibodies rescued from B6.Sle1 mice
| Mice | mAb 1 | Isotype | Nucleosome | dsDNA | ssDNA | Histone |
|---|---|---|---|---|---|---|
| B6.Sle1 z #1 | 1A1G12a | IgG2a | ++ | - | + | - |
| B6.Sle1 z #2 | 1BD2b | IgG2a | ++ | ++ | ++ | - |
| B6.Sle1 z #3 | 1CA4c | IgG2a | ++ | + | ++ | - |
| 1CB3 | IgG2a | ++ | - | - | - | |
| 1CD3 | IgG2a | + | - | - | ++ | |
| 1CE6 | IgM | ++ | - | - | - | |
| 1CE7 | IgG2a | ++ | + | - | - | |
| B6.Sle1 z #4 | 1DB2 | IgG2b | ++ | - | - | - |
| 1DC1e | IgM | ++ | - | - | - | |
| 1DC3 | IgM | + | + | + | + | |
| 1DC5 | IgM | ++ | ++ | - | - | |
| 1DC7f | IgM | ++ | + | - | + | |
| 1DC9 | IgG2a | + | + | ++ | ++ | |
| 1DE1 | IgM | (−/+) | + | ++ | - | |
| 1DE4 | IgG2b | ++ | + | - | - | |
| 1DG5d | IgG2a | ++ | + | + | ++ | |
| B6.Sle1 z #5 | 1EA9 | IgG2a | (−/+) | + | + | + |
| 1EA10 | IgG1 | ++ | - | - | - | |
| 1EB2 | IgG2b | - | - | + | - | |
| 1EB5 | IgM | ++ | - | - | + | |
| 1EC1 | IgM | ++ | ++ | + | + | |
| 1ED6 | IgM | ++ | - | - | + | |
| 1ED7 | IgG2a | (−/+) | - | - | + | |
| 1ED9 | IgG2a | - | - | ++ | - | |
| 1EE5g | IgG2a | ++ | - | - | + | |
| 1EG4 | IgM | (−/+) | + | + | + | |
| 1EG8 | IgG2a | ++ | + | + | + | |
| 1EH1 | IgG2a | - | - | + | + | |
| 1EH2 | IgM | - | + | ++ | - | |
| 1EH4 | IgM | (−/+) | - | + | ++ | |
| 1EH5 | IgM | - | - | ++ | - | |
| 1EJ5 | IgM | + | - | ++ | + |
Listed are anti-nuclear mAbs generated from 5 B6.Sle1z mice. Clones with 2 or more members (as determined by shared HC CDR3 regions) are indicated with alphabets in superscripts, and are represented by the most mutated clone (in order to capture as much of the mutational information as possible).
Clonal sizes are as follows: a – 5 (i.e., 5 mAbs were clonally related); b – 4; c – 2; d – 5; e – 2; f – 2; g – 5. More detailed CDR sequence information is presented in Tables 2 and 3.
The “+” nomenclature used to describe the strength of nuclear antigen binding is detailed in Methods. Comparison of the usage frequencies of HC and LC to those noted among non-ANAs are summarized in Fig, 1.
2.3 Antibody sequence analysis
Sequences were aligned using OMIGA 3.0 (Oxford Molecular, Oxford, UK), blasted against public databases of mouse Ig sequences (http://www.ncbi.nlm.nih.gov/igblast), assigned to their respective germline origins as described (Gu et al., 1991; Haines et al., 2001), and deposited into Genbank (accession numbers AY436820-AY436914).The control databases of non-ANA sequences described in this study represent recently assembled collections of non-ANA HC and LC sequences drawn from the Genbank (Liang et al., 2003; Sedrak et al., 2003). Importantly, these abridged databases had no clonal replicates, and no 2 Abs shared the same antigen specificity. For the statistical comparisons, multi-member clones were represented by one member each so as to minimize the impact of clonal bias. Specifically, the single clone selected was the most mutated clone, so that as much of the mutational information was preserved. The respective frequencies of VH and Vk gene usage, as well as the frequencies of individual amino acid residues at the different CDR positions were compared between the ANAs and control Abs using Chi square tests (with Yates correction, where appropriate), or Fisher’s exact test. The Student’s t-test was used for comparing group means. All statistical comparisons were performed using SigmaStat (Jandel Scientific).
3. Results
3.1 Monoclonal ANAs from B6.Sle1z mice
Table 1 lists the mAbs rescued from 5 B6.Sle1z mice, named B6.Sle1z #1 to #5. The percentage of singleton wells that tested positive for histone/DNA binding in the fusions from the 5 mice ranged from 6.9% to 22.0%, with an average of 17.2%. All mAbs that were reactive with histone/DNA complexes, nucleosomes, dsDNA, ssDNA or histones were retained, and sub-cloned twice, resulting in 50 nuclear antigen binding mAbs, of which only the clonally independent mAbs have been listed in Table 1. In addition, 72 non-nuclear antigen binding control mAbs were also saved (not tabulated). The vast majority of the retrieved clones were IgM or IgG2a in isotype, consistent with the serum isotype profile noted in this strain (Mohan et al., 1998; Morel et al., 1997). Almost all of the mAbs derived from B6.Sle1z mice were reactive with nucleosomes, but less so with dsDNA (Table 1). In addition, about a third of the generated mAbs showed reactivity with DNA-free histones, or with ssDNA. Overall, the antigen reactivity profiles of the generated mAbs closely resembled the serum ANA profile reported in this strain (Mohan et al., 1998; Morel et al., 1997). The exception to this rule were the mAbs derived from B6.Sle1z #2, a mouse which was seropositive for anti-dsDNA (as well as anti-nucleosome Abs); indeed about 30% of B6.Sle1z mice have been reported to be seropositive for anti-dsDNA Abs, in addition to possessing anti-nucleosome Abs (Mohan et al., 1998).
As indicated in Table 1 and Table 2, there was a strong degree of clonal expansion (based on shared HC CDR3 sequences), particularly in the first two mice studied. Unlike these 2 mice, which apparently exhibited a monoclonal expansion of ANAs, the anti-nuclear Ab repertoires in the remaining three mice were more polyclonal, albeit with several examples of oligoclonal expansion (as indicated in Table 1 and Table 2). Table 2 and Table 3 detail the Ig HC and LC CDR sequences of the ANAs generated from the B6.Sle1z congenics, with multi-member clones being listed once each only. Sequence comparisons were next made to 2 sets of non-ANA controls – non-nuclear antigen binding mAbs derived from NCBI/Genbank (Liang et al., 2003; Sedrak et al., 2003), and also to non-ANA mAbs generated from the same 5 B6.Sle1z congenics. The former control databases comprised of a large number of mAbs with a wide spectrum of non-overlapping, non-nuclear antigen specificities, extracted in an unbiased fashion following a comprehensive screen of all mAbs deposited into the public Ab databases (Liang et al., 2003; Sedrak et al., 2003). One caveat pertaining to the use of these databases relates to the fact that most of these mAbs were not of B6 origin, but had been generated from several other strains, notably BALB/c. In contrast, the second control database of non-ANAs that we used in this study overcame this limitation as they had been generated from the same B6.Sle1z congenic mice as the ANAs.
Table 2.
Immunoglobulin heavy chain usage by B6.Sle1-derived ANAs
| VH gene usaCDR13 |
CDR2 |
CDR3 |
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | mAb1 | GL | Jh | 31 | 32 | 33 | 34 | 35 | 50 | 51 | 52 | a | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 95 | 96 | 97 | 98 | 99 | 100 | a | b | c | d | e | f | i | j | k | 101 | 102 |
| B6.Sle1 z-#1 | 1A1G12a | J558.75.177 | 4 | R | Y | W | L | H | R | I | D | P | N | S | G | A | A | K | Y | N | E | K | F | K | I | P | Y | S | N | Y | G | G | A | M | D | Y | ||||||
| B6.Sle1 z -#2 | 1BD2b | J558.53.146 | 2 | T | Y | W | I | H | N | I | K | P | S | N | G | N | T | N | Y | N | E | N | F | N | K | R | R | Y | N | Y | Y | D | L | D | Y | |||||||
| B6.Sle1 z -#3 | 1CA4c | J558.75.177 | 2 | N | Y | W | I | H | R | I | D | L | N | S | G | G | I | K | Y | N | E | N | F | K | N | E | G | F | D | G | V | D | Y | |||||||||
| 1CB3 | J558.55.149 | 2 | S | Y | W | I | T | D | I | Y | P | G | S | R | S | T | N | Y | N | E | K | F | K | N | S | K | R | I | W | R | N | S | F | D | Y | |||||||
| 1CD3 | 7183.19.36 | 4 | D | F | Y | M | A | H | I | N | Y | D | G | S | N | T | Y | Y | L | D | S | L | K | S | A | Q | L | R | N | Y | A | M | D | Y | ||||||||
| 1CE6 | J558.72.173 | 2 | S | Y | W | M | H | E | I | Y | P | S | D | S | Y | T | N | Y | N | Q | K | F | K | G | R | G | N | L | G | K | V | F | D | Y | ||||||||
| 1CE7 | 36–60.6.70 | 4 | S | Y | Y | W | N | Y | I | S | Y | D | G | S | N | N | Y | N | P | S | L | K | N | D | D | Y | D | Y | D | G | D | G | Y | Y | Y | A | M | D | Y | |||
| B6.Sle1 z -#4 | 1DB2 | J558.26.116 | 2 | D | Y | Y | M | H | D | F | N | P | N | N | D | V | T | S | Y | N | Q | K | F | K | G | R | G | L | W | L | R | R | F | Y | F | L | D | Y | ||||
| 1DC1e | J558.26.116 | 3 | D | N | Y | I | N | D | I | N | A | K | N | G | G | S | R | Y | S | Q | K | F | K | G | E | D | R | D | Y | D | G | G | F | A | Y | |||||||
| 1DC3 | J558.84.190 | 3 | S | Y | G | I | S | E | I | Y | P | R | S | G | N | T | Y | Y | N | E | K | F | K | G | R | A | W | F | A | Y | ||||||||||||
| 1DC5 | J558.83.189 | 4 | N | Y | W | M | N | Q | I | Y | P | G | D | G | D | T | N | Y | N | G | K | F | K | G | H | D | Y | D | G | A | W | F | A | Y | ||||||||
| 1DC7f | J558.26.116 | 3 | D | Y | Y | M | N | D | I | N | P | N | N | G | G | T | S | Y | N | Q | K | F | K | G | S | R | G | N | A | W | F | A | Y | |||||||||
| 1DC9 | J558.42.132 | 1 | G | Y | Y | M | N | E | I | N | P | T | N | G | I | T | T | Y | N | Q | K | F | K | A | K | T | D | V | ||||||||||||||
| 1DE1 | J606.1.79 | 3 | N | Y | W | M | N | Q | I | R | S | D | N | Y | A | T | H | Y | A | E | S | V | K | G | L | I | Q | G | F | A | Y | |||||||||||
| 1DE4 | 7183.19.36 | 4 | D | Y | Y | M | T | N | I | N | Y | D | G | S | I | T | Y | Y | L | D | S | L | K | S | A | Q | L | R | N | Y | A | M | D | Y | ||||||||
| 1DG5d | J558.55.149 | 2 | S | Y | W | I | T | D | I | Y | P | G | S | R | S | T | N | Y | N | E | K | F | K | N | S | K | R | I | W | R | N | S | F | D | Y | |||||||
| B6.Sle1 z -#5 | 1EA9 | J558.72.173 | 2 | S | Y | W | T | H | E | I | D | P | S | D | S | Y | T | N | Y | N | Q | K | F | K | D | R | G | S | S | F | D | Y | ||||||||||
| 1EA10 | J558.53.146 | 2 | S | Y | W | M | H | D | I | N | P | S | N | G | G | T | N | Y | N | E | K | F | K | T | S | S | A | F | I | N | S | F | D | Y | ||||||||
| 1EB2 | J558.67.166 | 4 | T | Y | W | M | H | V | T | H | P | N | S | G | F | T | N | Y | S | G | K | F | K | R | T | N | W | E | R | N | Y | A | M | D | Y | |||||||
| 1EB5 | 7183.20.37 | 4 | D | Y | G | M | H | Y | I | S | S | G | S | S | T | I | Y | Y | A | D | T | V | K | G | R | G | Y | G | Y | A | M | D | Y | |||||||||
| 1EC1 | J558.84.190 | 1 | S | Y | G | I | S | E | I | Y | P | R | S | G | N | T | Y | Y | N | E | K | F | K | G | E | R | G | L | R | R | Y | F | D | V | ||||||||
| 1ED6 | J558.22.112 | 4 | D | Y | N | M | H | Y | I | N | P | N | N | G | G | T | N | Y | N | Q | K | F | K | G | V | N | W | D | A | A | M | D | Y | |||||||||
| 1ED7 | 7183.14.25 | 2 | N | Y | A | M | S | Y | I | S | S | G | G | D | Y | I | Y | Y | G | D | T | V | Q | G | E | G | E | I | E | I | Y | D | Y | |||||||||
| 1ED9 | J558.72.173 | 2 | S | Y | W | M | H | K | I | D | P | S | D | S | Y | T | N | Y | N | Q | K | F | K | G | G | S | S | R | Y | F | D | Y | ||||||||||
| 1EE5g | J558.26.116 | 4 | D | H | Y | V | N | D | I | N | P | N | N | G | G | T | S | Y | N | Q | K | F | K | D | T | W | G | G | T | N | G | M | D | Y | ||||||||
| 1EG4 | SM7.2.49 | 1 | D | Y | Y | M | H | R | I | D | P | E | D | G | E | T | K | Y | A | P | K | F | Q | G | E | P | I | Y | Y | G | Y | H | V | G | Y | F | D | V | ||||
| 1EG8 | Q52.9.29 | 4 | S | Y | G | I | S | V | I | W | T | G | E | G | T | Y | Y | N | S | A | L | K | S | T | Y | S | N | Y | R | T | M | D | Y | |||||||||
| 1EH1 | J558.4.93 | 2 | S | Y | W | M | H | Y | I | N | P | S | S | G | Y | T | K | Y | N | Q | K | F | K | G | T | Y | Y | S | N | Y | F | V | D | Y | ||||||||
| 1EH2 | J558.4.93 | 4 | G | Y | W | M | H | Y | I | N | P | S | T | N | Y | P | N | Y | N | Q | K | F | K | D | S | T | G | A | M | D | Y | |||||||||||
| 1EH4 | J558.85.191 | 3 | S | S | W | M | N | R | I | Y | P | G | D | G | D | T | N | Y | N | G | K | F | K | G | S | E | G | G | Y | R | F | A | Y | |||||||||
| 1EH5 | 7183.19.36 | 2 | D | Y | Y | M | A | N | I | N | Y | D | G | S | S | T | Y | Y | L | D | S | L | K | S | V | K | Y | S | N | Y | D | Y | ||||||||||
| 1EJ5 | J558.67.166 | 2 | S | Y | W | M | F | M | I | P | P | N | S | G | N | T | N | Y | N | E | K | F | K | R | R | N | L | Y | G | T | L | D | Y | |||||||||
1 Footnotes are as listed in Table 1.
Displayed are the HC CDR residues, with mutated residues in bold. H52b and H52c have been omitted as only two Abs had residues at these positions.
Table 3.
Immunoglobulin light chain usage by B6.Sle1-derived ANAs
| V-kappa usage2 |
CDR13 |
CDR2 |
CDR3 |
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | mAb1 | Jk | 24 | 25 | 26 | 27 | a | b | c | d | e | f | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | |
| B6.Sle1 z -#1 | 1A3F1a | 23–48 | 5 | R | A | S | Q | N | V | G | T | N | V | H | S | A | S | K | S | F | S | Q | Q | S | K | T | W | P | M | L | ||||||
| B6.Sle1 z -#2 | 1BD2 b | 12–44 | 5 | Q | A | S | E | N | I | A | S | D | L | P | D | A | K | T | L | A | D | Q | H | Y | Y | G | T | P | L | T | ||||||
| B6.Sle1 z -#3 | 1CA4 c | ai4 | 5 | T | A | R | S | S | V | S | S | S | Y | L | H | R | T | S | N | L | A | S | H | Q | Y | H | R | S | P | L | T | |||||
| 1CB3 | 21–10 | 5 | R | A | S | E | S | V | D | S | Y | G | N | S | F | M | H | L | A | S | N | L | E | S | Q | Q | N | N | E | D | P | L | T | |||
| 1CD3 | ai4 | 2 | T | A | S | S | S | V | S | S | S | S | L | H | S | T | S | I | L | A | S | H | Q | Y | R | R | S | P | P | I | ||||||
| 1CE6 | ||||||||||||||||||||||||||||||||||||
| 1CE7 | ai4 | 2 | T | A | S | S | S | V | T | S | S | R | L | H | A | T | S | N | L | A | S | H | Q | Y | H | R | S | P | P | I | ||||||
| B6.Sle1 z -#4 | 1DA2 e | 19–23 | 1 | K | A | S | Q | D | V | G | T | A | V | A | W | A | T | T | R | H | T | Q | Q | Y | S | S | Y | P | - | T | ||||||
| 1DB2 | ce9 | 2 | R | A | G | Q | D | I | S | N | Y | L | N | F | T | S | K | L | H | S | Q | Q | G | N | S | L | P | Y | T | |||||||
| 1DC1 e | 23–39 | 5 | R | A | S | Q | S | I | S | D | Y | L | H | Y | A | S | Q | S | I | S | Q | N | G | H | S | F | P | L | T | |||||||
| 1DC5 | ai4 | 5 | T | A | R | S | S | V | S | S | S | Y | L | H | S | T | S | N | L | A | S | H | Q | Y | H | R | S | P | L | T | ||||||
| 1DC7 f | ai4 | 2 | T | A | R | S | S | V | S | S | R | Y | L | H | S | T | S | S | L | A | S | H | Q | Y | H | R | S | P | Y | T | ||||||
| 1DC9 | 21-2 | 4 | R | A | S | E | S | V | D | N | Y | G | I | S | F | M | D | A | A | S | N | Q | R | S | Q | Q | T | K | E | I | P | F | T | |||
| 1DD10f | 19-15 | 2 | K | A | S | Q | N | V | G | T | N | V | A | S | A | S | Y | R | Y | S | Q | Q | Y | N | S | Y | P | Y | T | |||||||
| 1DE1 | cr1 | 1 | R | S | S | Q | S | I | V | H | S | N | G | N | T | Y | L | E | K | V | S | N | R | F | S | F | Q | G | S | H | V | P | W | T | ||
| 1DE4 | ai4 | 2 | T | A | S | S | S | V | T | S | S | R | L | H | A | T | S | N | L | A | S | H | Q | Y | H | R | S | P | P | I | ||||||
| B6.Sle1 z -#5 | 1EA9 | ai4 | 2 | R | A | S | E | N | I | Y | S | Y | L | T | S | T | S | N | L | A | S | L | Q | Y | H | R | S | P | Y | T | ||||||
| 1EA10 | 12–44 | 5 | T | A | S | S | S | V | R | S | S | Y | L | H | N | A | K | T | L | A | D | Q | H | H | F | G | T | P | L | T | ||||||
| 1EB2 | 23–39 | 5 | R | A | S | Q | S | I | S | D | Y | L | H | Y | A | S | Q | S | I | S | Q | N | G | H | S | F | P | L | T | |||||||
| 1EB5 | ai4 | 2 | T | A | S | S | S | V | R | S | I | Y | L | Y | N | T | S | N | L | T | S | H | Q | H | H | R | S | P | Y | T | ||||||
| 1EC1 | bb1 | 5 | R | S | S | Q | S | L | V | H | S | N | G | N | T | Y | L | H | K | V | S | N | R | F | S | S | Q | S | T | H | V | P | L | T | ||
| 1ED6 | 23–39 | 1 | R | A | S | Q | S | I | S | D | Y | L | H | Y | A | S | Q | S | I | S | Q | N | G | H | S | F | P | P | T | |||||||
| 1ED7 | at4 | 1 | S | A | S | S | S | V | S | Y | M | Y | D | T | S | N | L | A | S | Q | Q | W | S | S | Y | P | W | T | ||||||||
| 1ED9 | 23–43 | 2 | R | A | S | Q | S | I | S | N | N | L | H | Y | A | S | Q | S | I | S | Q | Q | S | N | S | W | Y | - | T | |||||||
| 1EE5g | ai4 | 2 | T | A | R | L | S | V | R | S | S | Y | L | H | S | T | S | S | L | A | S | H | Q | C | H | R | S | P | Y | T | ||||||
| 1EG4 | 12–44 | 4 | R | A | S | E | N | I | Y | S | F | L | A | N | A | K | T | L | A | E | H | H | H | Y | G | T | P | F | T | |||||||
| 1EG8 | kn4 | 1 | S | A | S | S | S | I | S | Y | M | H | D | T | S | K | L | A | S | H | Q | R | S | S | C | P | W | T | ||||||||
| 1EH1 | bb1 | 4 | R | S | S | Q | S | L | V | H | S | N | G | N | T | Y | L | H | K | V | S | N | R | F | S | S | Q | S | T | H | V | T | W | T | ||
| 1EH2 | bb1 | 2 | R | S | S | Q | S | L | L | H | S | N | G | N | T | Y | L | H | K | V | S | N | R | F | S | S | Q | S | T | H | V | P | Y | T | ||
| 1EH4 | ||||||||||||||||||||||||||||||||||||
| 1EH5 | 8–21 | 4 | K | S | S | Q | S | L | L | N | S | R | T | R | K | N | Y | L | A | W | A | S | T | R | E | S | K | Q | S | Y | N | L | F | - | T | |
| 1EJ5 | bv9 | 1 | R | A | S | Q | D | I | G | S | S | L | N | A | T | S | S | L | D | S | L | Q | Y | A | T | A | W | - | T | |||||||
1 The entries for 1EH4, 1CE6, and clone (d) have been omitted as these mAbs used λ LCs. All other details are as listed in Table 1.
3.2 Ig LC usage by B6.Sle1z ANAs
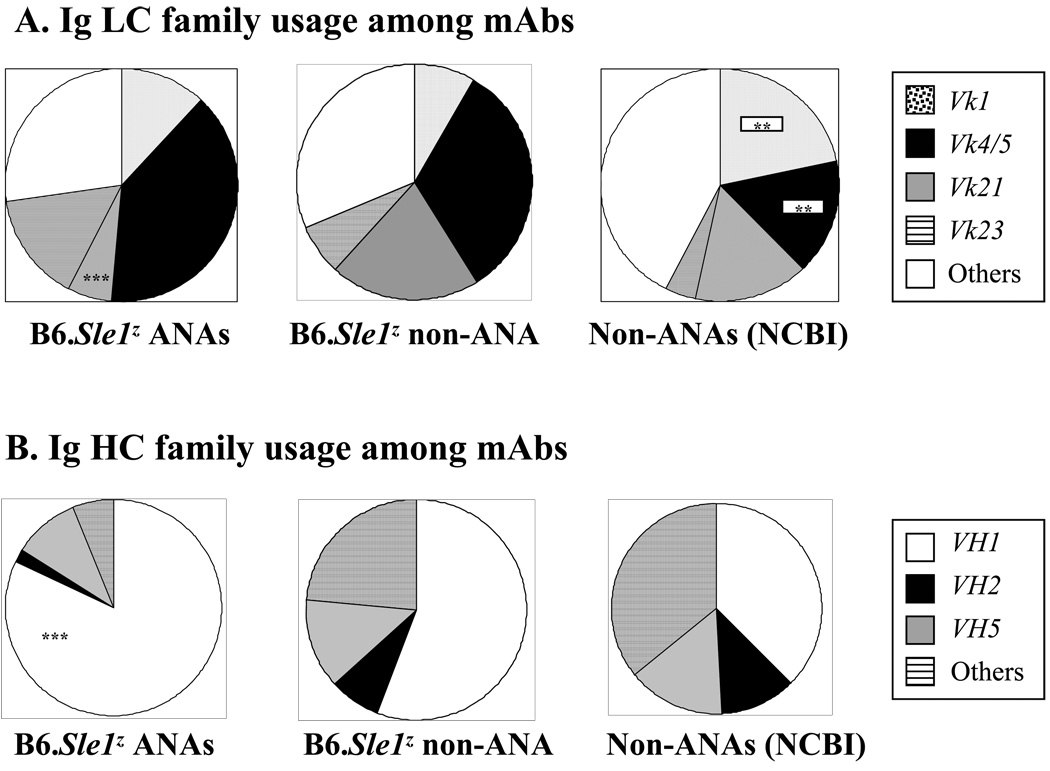
When the Ig Vk repertoire of B6.Sle1z-derived ANAs were compared to the NCBI database of non-ANA control Abs (Liang et al., 2003), ANAs exhibited a significant expansion of 2 Vk families – Vk4/5 and Vk23, and this was totally attributable to the increased usage of 2 particular Vk germline genes, Vk ai4 (33.3% vs. 3.4%, P <0.001), and Vk 23–39 (9.1% vs. 0.7%, P <0.05) (summarized in Fig. 1A, and Table 3). In contrast, B6.Sle1z-derived ANAs and non-ANAs isolated from the same set of 5 mice did not differ significantly in Vk usage, with one exception - ANAs exhibited significantly reduced frequencies of Vk21, compared to both groups of non-ANAs (Fig. 1A), and this might relate to the reported role of this LC in vetoing DNA-binding (Li et al., 2001). Finally, it was intriguing that both the ANAs and non-ANAs derived from the B6.Sle1z congenics exhibited increased usage of Vk4/5, and reduced frequencies of Vk1, compared to the NCBI-derived non-ANAs, possibly reflecting the contribution of strain-specific factors.
Figure 1. Ig HC and LC V-gene usage by the different groups of antibodies examined in this study A.
The Ig Vk LC gene family usage of B6.Sle1z-derived nuclear antigen reactive mAbs (as listed in Table 1) was compared to that of non-ANAs derived from the same strain or non-ANAs assembled from the NCBI/Genbank database, as described previously (Liang et al., 2003). In all 3 Ab groups, clonal replicates were represented by one member each; this resulted in a final panel of 32 ANAs, 73 non-ANA, and 145 non-ANA mAb LC sequences, respectively. Where ANAs differed significantly from B6.Sle1z non-ANAs (as determined using Chi squared test) this is indicated within the left-most pie; where the NCBI/Genbank-derived mAbs differed from the B6. Sle1z-derived mAbs, this is indicated in the right-most pie (**, P < 0.01; ***, P < 0.001).
B. The Ig VH gene family usage of B6.Sle1z-derived nuclear antigen reactive mAbs (as listed in Table 1) was compared to that of non-ANAs derived from the same strain or non-ANAs assembled from the NCBI/Genbank database, as derived previously (Sedrak et al., 2003). In all 3 Ab groups, clonal replicates were represented by one member each; this resulted in a final panel of 32 ANAs, 58 non-ANAs and 165 non-ANA mAb HC sequences, respectively. Where ANAs differed significantly from the non-ANAs (as determined using Chi sequared test) this is indicated within the left-most pie (***, P < 0.001). No other differences reached statistical significance.
Next, when the residue usage frequencies among B6.Sle1z-derived ANA LCs were compared against the NCBI-derived non-ANAs, the ANAs exhibited increased frequencies of “T” at L24, “R” at H26, “R” at L27c, “S” at L27d, “H” at L89, and “R” at L93 (data not shown). However, all of these differences were accounted for by the differential usage of Vk family/germline genes (notably Vk4/5 and Vk1) between these 2 sets of Abs, as noted above. In contrast, no residue usage differences emerged when the LCs of B6.Sle1z–derived ANAs and non-ANA mAbs were compared (data not shown). These observations resonate well with the reported absence of any statistically significant molecular signatures in previously reported ANA LC sequence comparison studies (Chen et al., 2002; Liang et al., 2003).
3.3 Ig HC usage and sequence motifs by B6.Sle1z ANAs
With respect to the Ig HC usage profiles, the ANAs exhibited significantly increased usage of VH1/J558 HC genes compared to both control groups of non-ANAs, with this increased frequency being compensated by reduced usage frequencies of most other VH family members (Fig. 1B). The ANA HCs did not differ significantly from the non-ANAs in terms of their CDR3 lengths or frequencies of D:D fusions (data not shown). Most HC residues were germline-encoded, with the frequencies of mutations being similar among the ANAs and non-ANAs (Table 4). When residue usage frequencies across the HC CDR and FR positions in the three Ab databases were compared, three different sets of molecular differences of statistical significance emerged, all confined to the CDR regions.
Table 4.
Frequency of non-germline amino acid residues in B6.Sle1z-derived ANAs and non-ANA control Abs
| Total number of clonally independent HCs |
Frequency of mutations in HC1 |
Frequency of mutations in HC CDR1 |
Frequency of mutations in HC CDR2 |
|
|---|---|---|---|---|
| B6.Sle1z ANAs | 32 | 7.5% | 16.4% | 13.4% |
| B6.Sle1z- non-ANAs | 58 | 5.9% | 10.9% | 12.0% |
| NCBI- non-ANAs | 165 | 7.2% | 15.7% | 13.0% |
Indicated are the frequencies of amino-acid residues that are non-germline, when compared to the NCBI/IgBlast database.
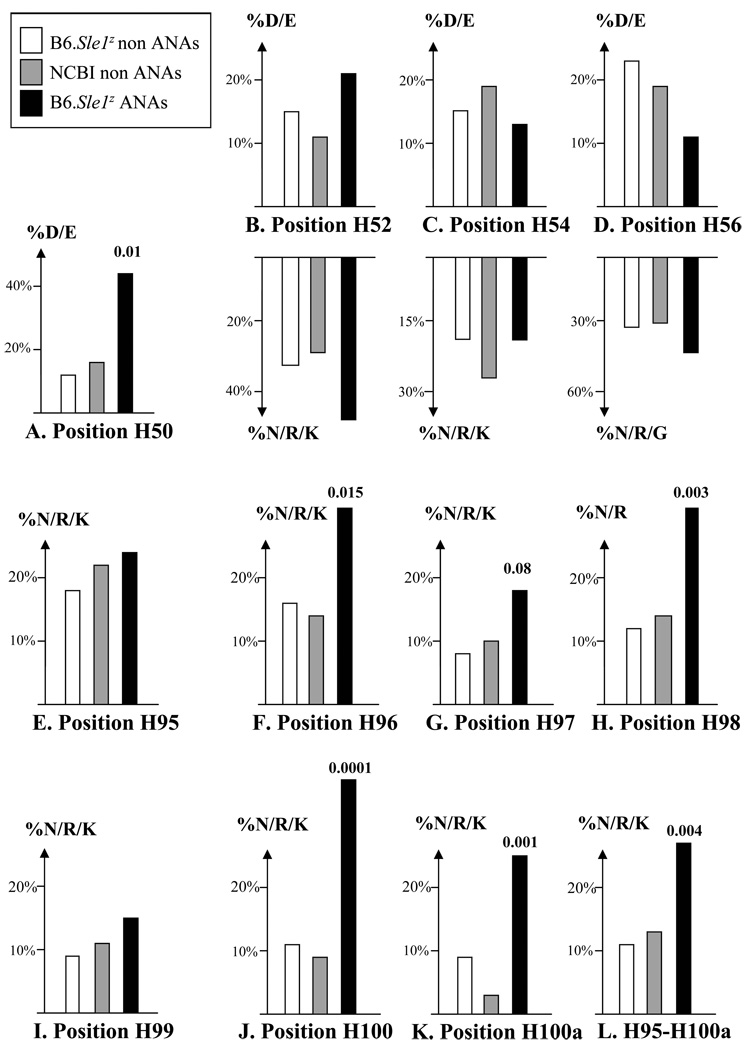
First, B6.Sle1z-derived ANA HCs exhibited significantly increased usage of cationic residues, notably “R”, in their HC CDR3 regions, compared to both databases of non-ANAs, with these differences becoming statistically significant at several positions, including H96, H97, H98, H100 and H100a (Fig. 2). Indeed, three quarters of the ANAs exhibited at least one cationic residue in their CDR3 regions (referred to in this manuscript as motif “A”), compared to ~ 50% of non-ANAs, with this difference being statistically significant (Table 2). The presence of this motif in these Abs conferred a 1.4 fold increased “risk” of nuclear antigen reactivity (Fig. 3). It was intriguing that positions H96, H98, H100 and H100a exhibited the highest peaks of cationicity within the HC CDR3 regions, with the intervening positions H95, H97 and H99 showing relatively lower increases in N/R/K usage (Fig. 2), a sequence pattern that resembles the cationicity profiles previously reported among NZM2410-derived ANAs (Liang et al., 2004). Somatic recombination with N-nucleotide addition accounted for 74% of all CDR3 “R” residues among the ANA HCs (data not shown).
Figure 2. The frequencies of polar/charged residues in the HC CDR2 and CDR3 regions of B6.Sle1z monoclonal ANAs and non-ANA controls derived from 2 sources.
Depicted are the respective frequencies of the indicated amino acid residues at the HC CDR2 positions, H50, H52, H54, H56 (A–D), and CDR3 positions H95-H100a (E–L), observed among B6.Sle1z derived ANAs (as listed in Table 1) and non-ANAs, as well as NCBI/Genbank derived non-ANA control Abs (Sedrak et al., 2003). In all 3 Ab groups, clonal replicates were represented by one member each; this resulted in a final panel of 32 B6.Sle1z ANAs, 58 B6.Sle1z non-ANAs, and 165 non-ANA mAb HC sequences from NCBI/Genbank. Indicated P-values pertain to Chi-square test or Fisher’s exact test comparisons of ANAs against both groups of non-ANAs, pooled.
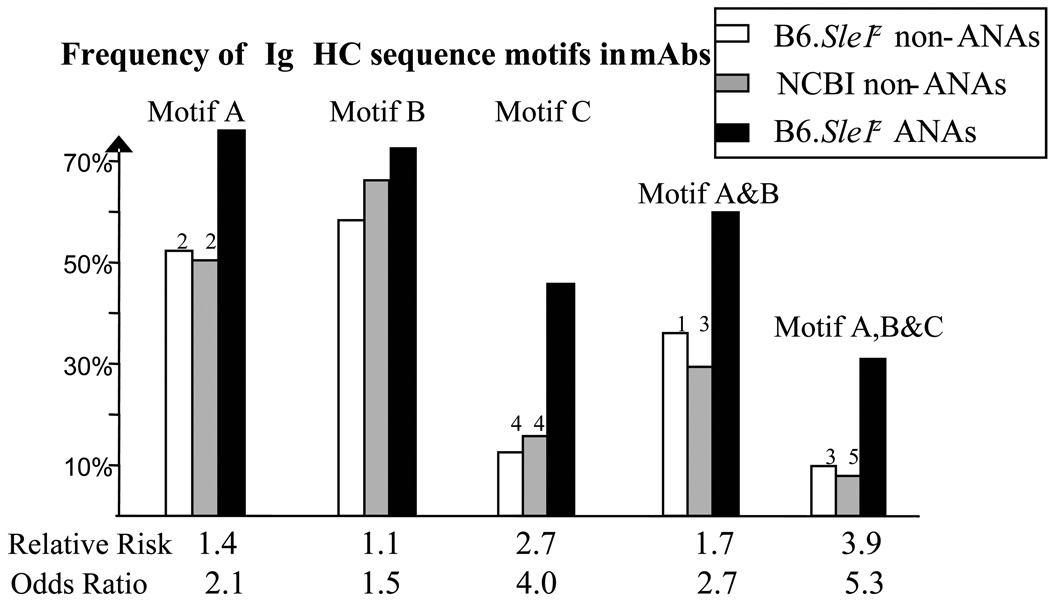
Figure 3. The frequencies of ANA-associated HC sequence motifs, “A”, “B”, and “C” among B6.Sle1z monoclonal ANAs and non-ANA controls.
The ANA and 2 non-ANA control datasets of Ig HCs detailed in Fig. 1B and Table 1 were examined for the frequencies of motif A (i.e., presence of at least 1 N/R/K residue within CDR3 H95-H100a), motif B (i.e., the absence of D/E residues across H52-H56), or motif C (i.e., D residue at H50) in the HC sequences. Clonal replicates have been removed from all datasets. Indicated P-values pertain to Chi-square test or Fisher’s exact test comparisons of each non-ANA control against the ANAs (1, P < 0.05; 2, P < 0.01; 3, P < 0.001; 4, P < 0.0001; 5, P < 0.00001). Indicated below each motif is the relative risk and odds ratio pertaining to the likelihood of the Ab being nuclear-antigen reactive if it were to possess that particular motif or combination of motifs. Note: the co-presence of motifs A and C, or motifs B and C, did not significantly increase the likelihood of an AB being nuclear antigen reactive; hence these combinations are not plotted.
Second, ANAs tended to use less anionic residues and more cationic residues within H52-H56. The frequency differences of charged residues at each of these positions were not significant when the different Ab databases were compared (Fig. 2); indeed the presence of motif “B” (defined as the absence of D/E residues across H52-H56) by itself did not confer significantly increased risk towards being nuclear-antigen reactive (Fig. 3). However, when one considered the co-expression of both motifs, A and B (i.e., HC with cationic CDR3 motifs and also non-anionic CDR2 regions), the “relative risk” of being nuclear antigen reactive increased to 1.7 (Fig. 3).
A third motif, “C”, that distinguished ANAs from both sets of non-ANA HCs was the increased presence of “D” residues at H50 in CDR2, conferring 2.7-fold increased “risk” towards nuclear antigen binding (Fig. 3). Importantly, the co-ordinate presence of all 3 HC sequence motifs, A, B, and C, rendered the Abs 3.9-fold more likely to be nuclear-antigen reactive, with an odds ratio exceeding 5 (Fig. 3). A fourth pattern, the increased presence of charged residues at H31 in CDR1 among the ANAs, did not attain statistical significance, and hence was not pursued further. To sum, 3 sequence motifs, distinguished ANA HCs from non-ANA HCs, particularly when acting in concert. None of the above motif differences were the result of any potential data-skewing by expanded Ab clones, since all multi-member clonal families were represented only once, before executing these comparisons.
4. Discussion
Whereas most other lupus-prone strains exhibit high levels of anti-dsDNA Abs (and other Ab specificities), the serology in B6.Sle1z congenic mice is rather unique in being skewed towards recognizing nucleosomes, as a consequence of polymorphisms in Ly108 that infringe B-cell tolerance (Kumar et al., 2006). This gave us the unique opportunity to define the molecular signatures of anti-nuclear Abs arising in this strain, compared to non-ANAs derived from the same congenics. Consistent with previous reports in the literature, the present work has uncovered several signatures associated with nuclear antigen reactivity. In addition, signature motifs not previously described in the ANA literature have also emerged from these studies.
The presence of “R” residues in HC CDR3 (i.e., motif A) is perhaps the most commonly reported molecular signature among ANAs, particularly at H96 and H98-H100a (Chen et al., 2002; Eilat and Anderson, 1994; Kieber-Emmons et al., 1994; Koelsch et al., 2007; Koutouzov et al., 1997; Liang et al., 2004; Marion et al., 1992; Radic and Weigert, 1994; Shlomchik et al., 1990), further bolstered by structural studies (Barry et al., 1994; Cygler et al., 1987; Herron et al., 1991; Mol et al., 1994; Pokkuluri et al., 1994; Seeman et al., 1976). Our observation that “R” residues in ANA HCs reach the highest usage frequencies at H96, H98, H100, and H100a in CDR3, and the finding that they are necessary for maximal anti-nuclear reactivity resonate well with previously published reports (Chen et al., 2002; Eilat and Anderson, 1994; Martin et al., 1994; Radic and Seal, 1997; Radic and Weigert, 1994; Wloch et al., 1997). Interestingly, anti-dsDNA ANAs have been reported to exhibit relatively lower increases of “R” usage at H97, as well as H99 (Chen et al., 2002; Krishnan et al., 1996; Krishnan and Marion, 1998; Liang et al., 2004; Tillman et al., 1992). Krishnan et al. have suggested that an “R” residue at H97 might actually destabilize key structural components of the antigen-binding groove, thus precluding dsDNA reactivity (Krishnan et al., 1996; Krishnan and Marion, 1998). Taking all of the published and current findings together, “R” at H96, H98, H100-100a in HC CDR3 appear to be particularly important for DNA-binding, while “R” at the intervening positions may serve to preferentially augment nucleosome-binding (over DNA-binding).
Charged residues have also been previously shown to be important in HC CDR2 regions of ANAs (Chen et al., 2002; Katz et al., 1994; Radic et al., 1993; Radic and Seal, 1997). Of note, deliberate mutagenesis of “D” at H56 to “R” profoundly enhanced DNA binding (Li et al., 2001). Conversely, “D” residues at these positions have been suggested to augment nucleosome/histone binding (Chen et al., 2002; Monestier, 1991; Monestier et al., 1993). Given the modest “relative risk” this motif alone contributes towards nuclear antigen binding (Fig. 3), we postulate that this motif might influence nuclear antigen binding most effectively when operating in concert with motif A.
In contrast to the above 2 motifs, no information is available in the literature concerning how motif “C” (i.e., “D” residues at H50) might impact anti-nuclear reactivity. In reviewing the origins of “D” at this position, they appear to be largely germline-encoded among the ANAs, though somatic mutation appears to contribute in part (Table 2, Table 4). Besides directly influencing nuclear antigen contact, it is also possible that the “D” residue at H50 might indirectly influence nuclear antigen specificity through potential intra-molecular salt bridges that impact the 3-dimensional conformation of the antigen binding pocket.
A further point of interest is the tremendous degree of clonal expansion noted among the ANAs in B6.Sle1z mice. Indeed, 2 of the 5 mice studied exhibited a monoclonal ANA repertoire, with the remaining mice showing evidence of oligoclonal expansions. Importantly, the ANA-associated HC sequence motifs described in this communication are over-expressed among the expanded ANA-secreting clones within these mice. Whereas the expanded ANA clones d, e, and f exhibited all 3 motifs and bound nucleosomes predominantly, clone b that exhibited motifs A and B, but not “D” at H50, bound DNA in addition to nucleosomes (Table 1 and Table 2). An examination of the remaining expanded ANA clones indicates that additional molecular events (besides motifs A, B, and C) may also be playing a role in shaping the anti-nuclear Ab repertoire in lupus, an observation that warrants future study.
Collectively, these studies indicate that cationic CDR3 residues, non-anionic CDR2 regions, and an increased frequency of aspartate residues at H50 constitute 3 specific Ig HC sequence motifs that may be critical for nucleosome and/or DNA binding. Site-directed mutagenesis to further validate these predictions is clearly warranted. Further studies aimed at defining the Ig HC (and LC) residues that dictate whether ANAs bind nucleosomes or dsDNA preferentially are also in order. Finally, it would be important to elucidate if the molecular signatures described in this communication are already inscribed in the primary Ig repertoire, possibly as a consequence of defects in early B-cell tolerance, as suggested by leads from recent genetic studies (Kumar et al., 2006). If it indeed becomes established that the V-gene usage repertoire (either at the naïve B-cell level, or as reflected by the expressed Abs) is significantly different in B6.Sle1z mice compared to B6 controls, we can be confident that these differences are not due to structural or regulatory differences at the Ig VH gene locus, because both strains are identical at this locus. Rather, these findings would imply that the Ig gene usage repertoire differences observed in lupus evolve as a consequence of B-cell selection events, such as those impacted by the key disease gene within the Sle1z locus, Ly108 (Kumar et al., 2006).
Acknowledgements
This work was supported by grants from the NIH (AR44894). We acknowledge the technical assistance provided by Kelvin Hsu.
References
- Barry MM, Mol CD, Anderson WF, Lee JS. Sequencing and modeling of anti-DNA immunoglobulin Fv domains. Comparison with crystal structures. J Biol Chem. 1994;269:3623–3632. [PubMed] [Google Scholar]
- Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chang S, Mohan C. Molecular signatures of antinuclear antibodies-contributions of heavy chain CDR residues. Mol Immunol. 2002;39:333–347. doi: 10.1016/s0161-5890(02)00110-4. [DOI] [PubMed] [Google Scholar]
- Cygler M, Boodhoo A, Lee JS, Anderson WF. Crystallization and structure determination of an autoimmune anti-poly(dT) immunoglobulin Fab fragment at 3.0 A resolution. J Biol Chem. 1987;262:643–648. [PubMed] [Google Scholar]
- Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Eilat D, Anderson WF. Structure-function correlates of autoantibodies to nucleic acids. Lessons from immunochemical, genetic and structural studies. Mol Immunol. 1994;31:1377–1390. doi: 10.1016/0161-5890(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Fitzsimons MM, Chen H, Foster MH. Diverse endogenous light chains contribute to basement membrane reactivity in nonautoimmune mice transgenic for an anti-laminin Ig heavy chain. Immunogenetics. 2000;51:20–29. doi: 10.1007/s002510050004. [DOI] [PubMed] [Google Scholar]
- Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- Haines BB, Angeles CV, Parmelee AP, McLean PA, Brodeur PH. Germline diversity of the expressed BALB/c VhJ558 gene family. Mol Immunol. 2001;38:9–18. doi: 10.1016/s0161-5890(01)00049-9. [DOI] [PubMed] [Google Scholar]
- Herron JN, He XM, Ballard DW, Blier PR, Pace PE, Bothwell AL, Voss EW, Jr, Edmundson AB. An autoantibody to single-stranded DNA: comparison of the three-dimensional structures of the unliganded Fab and a deoxynucleotide-Fab complex. Proteins. 1991;11:159–175. doi: 10.1002/prot.340110302. [DOI] [PubMed] [Google Scholar]
- Ibrahim SM, Weigert M, Basu C, Erikson J, Radic MZ. Light chain contribution to specificity in anti-DNA antibodies. J Immunol. 1995;155:3223–3233. [PubMed] [Google Scholar]
- Kantor AB, Merrill CE, Hillson JL. Construction of cDNA from single unstimulated mouse B lymphocytes: method and application to the study of expressed antibody repertoire in FACS-sorted murine B cell subsets. Blackwell Scientific; 1996. [Google Scholar]
- Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber-Emmons T, Foster MH, Williams WV, Madaio MP. Structural properties of a subset of nephritogenic anti-DNA antibodies. Immunol Res. 1994;13:172–185. doi: 10.1007/BF02918278. [DOI] [PubMed] [Google Scholar]
- Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Koutouzov S, Jovelin F, Brard F, Raux G, Tron F, Gilbert D. Comparison of structural characteristics of antisubnucleosome and anti-DNA monoclonal antibodies derived from lupus mice. Ann N Y Acad Sci. 1997;815:327–330. doi: 10.1111/j.1749-6632.1997.tb52076.x. [DOI] [PubMed] [Google Scholar]
- Krishnan MR, Jou NT, Marion TN. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol. 1996;157:2430–2439. [PubMed] [Google Scholar]
- Krishnan MR, Marion TN. Comparison of the frequencies of arginines in heavy chain CDR3 of antibodies expressed in the primary B-cell repertoires of autoimmune-prone and normal mice. Scand J Immunol. 1998;48:223–232. doi: 10.1046/j.1365-3083.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus: current concepts and continuing controversies. Arthritis Rheum. 1996;39:894–903. doi: 10.1002/art.1780390605. [DOI] [PubMed] [Google Scholar]
- Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- Liang Z, Chen C, Mohan C. Molecular signatures of anti-nuclear antibodies: contributions of specific light chain residues and a novel New Zealand Black V kappa 1 germline gene. J Immunol. 2003;171:3886–3894. doi: 10.4049/jimmunol.171.7.3886. [DOI] [PubMed] [Google Scholar]
- Liang Z, Xie C, Chen C, Kreska D, Hsu K, Li L, Zhou XJ, Mohan C. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J Exp Med. 2004;199:381–398. doi: 10.1084/jem.20030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion TN, Tillman DM, Jou NT, Hill RJ. Selection of immunoglobulin variable regions in autoimmunity to DNA. Immunol Rev. 1992;128:123–149. doi: 10.1111/j.1600-065x.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Martin T, Crouzier R, Weber JC, Kipps TJ, Pasquali JL. Structure-function studies on a polyreactive (natural) autoantibody. Polyreactivity is dependent on somatically generated sequences in the third complementarity-determining region of the antibody heavy chain. J Immunol. 1994;152:5988–5996. [PubMed] [Google Scholar]
- Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol CD, Muir AK, Cygler M, Lee JS, Anderson WF. Structure of an immunoglobulin Fab fragment specific for triple-stranded DNA. J Biol Chem. 1994;269:3615–3622. [PubMed] [Google Scholar]
- Monestier M. Variable region genes of anti-histone autoantibodies from a MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1991;21:1725–1731. doi: 10.1002/eji.1830210721. [DOI] [PubMed] [Google Scholar]
- Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- Paul E, Lutz J, Erikson J, Carroll MC. Germinal center checkpoints in B cell tolerance in 3H9 transgenic mice. Int Immunol. 2004;16:377–384. doi: 10.1093/intimm/dxh035. [DOI] [PubMed] [Google Scholar]
- Pisetsky DS. Anti-DNA and autoantibodies. Curr Opin Rheumatol. 2000;Vol. 12:364–368. doi: 10.1097/00002281-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Pokkuluri PR, Bouthillier F, Li Y, Kuderova A, Lee J, Cygler M. Preparation, characterization and crystallization of an antibody Fab fragment that recognizes RNA. Crystal structures of native Fab and three Fab-mononucleotide complexes. J Mol Biol. 1994;243:283–297. doi: 10.1006/jmbi.1994.1654. [DOI] [PubMed] [Google Scholar]
- Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–4977. [PubMed] [Google Scholar]
- Radic MZ, Seal SN. Selection of recurrent V genes and somatic mutations in autoantibodies to DNA. Methods. 1997;11:20–26. doi: 10.1006/meth.1996.0383. [DOI] [PubMed] [Google Scholar]
- Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- Sedrak P, Hsu K, Mohan C. Molecular signatures of anti-nuclear antibodies--contribution of heavy chain framework residues. Mol Immunol. 2003;40:491–499. doi: 10.1016/s0161-5890(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Seeman NC, Rosenberg JM, Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976;73:804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Xie C, Chang S, Zhou XJ, Tedder T, Mohan C. CD19 hyperexpression augments Sle1-induced humoral autoimmunity but not clinical nephritis. Arthritis Rheum. 2007;56:3057–3069. doi: 10.1002/art.22825. [DOI] [PubMed] [Google Scholar]
- Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Spatz L, Saenko V, Iliev A, Jones L, Geskin L, Diamond B. Light chain usage in anti-double-stranded DNA B cell subsets: role in cell fate determination. J Exp Med. 1997;185:1317–1326. doi: 10.1084/jem.185.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Boekel E, Melchers F, Rolink A. The status of Ig loci rearrangements in single cells from different stages of B cell development. Int Immunol. 1995;7:1013–1019. doi: 10.1093/intimm/7.6.1013. [DOI] [PubMed] [Google Scholar]
- Tillman DM, Jou NT, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB × NZW)F1 mice. J Exp Med. 1992;176:761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloch MK, Clarke SH, Gilkeson GS. Influence of VH CDR3 arginine and light chain pairing on DNA reactivity of a bacterial DNA-induced anti-DNA antibody from a BALB/c mouse. J Immunol. 1997;159:6083–6090. [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]