Abstract
Background
Filaggrin is important for skin barrier function and is mutated in 15-20% of patients with atopic dermatitis.
Objective
To examine whether filaggrin deficiency predisposes to skin inflammation and epicutaneous (EC) sensitization with protein antigen.
Methods
Skin histology in filaggrin-deficient ft/ft mice and WT controls was assessed by H&E staining and immunohistochemistry. Cytokine mRNA expression was examined by quantitative RT-PCR. Serum antibody levels and splenocyte secretion of cytokines were measured by ELISA.
Results
ft/ft mice developed eczematous skin lesions after age 28 weeks and a progressive increase in serum IgE and IgG1 levels. Normal appearing skin from 8-week-old ft/ft mice had epidermal thickening and increased dermal infiltration with CD4+ cells and expression of mRNA for IL-17, IL-6 and IL-23, but not IL-4, IL-13 or IFN-γ. Lesional skin of 32-week-old ft/ft mice exhibited qualitatively similar, but more pronounced, changes, and elevated IL-4 mRNA levels. EC application of ovalbumin (OVA) to shaved skin of 8-week-old ft/ft mice, but not WT mice, resulted in increased epidermal thickening, dermal infiltration by CD4+ cells, but not eosinophils, and expression of IL-17, IL-6, IL-23, IL-4 and IFN-γ, but not IL-5 or IL-13, mRNA. Splenocytes from EC sensitized ft/ft mice, but not controls, secreted cytokines in response to OVA stimulation and their sera, but not those of controls, contained OVA specific IgE and IgG1 antibodies.
Conclusions
Filaggrin deficient mice exhibit Th17-dominated skin inflammation, eczematous changes with age, and are permissive to EC sensitization with protein antigen.
Keywords: ft/ft mice, filaggrin, atopic dermatitis, IL-17, skin, allergen
Introduction
Patients with atopic dermatitis (AD) exhibit impaired skin barrier function and abnormal structure and chemistry of the stratum corneum (SC)1. Furthermore, defects in skin barrier function in mice are associated with an AD-like phenotype2. Alteration of the skin barrier in AD is evidenced by reduction in the water content of the SC and by increased transepidermal water loss (TEWL)2. Mechanical injury inflicted by scratching and skin inflammation are likely to aggravate skin barrier dysfunction in AD, as suggested by the observation that the defect in TEWL improves with decreased disease activity3. Impaired barrier function increases transepidermal penetration of environmental allergens. This is supported by the observation that children with AD exposed to topical creams and lotions containing peanut protein have a significantly increased risk of peanut sensitization4. AD has been considered a Th2 mediated disease, characterized by elevated IgE and Th2 cytokine expression in acute skin lesions, Several recent observations suggest the presence of IL-17-producing cells infiltrating the dermis in acute AD lesions and in the peripheral blood of AD patients5, 6. Furthermore, epicutaneous (EC) sensitization of tape stripped mouse skin with OVA results in local and systemic Th17 as well as Th2 responses7.
AD shows strong genetic linkage to Chromosome 1q21, which contains the human Epidermal Differentiation Complex (EDC) of genes that encode keratinocyte structural proteins, including filaggrin8 plays a critical role in skin hydration9. Mutations in the filaggrin gene (FLG), have been identified in ichthyosis vulgaris10 and in AD11. The majority of FLG mutations in AD are heterozygous. Two loss-of-function mutations (R510X and 2282del4) account for the majority of FLG mutations in European patients with AD and are major risk factors for AD, and AD-associated asthma, but not for asthma alone11, 12. There is a strong association between FLG mutations and extrinsic AD12. Filaggrin expression is also reduced in AD patients with no FLG mutations, possibly due to local expression of the Th2 cytokines, IL-4 and IL-13, which downregulate FLG expression in keratinocytes13. Decreased filaggrin expression in AD skin is associated with decreased hydration of the SC14.
Flaky tail (ft) is a spontaneously arising autosomal recessive mutation located at chromosome 3 within the mouse EDC, and is in close linkage with another mutation (ma) that causes hair matting15. Homozygous ft/ft mice have dry, flaky skin which expresses reduced amounts of profilaggrin mRNA and an abnormal profilaggrin protein that is not processed to filaggrin monomers. ft/ft mice have increased TEWL. In this study, we demonstrate that ft/ft mice develop Th17-dominated skin inflammation and eczematous skin lesions and are permissive to EC sensitization with protein antigen.
Materials and Methods
Mice
Flaky tail (ft) mice were from Jackson laboratory. C57BL6 and BALB/c wild-type (WT) mice were from Charles River Laboratories. All mice were bred in our animal facility, and kept in a specific pathogen-free environment and fed an OVA-free diet. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Children's Hospital Boston.
EC sensitization and intraperitoneal immunization
The skin of anesthetized eight-week-old female mice was shaved then 100 μg OVA (Grade V; Sigma) in 100 μL of normal saline, or placebo (100 μL of normal saline), were placed on a patch of sterile gauze (1×1 cm), which was secured to dorsal skin with a transparent bio-occlusive dressing (Tegaderm, Westnet Inc.). Each mouse had a total of three one-week exposures to the patch separated by two-week intervals. Mice were euthanized at the end of the third cycle of sensitization (day 49). Four to six-week-old female mice were sensitized on day 0, 14, and 21 by intraperitoneal injection of 50 μg OVA emulsified in 2 mg of Imject Alum (Pierce) in 100 μL saline and sacrificed at day 28.
Histological and immunohistochemical analysis of mouse skin
This was performed as previously described16. CD4+ cells were counted blinded in 10-15 high-power fields (HPFs) at a magnification of 400×. Epidermal thickness of 6 different randomly chosen sites was measured in each skin section from each mouse.
Quantitative RT-PCR for cytokines
Quantitative RT-PCR for cytokines was performed as previously described, using the housekeeping gene β2-microglobulin as a control17.
In vitro cytokine production
Spleen single cell suspensions were cultured at 2 × 106/ml in the presence of OVA (200 μg/ml) for 96 hrs as previously described16. Cytokine levels were measured by ELISA (BD Bioscience).
Serum antibody determination
Total IgE, IgG1 levels and OVA specific serum antibodies were determined by ELISA as previously described16.
FACS analysis of ear cells
Ears were floated on 0.25 % trypsin containing 2.2 mM EDTA for 45 min at 37°C, then incubated in RPMI 1640 containing 10% FCS at 37°C for 2 hours. Single ear cell suspensions were stained with mAbs to CD11c, CD11b, Gr-1, CD3, and CD4 (all from BD Bioscience) followed by FACS analysis.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine changes in skin infiltrating CD4+ T cells, splenocyte cytokine production, mRNA levels of cytokines, and levels of serum IgG1 and IgE. Statistical analysis was performed with the Graphpad Prism version 4.0 (Graphpad Software). Two-tailed values of p<0.05 were considered statistically significant for all comparisons.
Results
ft/ft mice develop eczematous skin lesions
Neonatal ft/ft mice appear normal at birth but have increased TEWL18. The flaky tail phenotype appears at about 3 days of age with the skin having a dry, scaly appearance15. There is gradual improvement of the skin condition, and three-week-old ft/ft pups appear normal, except for shortened ears and loss of tail tips in some mice. Because ft/ft mice are not on a homogenous C57BL6 background, we used both C57BL6 and BALB/c mice as controls, since these two strains lie on opposite ends of the spectrum of T helper responses with BALB/c mice more prone to Th2 and Th17 responses, and C57BL6 mice more prone to Th1 responses19.
Antigen entry via a disrupted skin barrier in ft/ft mice may lead to development of eczematous skin lesions with age. Fig. 1A shows that ft/ft mice have dark brown to black hair, which was matted compared to hair of C57BL6 mice. There were no visible skin lesions in ft/ft mice at 4, 8 and 16 weeks of age. Eczematous skin lesions appeared after age 28 weeks, with all ft/ft mice being affected at 32 weeks of age and typically exhibiting scaly pink eczematous skin lesions on the face and periauricular areas, periorbital swelling, and patches of scaly eczematous skin on the neck and trunk, with thinning of the overlying hair (Fig. 1B). No skin lesions were observed in age-matched controls maintained in the same environment (data not shown).
Figure 1. Eczematous skin lesions and serum IgE and IgG1 levels in ft/ft mice.
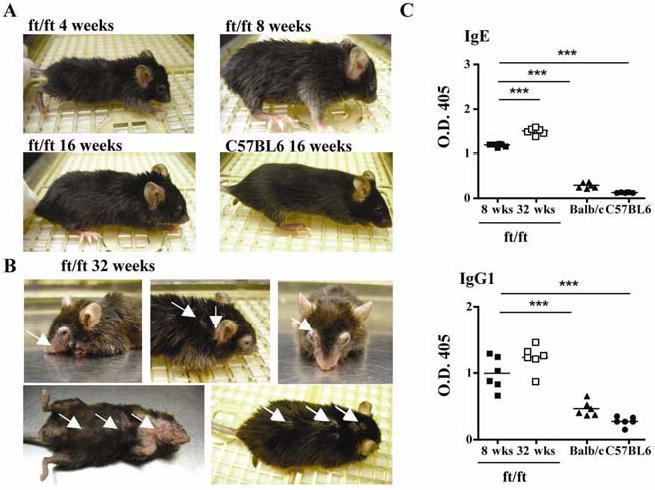
(A, B) Gross appearance of 4-, 8-, and 16-week-old ft/ft mice and 16-week-old C57BL6 (A) and of 32-week-old ft/ft mice (B). Arrows point to skin lesions. (C) Serum IgE and IgG1 levels in 8-, and 32-week-old ft/ft mice and 8 week-old BALB/c and C57BL6 mice. ***p<0.001.
ft/ft mice exhibited elevated levels of serum IgE and IgG1 at 8 weeks of age (Fig. 1C). Serum IgE, but not IgG1 levels rose significantly further by 32 weeks of age. ft/ft mice have the same IgG2a allotype as BALB/c mice, and serum IgG2a levels were comparable in the two strains (data not shown). Because serum IL-4 drives IgE and IgG1 production in mice20, these findings suggest that ft/ft mice were undergoing a systemic Th2 driven allergic response.
Th17 dominated skin inflammation in young ft/ft mice
The skin of newborn ft/ft pups exhibits hyperkeratosis with mild acanthosis, a markedly attenuated granular layer, but normal basal and spinous layers, and no evidence of inflammation18. Histological examination of normal appearing back skin from 8-week-old ft/ft mice, revealed hyperkeratosis, acanthosis (epidermal thickening), and occasional foci of parakeratosis (retention of keratinocyte nuclei within the SC) (Fig. 2A). The dermis of ft/ft mice exhibited significantly increased infiltration by mononuclear cells compared to skin from age-matched controls and the presence of rare neutrophils, but not eosinophils (Fig. 2A). These changes are compatible with a mild chronic eczematous dermatitis, as typically occurs in human AD. Immunohistochemistry revealed increased dermal infiltration with CD4+ cells in ft/ft skin (Fig. 2B). FACS analysis of cells isolated from ear skin confirmed that ft/ft skin contained significantly increased percentages of CD4+ T cells and Gr-1+ neutrophils, but not CD11c+ dendritic cells, compared to ear skin from controls (Fig. 2C). There was no increased expression of CD40, CD80, CD86 and OX40 by skin DCs from ft/ft mice, but MHC class II expression was reduced (data not shown), consistent with increased antigen uptake secondary to a disrupted skin barrier.
Figure 2. Spontaneous Th17-dominated skin inflammation in 8 week-old ft/ft mice.
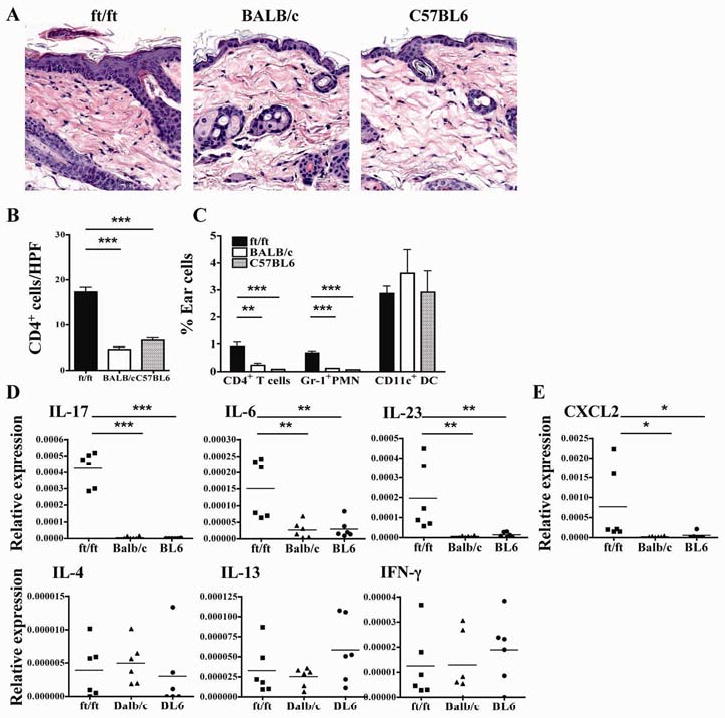
(A) Representative photomicrographs of H&E sections. (B) Numbers of CD4+ cells/HPF (400×). (C) FACS analysis of CD3+CD4+ cells and CD11b+Gr-1+ neutrophils in ear skin. (D, E) Cytokine (D) and CXCL2 (E) mRNA expression in the skin. *p<0.05, **p<0.01, ***p<0.001.
Quantitative PCR (Q-PCR) analysis revealed significantly increased expression of mRNA for IL-17, and the IL-17 promoting cytokines IL-6 and IL-23 (p19)21, in ft/ft skin mice compared to C57BL6 and BALB/c skin, which virtually had no detectable levels of IL-17 or IL-23 mRNA (Fig. 2D). There was also significant increase in mRNA expression of the IL-17 inducible neutrophil attractant chemokine CXCL2 in ft/ft skin (Fig. 2E). In contrast, there was no increased expression of mRNA for the Th2 cytokines IL-4 and IL-13 and the Th1 cytokine IFN-γ in ft/ft skin.
Analysis of lesional skin in older ft/ft mice
Histological examination of skin lesions from 32-week old ft/ft mice revealed changes qualitatively similar, but more pronounced, than those observed in 8-week-old ft/ft mice. These included focally pronounced hyperkeratosis and acanthosis, focal parakeratosis, and prominent dermal infiltration predominantly by lymphocytes with absence of detectable eosinophils (Fig. 3A). There were many plasmacytoid cells and some eosinophils in draining lymph nodes (data not shown). Immunohistochemistry revealed markedly increased dermal infiltration with CD4+ cells compared to skin from 8 week old ft/ft mice (Fig. 3B). Q-PCR analysis revealed significantly increased mRNA expression for IL-17, IL-6, IL-23 and CXCL2 in skin lesions from 32-week-old ft/ft mice, compared to skin from 8 week old ft/ft mice (Fig. 3C,D). There was a significant increase in IL-4, but not IL-13 or IFN-γ mRNA expression (Fig. 3C,D).
Figure 3. Analysis of skin lesions in aged 32-week-old ft/ft mice.
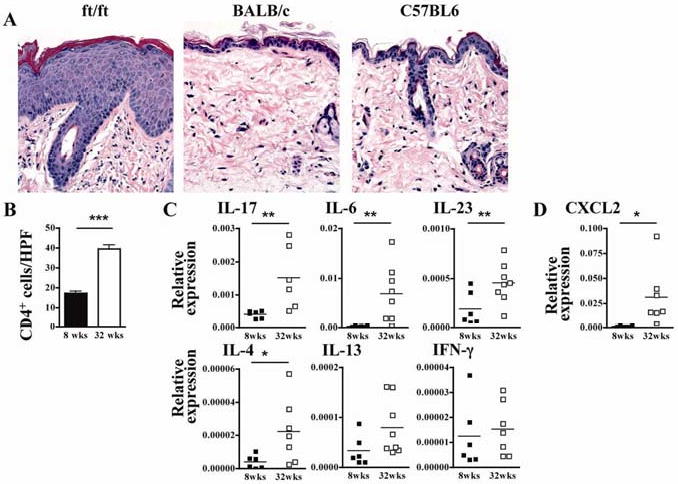
(A) Representative photomicrographs of H&E sections. (B) Numbers of CD4+ cells/HPF (400×). (C and D) Cytokine (C) and CXCL2 chemokine (D) mRNA expression. *p<0.05, **p<0.01, ***p<0.001.
OVA application to skin of ft/ft mice results in increased skin inflammation with expression of Th1, Th2 and Th17 cytokines
Since ft/ft mice have impaired skin barrier function, their skin may be permissive to EC sensitization by protein antigens normally excluded from skin. To test this hypothesis, OVA or saline was applied using a patch to shaved, but not tape stripped, skin, and the mice were examined for the development of skin inflammation. Skin of ft/ft mice, but not of C57BL6 and BALB/c mice, exhibited significantly increased epidermal thickening, hyperkeratosis, focal parakeratosis, and mild spongiosis following EC sensitization with OVA compared to saline (Fig. 4A,B). OVA sensitized skin of ft/ft, but not C57BL6 or BALB/c, mice, also exhibited increased mononuclear cells in the dermis, that included lymphocytes with dense nuclei, and macrophages with infolded nuclei and abundant, focally vacuolated cytoplasm. There was lymphocyte exocytosis into the epidermis, prominence and apparent increase in numbers of mast cells, and intravascular aggregation of neutrophils (Fig. 4A). No eosinophil infiltration was detectable. There was a significant increase in dermal infiltration by CD4+ cells in OVA sensitized ft/ft, but not BALB/c or C57BL6, skin (Fig. 4C). mRNA levels for IL-17, IL-6, IL-23, IL-4, IFN-γ and CXCL2, but not IL-5 and IL-13, were significantly higher in OVA-sensitized, compared to saline-sensitized, ft/ft skin (Fig. 4D and Supplemental Fig. 1). In contrast, there was no detectable upregulation in mRNA expression for any of these cytokines in BALB/c or C57BL6 skin following EC sensitization with OVA.
Figure 4. Histology and cytokine expression of EC sensitized skin.
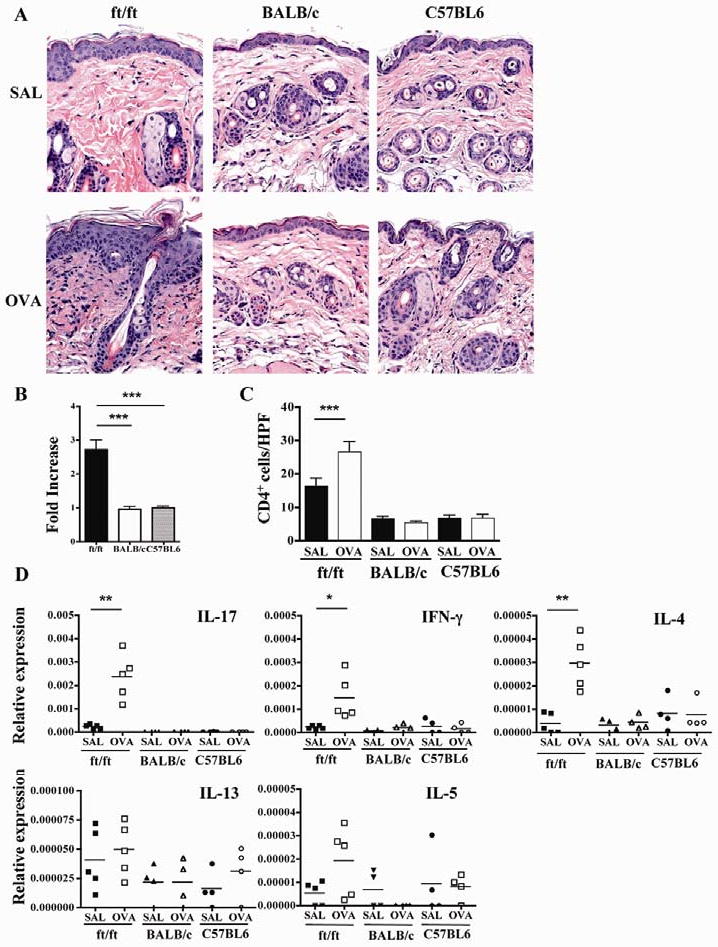
(A) Representative photomicrographs of H&E sections of skin. (B) Epidermal thickness of OVA sensitized skin. Results are expressed as fold increase over saline sensitized skin. (C) Numbers of CD4+ cells/HPF (400×). (D) Cytokine mRNA expression. *p<0.05, **p<0.01, ***p<0.001.
OVA application to skin of ft/ft mice elicits a systemic immune response to OVA antigen
To examine whether application of OVA to the skin elicits a systemic immune response in ft/ft mice, we measured OVA specific serum antibody levels and secretion of cytokines by splenocytes following in vitro stimulation with OVA. OVA application to shaved, nonstripped skin elicited OVA specific IgG1 IgE and IgG2a antibodies in ft/ft mice, but not in BALB/c or C57BL6 controls (Fig. 5A and data not shown). Splenocytes from ft/ft mice, EC sensitized with OVA secreted IL-17, IL-4, IL-13 and IFN-γ in response to in vitro stimulation with OVA (Fig. 5B). In contrast, splenocytes from C57BL6 or BALB/c mice EC sensitized with OVA failed to secrete these cytokines in response to OVA stimulation.
Figure 5. Systemic immune responses to EC sensitization with OVA.
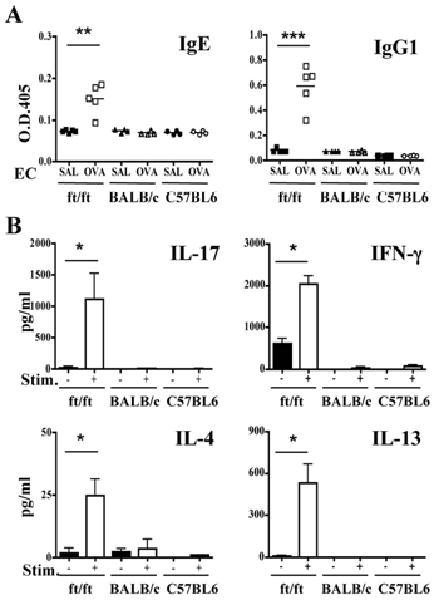
(A) Serum levels of OVA-specific IgE and IgG1. (B) Cytokine secretion by splenocytes in response to OVA stimulation in vitro. *p<0.05, **p<0.01, ***p<0.001.
Intraperitoneal immunization elicits a comparable immune response in ft/ft mice, C57BL6 and BALB/c mice
The systemic immune response of ft/ft mice to EC sensitization with OVA could be due to a generalized heightened immune response. We compared the response of ft/ft, C57BL6 and BALB/c mice to intraperitoneal (i.p.) immunization with OVA, an immunization route that bypasses the skin. The IgE antibody response of ft/ft mice to i.p. immunization was comparable to that of C57BL6 mice, and significantly lower than that of BALB/c mice (Fig. 6A). Their IgG2a antibody response was comparable to that of BALB/c controls (data not shown). There were no significant differences in the IgG1 antibody response of the three strains to i.p. immunization (Fig. 6A). Splenocytes from i.p. immunized ft/ft mice produced levels of IL-17, IL-4 and IL-13 that fell in between those secreted by splenocytes from C57BL6 and BALB/c mice, but like C57BL6 splenocytes, secreted significantly more IFN-γ than splenocytes from BALB/c mice (Fig. 6B). As expected, splenocytes from BALB/c mice produced significantly more IL-13, IL-4 and IL-17 and significantly less IFN-γ, than splenocytes from C57BL6 mice.
Figure 6. Systemic immune responses to i.p. OVA-immunization with OVA.
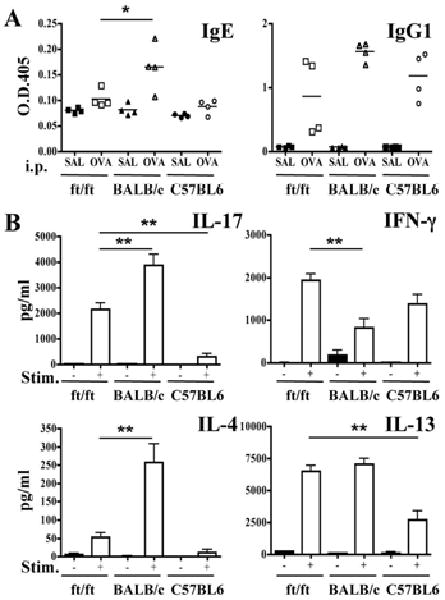
(A) Serum levels of OVA-specific IgE and IgG1. (B) Cytokine secretion by splenocytes in response to OVA stimulation in vitro. *p<0.05, **p<0.01.
Discussion
We demonstrate that filaggrin deficiency is associated with Th17-dominated skin inflammation, development of eczematous skin lesions with age, and permissiveness to EC sensitization with protein antigen.
Except for matted hair, there were no discernible skin lesions in ft/ft mice until about 28 weeks of age. By 32 weeks of age, ft/ft mice developed scaly eczematous skin lesions (Fig. 1A). Skin lesions have not been previously described in ft/ft mice18, possibly because previous reports may not have investigated older mice. Alternatively, as in Nc/Nga mice22, development of skin lesions in ft/ft mice may be influenced by environmental factors, particularly microbial skin colonization, which may differ between animal facilities.
Normal appearing skin from 8-week-old ft/ft mice revealed skin inflammation characterized by hyperkeratosis, acanthosis, dermal infiltration with CD4+ cells and rare neutrophils, with notable absence of eosinophils, and increased expression of mRNA for IL-17, the IL-17 promoting cytokines IL-6 and IL-23, and the IL-17 inducible chemokine CXCL2 (Fig. 2). More severe, but qualitatively similar, skin inflammation was observed in skin lesions that had developed spontaneously in 32-week-old ft/ft mice (Fig. 2). Keratinocytes, DCs, and macrophages are sources of IL-6 and IL-23, and release these cytokines in response to TLR ligands and IL-123-25. TLR2 ligands from S. aureus and other bacteria, which colonize skin are particularly potent at driving IL-23 expression26. We have found that mechanical skin injury by tape stripping causes rapid upregulation of IL-6 and IL-23 in the skin (MKO and RSG: unpublished observations). There was no significant elevation of IL-6 and IL-23 mRNA expression in the skin of 2-week-old ft/ft mice (data not shown) suggesting that lack of filaggrin by itself does not drive increased expression of these cytokines. The trigger for this increase could be microbial antigens, absorbed via a disrupted skin barrier, and/or a mechanically damaged skin. Elevated IL-17 expression may explain the neutrophil infiltration in ft/ft skin, as IL-17 induces expression of neutrophil chemotactic chemokines in epithelial cells, including CXCL227, which was upregulated in ft/ft skin. In addition to T cells, neutrophils are a source of IL-1719, and may contribute to increased IL-17 expression in ft/ft skin. IL-6 drives keratinocyte proliferation28, thus increased IL-6 expression in the skin may underlie epidermal thickening in ft/ft mice.
The skin of 8-week-old ft/ft mice exhibited no detectable evidence of Th2-driven inflammation. There was no significant infiltration with eosinophils or upregulation of Th2 cytokine mRNA expression (Fig. 2). There was also no evidence of Th1-driven inflammation, as there was no increase in IFN-γ mRNA expression. Lack of evidence of Th2 inflammation in the skin of ft/ft mice contrasts with elevation of their serum IgE and IgG1 levels, an indicator of a systemic Th2 response. High levels of IL-17 in the skin of ft/ft mice may have inhibited local expression of Th2 cytokines and subsequent recruitment of eosinophils29. Skin lesions in 32-week-old ft/ft mice exhibited qualitatively similar, but more pronounced, changes in histology and cytokine expression, compared to skin from 8-week-old ft/ft mice (Fig. 3) The exception was IL-4 mRNA levels, which were elevated in lesional skin of 32-week-old, but not 8-week-old mice, consistent with an ongoing Th2 response to cutaneously absorbed antigens.
The skin of ft/ft mice was permissive to EC sensitization of nonstripped skin with OVA, a protein antigen, normally excluded from penetrating the skin. Application of OVA to shaved, but non-tape stripped, skin elicited allergic skin inflammation and OVA specific systemic humoral and cellular immune responses in ft/ft mice, but not C57BL6 or BALB/c mice (Fig. 4 and 5). In previous studies, two additional strains, 129Sv and hr/hr hairless mice, failed to respond to OVA application to shaved skin (RSG: unpublished observations). Skin inflammation in EC sensitized ft/ft mice was characterized by a significant increase in epidermal thickness, dermal infiltration with CD4+ cells and expression of IL-4, IL-17 and IFN-γ, but not IL-13 or IL-5 mRNA. IL-13 plays an important in driving eotaxin expression in the skin30. IL-5 is important not only for eosinophil mobilization, but also for their survival in tissues31. Elevated IL-17 levels in ft/ft skin before, and more so after EC sensitization, may have inhibited of IL-13 and IL-5 expression, resulting in lack of eosinophil infiltration. Genetic background could have contributed to lack of IL-5 and IL-13 mRNA expression in EC sensitized skin of ft/ft mice.
EC sensitization of tape stripped skin of WT mice (BALB/c, C57BL6, 129Sv and BWF1) results in allergic skin inflammation and in OVA specific immune responses16, 32, 33. Since tape stripping disrupts skin barrier and allows antigen entry34, disrupted skin barrier function in ft/ft mice likely underlies their permissiveness to EC sensitization of nonstripped skin by protein antigen. This permissiveness did not reflect a generalized enhanced immune response, because the humoral and immune responses of ft/ft mice to i.p. immunization with OVA were comparable or lower than those of C57BL6 and BALB/c mice (Fig. 6). This is consistent with the observation that FLG is expressed exclusively in mouse skin, tongue, esophagus, and forestomach35. With their eczematous skin lesions that developed with age and expressed IL-4 mRNA, elevated serum IgE and IgG1 levels and permissiveness to EC sensitization of nonstripped skin with protein antigen, ft/ft mice share features with patients with extrinsic AD. However, while IL-17 expression is prominent in the skin of ft/ft mice and in psoriatic skin lesions, it is less prominent in AD skin lesions. There are two limitations to the interpretation of our results. Although mutation in FLG likely underlies the skin abnormalities in ft/ft mice, these mice are double homozygous of flaky tail and matted. Therefore, we cannot rule out a role for the ma mutation in the skin inflammation and susceptibility to EC sensitization of nonstripped skin of ft/ft mice. The other limitation is that the ft/ft mice we studied, are not on a homogeneous background. Nevertheless, the skin inflammation and susceptibility to EC sensitization of nonstripped skin we observed in these mice was not observed in four other strains we have studied to date. Thus, it is unlikely that genes other than FLG, or a closely linked gene, are implicated in the susceptibility of ft/ft mice to develop skin inflammation to protein antigens normally excluded by the skin barrier and to develop eczema.
While this manuscript was being reviewed, the mutation in FLG in ft/ft mice was identified to be a 1-bp deletion (5303delA), and ft/ft mice bred on C57BL6 background and lacking the ma mutation were demonstrated to be permissive to sensitization by cutaneous application of antigen, in agreement with our findings36.
Clinical Implications.
Filaggrin deficiency predisposes to skin inflammation, development of eczematous lesions and sensitization upon contact with protein antigens normally excluded by the skin barrier.
Supplementary Material
Acknowledgments
We thank Dr. Hans Oettgen for reading the manuscript.
Sources of funding: This work was supported by The Atopic Dermatitis and Vaccinia Immunization Network contract NO1 AI 40030, and USPHS grant AR-047417.
Abbreviations used
- AD
atopic dermatitis
- EDC
Epidermal Differentiation Complex
- EC
epicutaneous(ly)
- FLG
Filaggrin
- ft
Flaky tail
- IFN-γ
interferon γ
- OVA
ovalbumin
- TEWL
Transepidermal water loss
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–60. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 2.Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75:429–33. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto M, Sugiura H, Uehara M. Skin barrier function in patients with completely healed atopic dermatitis. J Dermatol Sci. 2000;23:178–82. doi: 10.1016/s0923-1811(00)00073-6. [DOI] [PubMed] [Google Scholar]
- 4.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 5.Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–81. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 6.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–30. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 7.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–22. doi: 10.1073/pnas.0706942104. Epub 2007 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–88. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 9.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 1:43–8. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–42. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 11.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. Epub 2006 Mar 19. [DOI] [PubMed] [Google Scholar]
- 12.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–9. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, et al. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128:2117–9. doi: 10.1038/jid.2008.29. [DOI] [PubMed] [Google Scholar]
- 15.Lane PW. Two new mutations in linkage group XVI of the house mouse. Flaky tail and varitint-waddler-J. J Hered. 1972;63:135–40. doi: 10.1093/oxfordjournals.jhered.a108252. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Oyoshi MK, Le Y, Bianchi T, Koduru S, Mathias CB, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J Clin Invest. 2009;119:47–60. doi: 10.1172/JCI32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–81. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182:3039–46. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–6. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 23.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–61. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen JM, Bonefeld CM, Poulsen SS, Geisler C, Skov L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J Allergy Clin Immunol. 2009;123:486–92. doi: 10.1016/j.jaci.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–15. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 26.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–9. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 27.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–11. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–94. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Alenius H, Laouini D, Woodward A, Mizoguchi E, Bhan AK, Castigli E, et al. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol. 2002;109:106–13. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- 33.Woodward AL, Spergel JM, Alenius H, Mizoguchi E, Bhan AK, Castigli E, et al. An obligate role for T-cell receptor alpha beta T+ cells but not T-cell receptor gamma delta T+ cells, B cells, or CD40/CD40L interactions in a mouse model of atopic dermatitis. Journal of Allergy and Clinical Immunology. 2001;107:359–66. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- 34.Smith W. Stratum corneum barrier integrity controls skin homeostasis. Int J Cosmet Sci. 1999;21:99–106. doi: 10.1046/j.1467-2494.1999.196562.x. [DOI] [PubMed] [Google Scholar]
- 35.Makino T, Takaishi M, Toyoda M, Morohashi M, Huh NH. Expression of hornerin in stratified squamous epithelium in the mouse: a comparative analysis with profilaggrin. J Histochem Cytochem. 2003;51:485–92. doi: 10.1177/002215540305100410. [DOI] [PubMed] [Google Scholar]
- 36.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


