Abstract
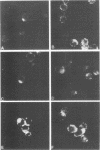
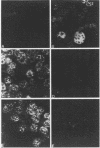
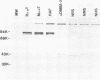
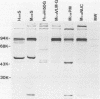
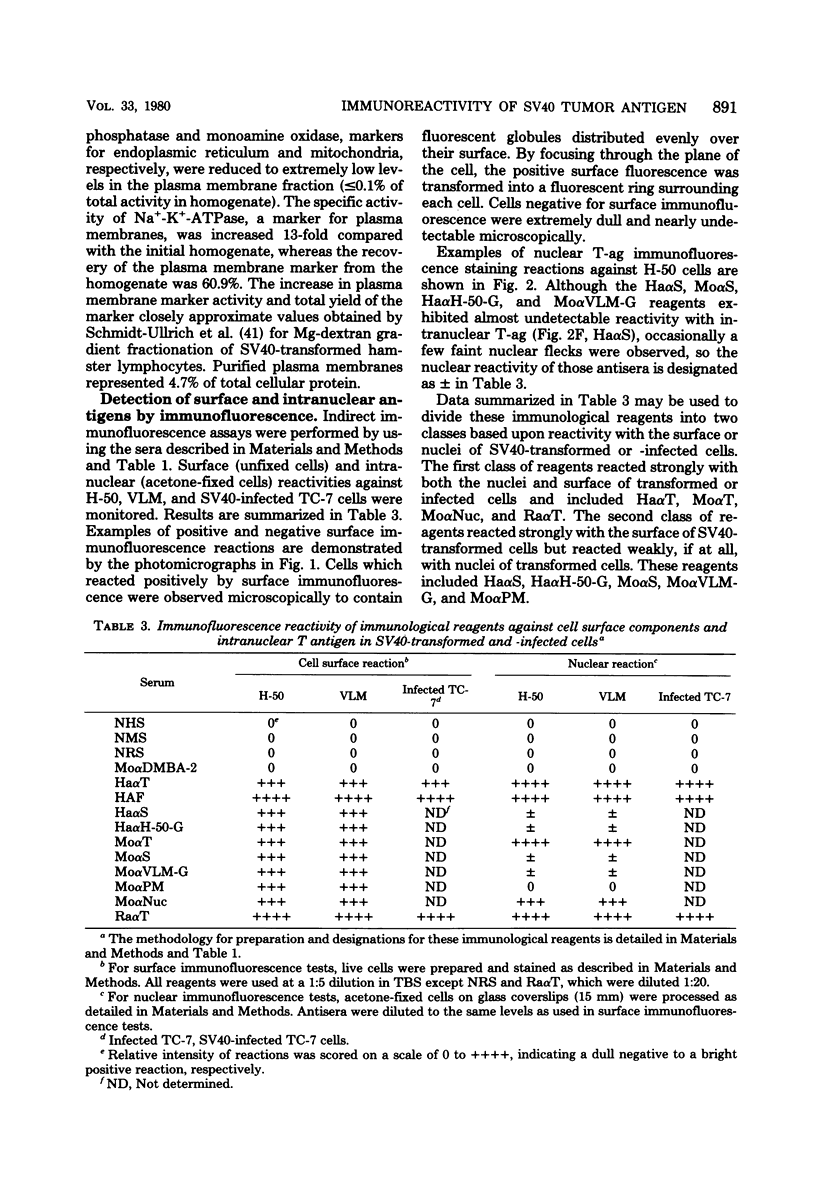
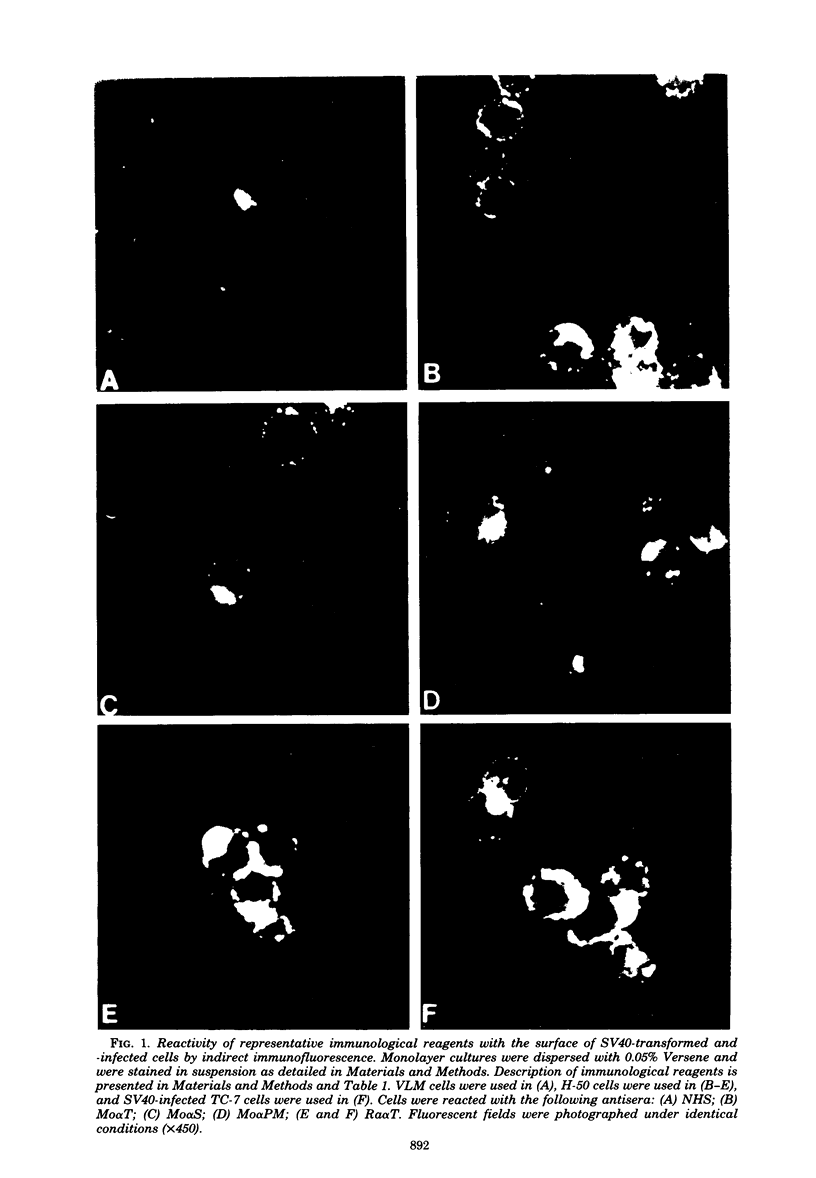
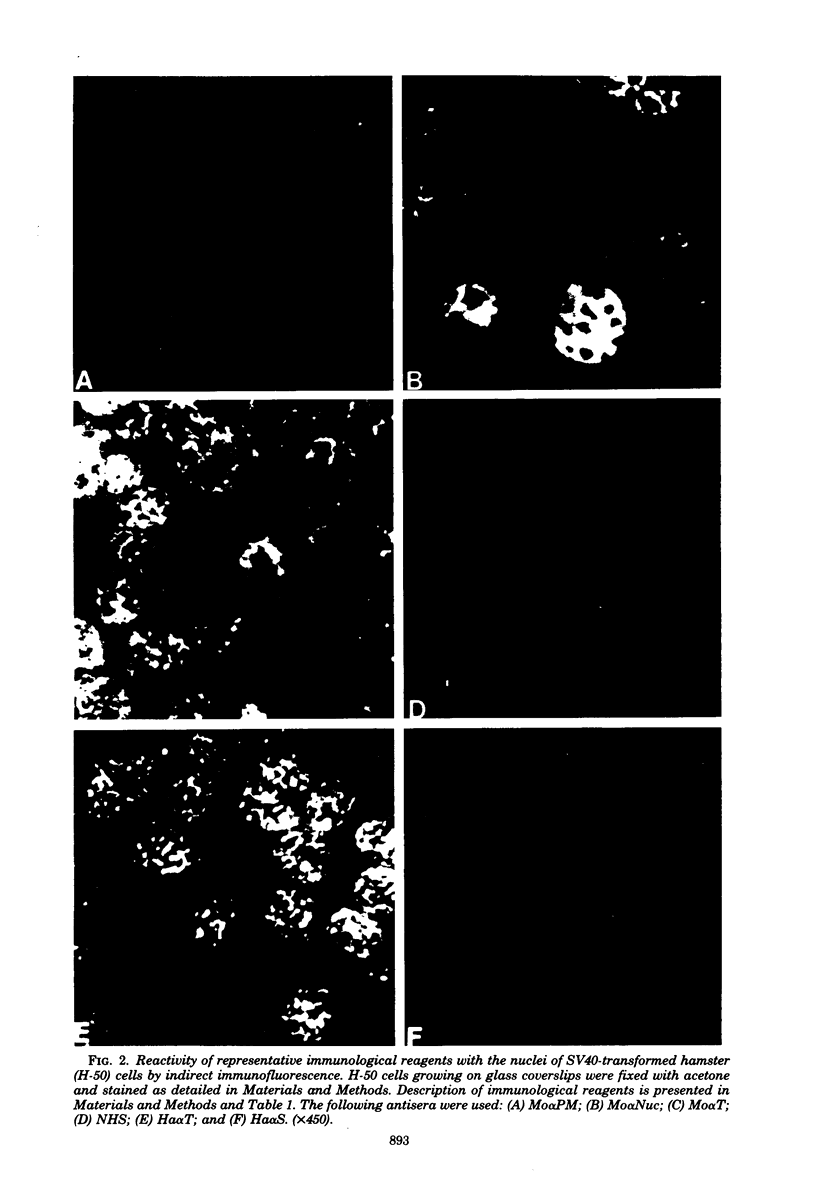
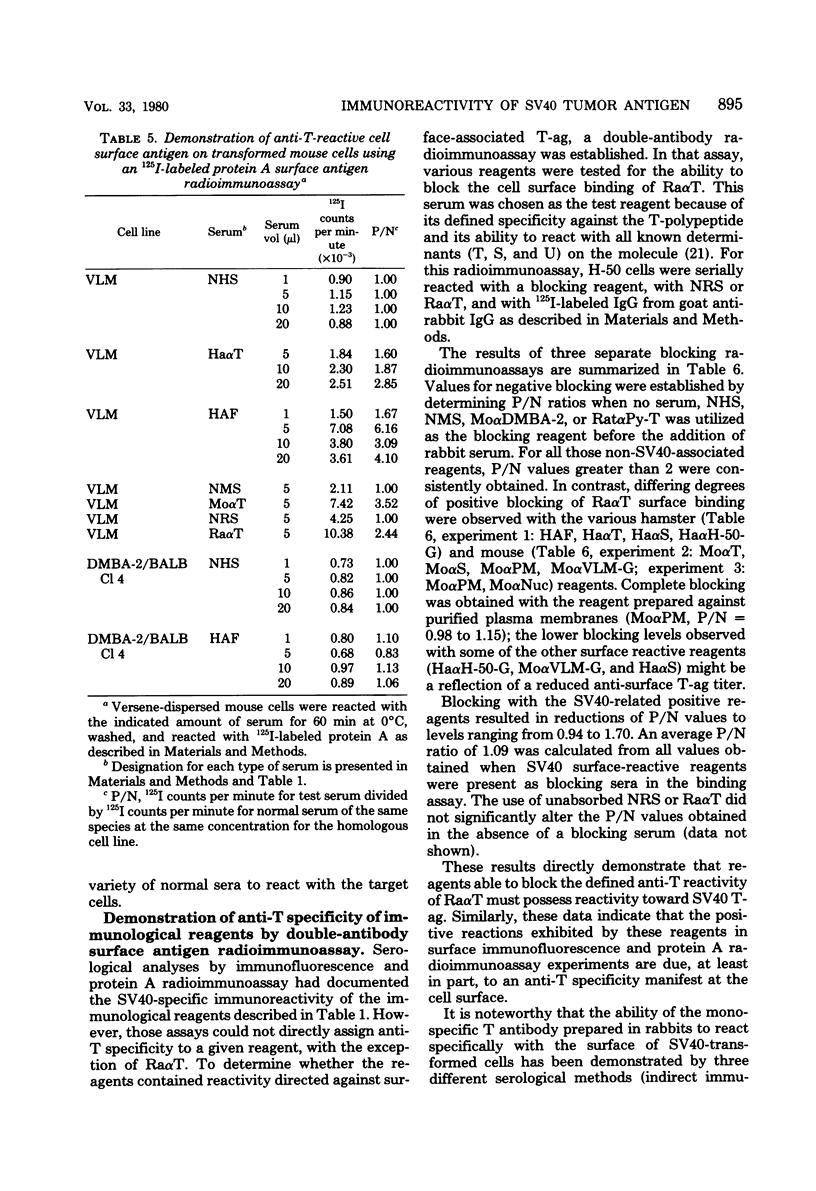
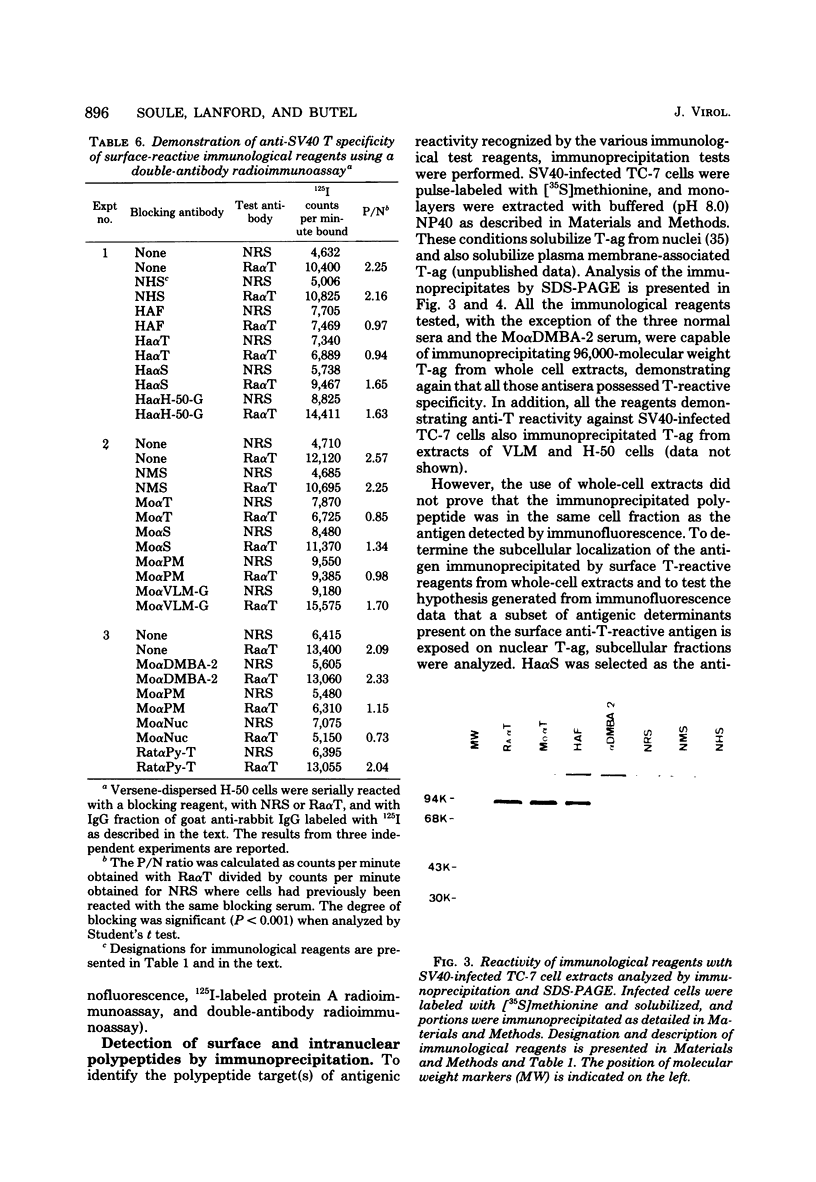
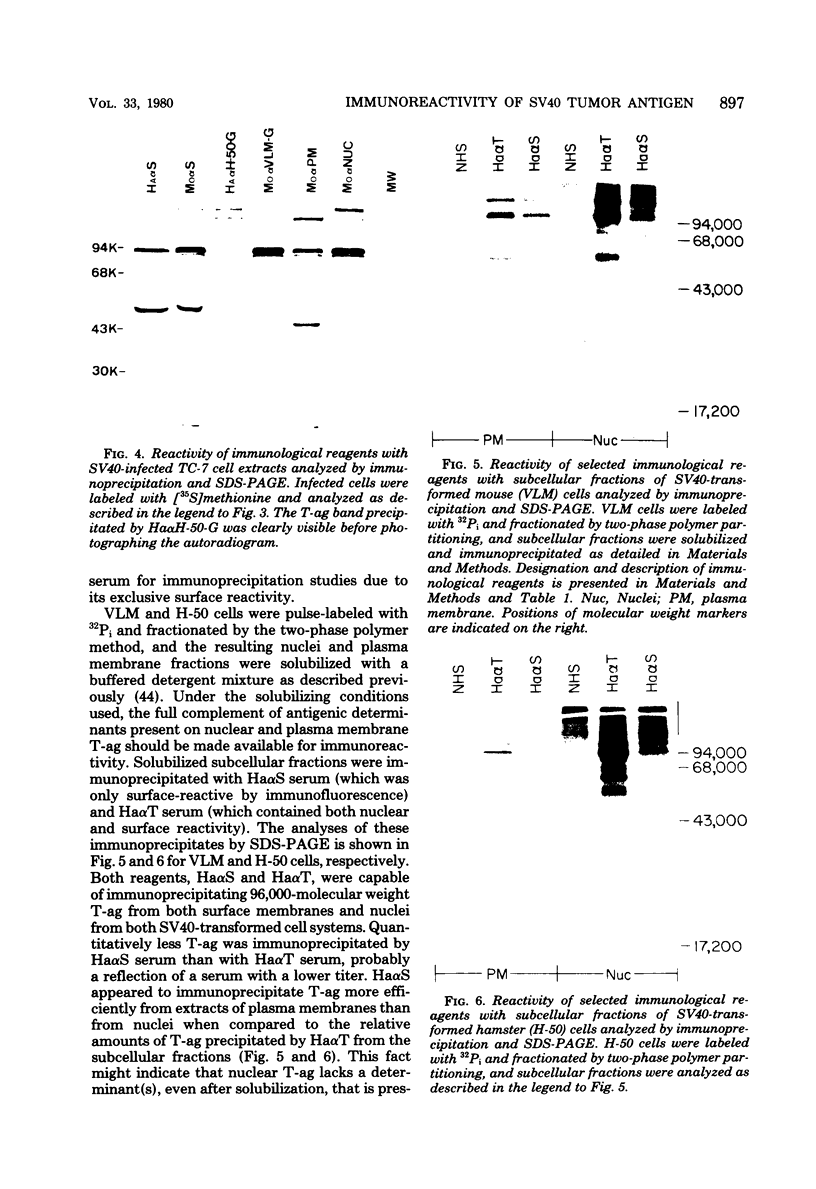
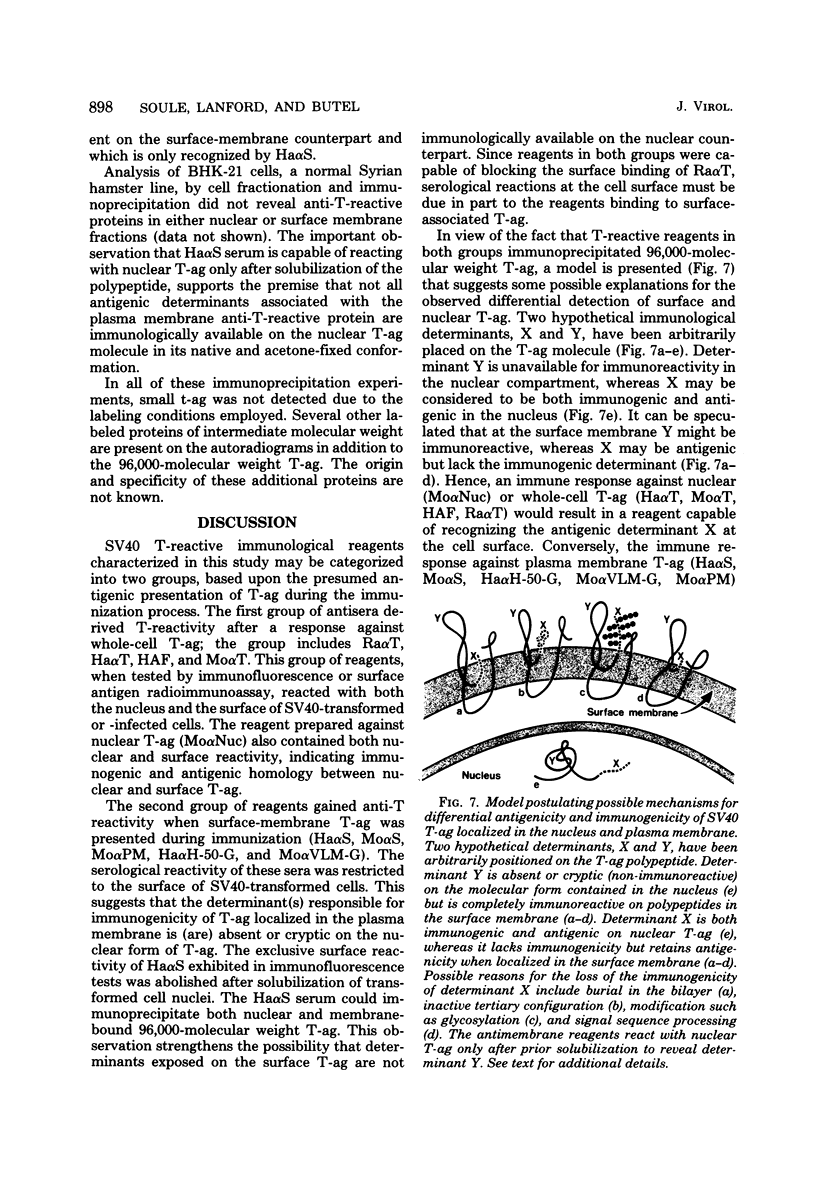
Antisera were prepared in syngeneic hosts against subcellular fractions of simian virus 40 (SV40)-transformed cells (MoαPM, MoαNuc), glutaraldehydefixed SV40-transformed cells (HaαH-50-G, MoαVLM-G), and electrophoretically purified denatured SV40 tumor antigen (T-ag) (RaαT). Immune sera were also collected from animals bearing tumors induced by SV40-transformed cells (HaαT, MoαT, HAF) and from SV40-immunized animals that had rejected a transplant of SV40-transformed cells (HaαS, MoαS). Immunological reagents prepared against cell surface (MoαPM, HaαS, MoαS, HaαH-50-G, MoαVLM-G) reacted exclusively with the surface of SV40-transformed cells by indirect immunofluorescence or protein A surface antigen radioimmunoassay. Immunological reagents prepared against the nuclear fraction (MoαNuc) or whole-cell determinants (HaαT, MoαT, HAF, RaαT) reacted with both the nuclei and surface of SV40-transformed or -infected cells. All reagents were capable of immunoprecipitating 96,000-molecular weight large T-ag from solubilized whole cell extracts of SV40-transformed cells. The exclusive surface reactivity of HaαS exhibited in immunofluorescence tests was abolished by solubilization of subcellular fractions, which then allowed immunoprecipitation of T-ag by HaαS from both nuclear and plasma membrane preparations. Specificity was established by the fact that all T-reactive reagents failed to react in serological tests against chemically transformed mouse cells, and sera from mice bearing transplants chemically transformed mouse cells (MoαDMBA-2) failed to react with SV40-transformed mouse or hamster cells. Reagents demonstrating positive surface immunofluorescence and protein A radioimmunoassay reactions against SV40-transformed cells were capable of blocking the surface binding of RaαT to SV40-transformed cells in a double-antibody surface antigen radioimmunoassay. This blocking ability demonstrated directly that a component specificity of each surface-reactive reagent is directed against SV40 T-ag. A model is presented which postulates that the differential detection of T-ag by the various serological reagents is a reflection of immunogenic and antigenic differences between T-ag polypeptides localized in nuclei and plasma membranes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Tumorigencity of simian papovavirus SV40 and of virus-transformed cells. J Natl Cancer Inst. 1963 Jun;30:1227–1265. [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Soule H. R. Role of the simian virus 40 gene A product in regulation of DNA synthesis in transformed cells. J Virol. 1978 Jun;26(3):584–594. doi: 10.1128/jvi.26.3.584-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Tevethia S. S., Melnick J. L. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res. 1972;15:1–55. doi: 10.1016/s0065-230x(08)60371-1. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Goldfine S. M., Melero J. A. Antiserum to polyacrylamide gel-purified simian virus 40 T antigen. Virology. 1978 Jun 1;87(1):194–198. doi: 10.1016/0042-6822(78)90171-x. [DOI] [PubMed] [Google Scholar]
- Chang C., Martin R. G., Livingston D. M., Luborsky S. W., Hu C. P., Mora P. T. Relationship between T-antigen and tumor-specific transplantation antigen in simian virus 40-transformed cells. J Virol. 1979 Jan;29(1):69–75. doi: 10.1128/jvi.29.1.69-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Pates R. Cell surface location of simian virus 40-specific proteins on HeLa cells infected with adenovirus type 2-simian virus 40 hybrid viruses Ad2+ND1 and Ad2+ND2. J Virol. 1979 Aug;31(2):522–536. doi: 10.1128/jvi.31.2.522-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Pates R. Simian virus 40 specific proteins on surface of HeLa cells infected with adenovirus 2--SV40 hybrid virus Ad2+ND2. Nature. 1979 Jan 25;277(5694):322–324. doi: 10.1038/277322a0. [DOI] [PubMed] [Google Scholar]
- Dorval G., Welsh K. I., Wigzell H. A radioimmunoassay of cellular surface antigens on living cells using iodinated soluble protein A from Staphylococcus aureus. J Immunol Methods. 1975 Jun;7(2-3):237–250. doi: 10.1016/0022-1759(75)90021-6. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. M. Isolation and characterization of membranes from normal and transformed tissue-culture cells. Biochem J. 1972 Dec;130(4):1113–1124. doi: 10.1042/bj1301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Processing of 60,000-dalton sarc gene protein synthesized by cell-free translation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4399–4403. doi: 10.1073/pnas.75.9.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khoury G., Carter B. J., Ferdinand F. J., Howley P. M., Brown M., Martin M. A. Genome localization of simian virus 40 RNA species. J Virol. 1976 Mar;17(3):832–840. doi: 10.1128/jvi.17.3.832-840.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitcher S. A., Siddle K., Luzio J. P. A method for the determination of glucose-6-phosphatase activity in rat liver with [U-14C]glucose 6-phosphate as substrate. Anal Biochem. 1978 Jul 15;88(1):29–36. doi: 10.1016/0003-2697(78)90395-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane D. P., Robbins A. K. An immunochemical investigation of SV40 T antigens. 1. Production properties and specificity of rabbit antibody to purified simian virus 40 large-T antigen. Virology. 1978 Jun 1;87(1):182–193. doi: 10.1016/0042-6822(78)90170-8. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Antigenic relationship of SV40 early proteins to purified large T polypeptide. Virology. 1979 Sep;97(2):295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Langone J. J. [125I]protein A: a tracer for general use in immunoassay. J Immunol Methods. 1978;24(3-4):269–285. doi: 10.1016/0022-1759(78)90131-x. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies on nondefective adenovirus-simian virus 40 hybrid viruses. I. A newly characterized simian virus 40 antigen induced by the Ad2+ND 1 virus. J Virol. 1971 Feb;7(2):189–197. doi: 10.1128/jvi.7.2.189-197.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. S., Schmidt-Ullrich R., Wallach D. F. Transformation by simian virus 40 induces virus-specific, related antigens in the surface membrane and nuclear envelope. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2495–2499. doi: 10.1073/pnas.74.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. S., Schmidt-Ullrich R., Wallach D. F. Transformation by simian virus 40 induces virus-specific, related antigens in the surface membrane and nuclear envelope. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2495–2499. doi: 10.1073/pnas.74.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., KHERA K. S., RAPP F. PAPOVAVIRUS SV40: FAILURE TO ISOLATE INFECTIOUS VIRUS FROM TRANSFORMED HAMSTER CELLS SYNTHESIZING SV40-INDUCED ANTIGENS. Virology. 1964 Jul;23:430–432. doi: 10.1016/0042-6822(64)90267-3. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P., Kelly T. J., Jr, Lewis A. M., Jr Mapping of simian virus 40 early functions on the viral chromosome. J Virol. 1973 Sep;12(3):653–658. doi: 10.1128/jvi.12.3.653-658.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan C. A., Brugge J. S., Butel J. S. Characterization of simian cells tranformed by temperature-sensitive mutants of simian virus 40. J Virol. 1976 Jun;18(3):1106–1119. doi: 10.1128/jvi.18.3.1106-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gluzman Y., Winocour E. Cellular and cell-free synthesis of simian virus 40 T-antigens in permissive and transformed cells. J Virol. 1978 Feb;25(2):587–595. doi: 10.1128/jvi.25.2.587-595.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., MELNICK J. L. VIRUS-INDUCED INTRANUCLEAR ANTIGEN IN CELLS TRANSFORMED BY PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1131–1135. doi: 10.3181/00379727-116-29472. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Law L. W. Immunogenic properties of a soluble tumor rejection antigen (TSTA) from a Simian virus 40-induced sarcoma. Int J Cancer. 1979 Jan 15;23(1):89–96. doi: 10.1002/ijc.2910230116. [DOI] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Sugden B., Keller W., Sharp P. A. Transcription of simian virus 40. 3. Mapping of "early" and "late" species of RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3711–3715. doi: 10.1073/pnas.70.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Thompson W. S., Wallach D. F. Antigenic distinctions of glycoproteins in plasma and mitochondrial membranes of lymphoid cells neoplastically transformed by simian virus 40. Proc Natl Acad Sci U S A. 1977 Feb;74(2):643–647. doi: 10.1073/pnas.74.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Thompson W. S., Wallach D. F. Biochemical and immunochemical characterization of two simian virus 40 (SV40)-specific glycoproteins in nuclear and surface membranes of SV40-transformed cells. Biochem Biophys Res Commun. 1979 Jun 13;88(3):887–894. doi: 10.1016/0006-291x(79)91492-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F., Davis F. D., 2nd Membranes of normal hamster lymphocytes and lymphoid cells neoplastically transformed by simian virus 40. I. High-yield purification of plasma membrane fragments. J Natl Cancer Inst. 1976 Nov;57(5):1107–1116. doi: 10.1093/jnci/57.5.1107. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F. Membrane proteins of cells neoplastically transformed by simian virus 40. Prog Exp Tumor Res. 1978;22:151–189. doi: 10.1159/000401201. [DOI] [PubMed] [Google Scholar]
- Silver J., Schaffhausen B., Benjamin T. Tumor antigens induced by nontransforming mutants of polyoma virus. Cell. 1978 Oct;15(2):485–496. doi: 10.1016/0092-8674(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEVETHIA S. S., KATZ M., RAPP F. NEW SURFACE ANTIGEN IN CELLS TRANSFORMED BY SIMIAN PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1965 Jul;119:896–901. doi: 10.3181/00379727-119-30330. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Tevethia S. S. Biology of SV40 transplantation antigen (TrAg). I. Demonstration of SV40 TrAg on glutaraldehyde-fixed SV40-infected African green monkey kidney cells. Virology. 1976 Feb;69(2):474–489. doi: 10.1016/0042-6822(76)90478-5. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Butel J. S. Induction of common transplantation antigen by various isolates of papovavirus SV40 and by virus rescued from transformed cells. Intervirology. 1973;2(3):200–205. doi: 10.1159/000149424. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Couvillionla, Rapp F. Development in hamsters of antibodies against surface antigens present in cells transformed by papovavirus SV40. J Immunol. 1968 Feb;100(2):358–362. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. I. Development of cellular immunity to simian virus 40 tumor-specific transplantation antigens during tumorigenesis by transplanted cells. J Natl Cancer Inst. 1973 Jan;50(1):137–147. doi: 10.1093/jnci/50.1.137. [DOI] [PubMed] [Google Scholar]