Most people would agree that mothers are influential in shaping their children’s outlook on life. A recent study by Mold and colleagues1 discloses a new facet of maternal influence: maternal immune cells “teach” those of the fetus how to balance the need for self-defense on the one hand and the requirement for immunologic tolerance on the other. Balance is critical. Too much restraint of immunity could lead to a lethal infection in the newborn; too little could lead to autoimmunity.
The study by Mold et al. offers a rare glimpse into the development of the human adaptive immune system, and it suggests that the balance is markedly shifted, in utero, toward tolerance by means of the preferential induction of lymphocytes known as regulatory T cells. The investigators compared leukocytes from the lymphoid tissues of fetuses of 20 weeks’ gestation with leukocytes from umbilical-cord blood, the thymuses of infants, and the lymph nodes and peripheral blood of adults. They observed no bias in regulatory T cells in the fetal thymus. Just as in the thymuses of infants and adults, only a small number of CD4 T cells (5 to 10%) were regulatory, as indicated by expression of the regulatory T-cell marker, the forkhead box P3 (FOXP3) protein (a nuclear transcription factor). In contrast, they observed a bias toward regulatory T cells that was as high as 20 to 25% in the fetal lymph nodes and spleen, where mature T cells go to encounter antigens. This bias disappeared at birth and was not observed in adult lymph nodes. Maternally derived cells benefited from this transient immune deviation; this finding has potentially far-reaching consequences for human health and disease.
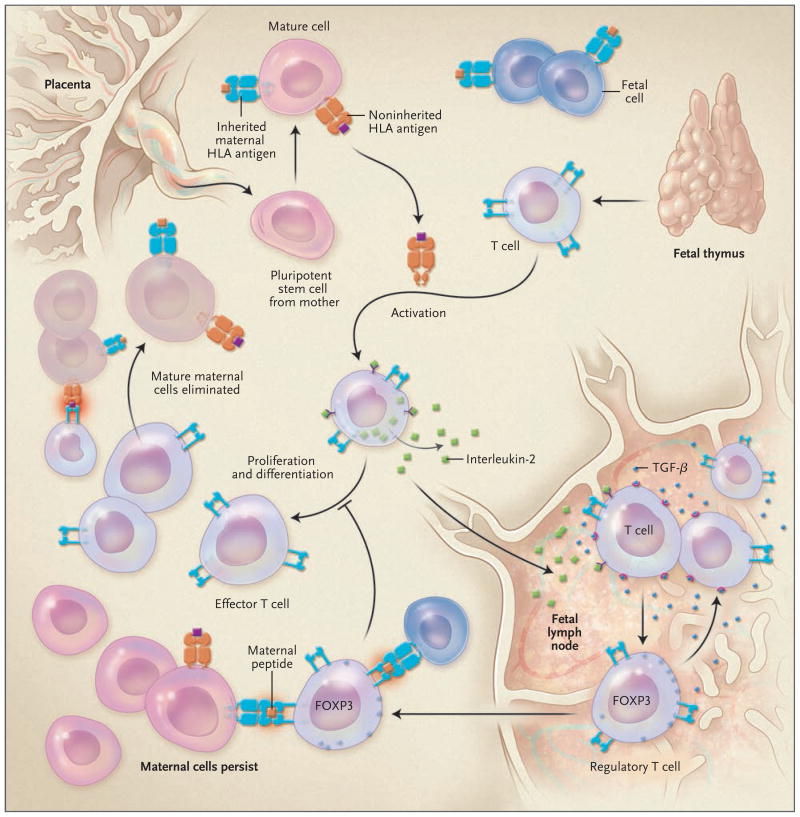
Whence comes this remarkable pro-tolerance bias in the adaptive immune system of the fetus? Fully competent T cells emerge from the human fetal thymus and head toward peripheral lymph nodes (Fig. 1). The newly arising T cells encounter maternal cells that are most likely to be the progeny of pluripotent stem cells that have crossed the placental barrier and managed to avoid elimination by fetal natural killer cells. Since a high proportion of cells in a mature T-cell repertoire are capable of recognizing foreign HLA antigens, many T cells will detect the maternal cells as well as soluble HLA antigen encoded by noninherited HLA alleles and undergo activation. Activated T cells make interlukin-2 and express the high-affinity interleukin-2 receptor, and so they will proliferate and differentiate into effector T cells. The latter could easily wipe out cells bearing unfamiliar HLA and non-HLA antigens, as happens routinely in adults who have received transfusions or transplants.
Figure 1. Immunity and the Maternal–Fetal Axis.
Mold and colleagues1 recently described a mechanism through which fetal tolerance of maternal antigens is initiated. Noninherited HLA antigens (orange) and inherited maternal HLA antigens (blue) are shown. Noninherited maternal HLA antigens include intact HLA recognized by fetal effector T cells and maternal peptides recognized by the FOXP3-positive regulatory T cells. The different results of effector T cells versus regulatory T-cell pathways with respect to the persistence of mature maternal cells (microchimerism) are indicated.
However, the authors found that the fetal lymph node did not cultivate strong immune responses to antigens; rather, it behaved more like an immune-privileged site,2 where the regulation of immunity allowing maternal T cells to survive, rather than antigen-specific sensitization, was favored. The fetal lymph node produced an abundance of transforming growth factor β (TGF-β). TGF-β, in combination with antigens and interleukin-2, stimulates FOXP3 expression and induction of regulatory T cells that are specific to maternal antigens. These suppressive cells, which might recognize a noninherited maternal peptide presented by the inherited HLA class II molecule, blocked the proliferation of effector T cells and spared the remaining maternal cells from destruction. The result was maternal microchimerism (up to 0.8% of maternal cells) in the fetus that was about 10 times greater than that previously reported in adults.
Shortly after birth, the lymph node is no longer such a hospitable environment for maternal cells. Nonetheless, Mold et al. found evidence of a lingering regulatory T-cell effect in some, but not all, healthy young adult offspring in a given family. Some FOXP3-positive regulatory T cells induced in the fetus may survive as long-lived memory T cells in the adult, as the authors suggest. Alternatively, the generation of new regulatory T cells may require additional antigen input from breast-feeding, ongoing maternal microchimerism, or both to sustain tolerance.
The expansion of antigen-specific regulatory T cells, both ex vivo and in vivo, is being developed as a clinical strategy to promote tolerance and thus to reduce or eliminate entirely the burden of immunosuppressive drugs on recipients of organ transplants. The findings of Mold et al. suggest that antigen-specific regulatory T cells and microchimerism are probably complementary. Regulatory T-cell expansion could be combined with stem-cell transplantation to maximize the induction and maintenance of tolerance. It will be important to determine precisely what type of maternal stem cell generates microchimerism and tolerance in the fetus. Currently, strategies to minimize the side effects of immunosuppressive drugs in renal transplantation (such as corticosteroid or cyclosporine withdrawal) are applied indiscriminately, with a significant risk of acute or chronic rejection of the graft. Perhaps patients, particularly those with living related donors, could be evaluated before transplantation to identify levels of microchimerism and associated regulatory T-cell activity, before they are enrolled in such a trial. Choosing donors with at least one HLA-DR and HLA-DQ match and appropriate maternal-antigen mismatches could also be of value.
The high success rate of clinical living-related organ and hematopoietic stem-cell transplantations when the donor or recipient expresses antigens that were not inherited from the mother3,4 can now be explained by the influence of developmentally induced regulatory T cells; this idea is strongly supported by a recent study in mice.5 Maternally induced regulatory T cells may also partly explain the reduced incidence of the formation of potentially harmful antibodies in patients who have been exposed to noninherited maternal antigens through pregnancy, blood transfusions, or transplantation; however, independent effects of maternal microchimerism on B cells may also have a role.
There is still much more to be learned about tolerogenic versus immunogenic forms of microchimerism, both of which have been reported, and the possibility that autoimmunity may result if maternal antigen–specific, TGF-β–producing regulatory T cells become Th-17 effectors in the presence of interleukin-6. For the time being, however, what a marvel are mothers and the pathway toward tolerance they map out for us before we are born.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Mold JE, Michaëlsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 3.Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–64. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 4.van Rood JJ, Loberiza FR, Jr, Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–7. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 5.Molitor-Dart ML, Andrassy J, Kwun J, et al. Developmental exposure to noninherited maternal antigens induces CD4 + T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–61. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]