Abstract
Many preclinical and clinical studies have implied a role for cholesterol in the pathogenesis of Alzheimer's disease (AD). In this review we will discuss the movement of intracellular cholesterol and how normal distribution, transport, and export of cholesterol is vital for regulation of the AD related protein, Aβ. We focus on cholesterol distribution in the plasma membrane, transport through the endosomal/lysosomal system, control of cholesterol intracellular signaling at the endoplasmic reticulum and Golgi, the HMG-CoA reductase pathway and finally export of cholesterol from the cell.
Keywords: Alzheimer's disease, amyloid, apolipoprotein E (apoE), APP, cholesterol, cholesterol ester, lipid rafts, sterol regulatory element binding protein (SREBP), SREBP cleavage activating protein (Scap), niemann-pick type C disease (NPC)
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized histologically by the presence of amyloid plaques and neurofibrillary tangles in the brain. The plaques consist of aggregated proteinaceous material, a major component of which is β-amyloid (Aβ). The neurofibrillary tangles are composed of paired helical filaments of the microtubule associated phosphoprotein, tau. For more than a decade, research has focused on how Aβ is generated, how it impacts cellular function, and how it promotes the pathobiology of tau.
The Amyloid Precursor Protein (APP), located on chromosome 21 was implicated as being a critical player in AD as the Aβ peptide that is found in amyloid plaques is derived from APP following a number of cleavage events, and Down syndrome patients, who have three copies of Chromosome 21, always develop AD. In 1991, the first mutations that cause familial forms of AD were identified in APP (reviewed in [1]). In 1995, a second genetic locus, presenilin 1 (PS-1) was found to be associated with familial AD. This was soon followed by the identification of mutations in the presenilin 2 (PS-2) gene. Mutations in APP and the presenilins have as their unifying feature the ability to alter the processing of APP such that more Aβ peptides are produced (reviewed in [1]). It is therefore thought that the accumulation and/or aggregation of Aβ underlies the etiology of the disease, and that preventing this accumulation is a valid therapeutic target. Multiple studies have indicated a role for cholesterol in maintaining normal levels of Aβ in vitro and in vivo. In this review we will discuss the movement of intracellular cholesterol and how normal distribution, transport, and export of cholesterol is vital for homeostatic regulation of Aβ. The major stages of intracellular cholesterol transport are shown in Figure 1.
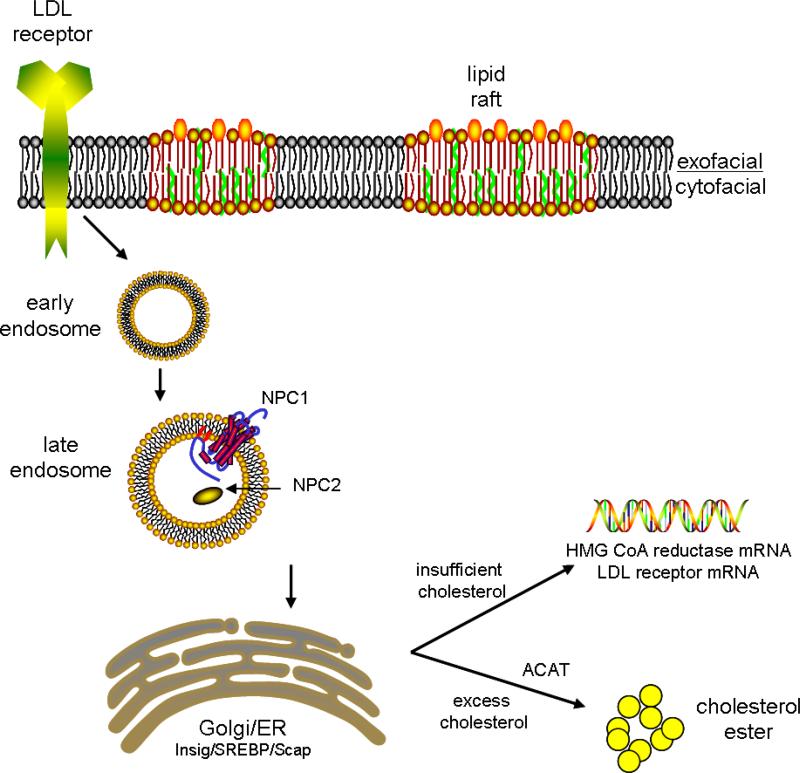
Fig 1.
Schematic representation of the intracellular cholesterol transport pathway. Extracellular cholesterol enters the cell via the LDL receptor and endocytosis. Transport from the endosomal system to the endoplasmic reticulum (ER) occurs via transport proteins such as Niemann-Pick Type C (NPC) protein. Once at the ER, excess cholesterol is converted to cholesterol ester by ACAT. If intracellular cholesterol levels drop, the N-terminus of the Sterol Regulatory Element Binding Protein (SREBP) is released and enters the cell nucleus, resulting in transcription of mRNA to increase cholesterol production and increase receptors for extracellular cholesterol.
Evidence for a role of cholesterol in Alzheimer's disease
Sporadic, late onset AD accounts for greater than 95% of all AD cases [2]. Within these sporadic cases, by far the greatest genetic risk factor is possession of the apolipoprotein E ε4 (APOE4) allele [3;4]. Not only does APOE genotype act as a risk factor for developing the disease, but also the age of onset of the disease. In an almost dose-respondent manner, the average age of onset for patients with 2 ε4 alleles is less than 70 years of age, with 1 ε4 allele, 80 years, whereas for those expressing no ε4 alleles it is 90 years [3].
How APOE genotype alters risk of developing AD remains an open question, but a primary role for apolipoproteins is cholesterol transport, and thus studies of AD began to examine the effects of cholesterol on APP processing. The brain is the most cholesterol rich organ in the body, but following development, CNS cholesterol is generated solely through de novo synthesis, with transfer from the periphery contributing little [5]. Cholesterol is essential in the CNS for, amongst other things, synapse formation, neuronal repair, myelin and neurosteroids production. ApoE plays an important role in the transport of cholesterol around the CNS, for repair, neurogenesis and myelin maintenance. Despite its important role, knockout of apoE does not cause severe impact on neurological function. This may be due to the fact that other apolipoproteins, such as apoD, can compensate when the APOE gene is removed [6].
The first evidence that cholesterol may impact Aβ production in the brain was provided in 1994, when Sparks and colleagues demonstrated that dietary cholesterol increases amyloid production in rabbits [7]. They demonstrated that a high cholesterol diet for as little as 4 weeks caused increased β-amyloid immunoreactivity in rabbit hippocampal neurons. This was followed by work on mice genetically modified to deposit cerebral β-amyloid, again showing that a cholesterol-enriched diet resulted in increased amyloid plaque deposition, increased Aβ and β-cleaved APP c-terminal fragment (CTF) production, and decreased α- cleaved secreted APP (sAPPα) [8]. Similar studies have confirmed these observations [9-12]. As mentioned above, very little cholesterol is transferred from the peripherary to the CNS, so the findings that increased dietary cholesterol can contribute to brain Aβ levels is puzzling. One possible explanation is the issue of blood brain barrier permeability, which is reported to be impaired in transgenic models of AD [13]. We have fed 5% cholesterol diets to the PS/APP transgenic mouse, and to nontransgenic mice. We found that only PS/APP mice had elevated brain Aβ, while Aβ levels in nontransgenic mice were unaltered by the diet (unpublished data).
Further evidence for a role for cholesterol in AD was found when cholesterol lowering drugs, statins, were shown to reduce both intracellular and extracellular levels of Aβ40 and Aβ42 peptides in primary cultures of hippocampal neurons transfected with human APP [14]. Studies by our group and others have shown that these drugs can also significantly reduce the levels of Aβ40 and Aβ42 in transgenic mice, wild-type mice and guinea pigs [14-16]. In addition, epidemiological studies indicated that statin use may decrease the risk of developing AD by up to 70% [17-19], and studies in humans have shown that statins use can reduce levels of Aβ in the plasma [20] and the β-cleaved fragment sAPPβ in CSF [17;21]. However prospective cohort studies have failed to demonstrate the protective effects of statins on dementia [22-24] and other studies have not replicated the Aβ lowering effect of statins in the CSF [25-27].
Plasma membrane
Cholesterol has many functions in animal cells, including a vital role in the plasma membrane. Lipids account for approximately 40% of the dry weight of plasma membranes, the remainder consisting of proteins. Phospholipids are the most abundant of these lipids (~70%) with cholesterol being the majority of the remainder (~21%). These proteins and lipids are arranged into bilayer leaflets, which display extraordinary fluidity. One of the major factors influencing this property is the cholesterol content of the membrane. An increased cholesterol concentration results in stiffening of the plasma membrane and reduced lateral motion of the membrane. This can influence basic membrane functions by preventing the translocation of substrates to proteins embedded in the membrane, and increasing endocytosis.
Early elegant experiments demonstrated the importance of membrane cholesterol for activity of the APP secretase enzymes, α- and β-secretase. Bodovitz and Klein were the first to demonstrate that increased cholesterol could impact α-secretase cleavage of APP [28]. They used a rapid delivery system that transfers cholesterol to the cell membrane in under 60 seconds [29]. This was followed by work that demonstrated that methyl-β-cyclodextrin, which removes cholesterol rapidly from the cell membrane, could decrease β-secretase cleavage of APP when combined with lovastatin [30]. Our lab has confirmed this work with methyl-β-cyclodextrin alone, and found that as cholesterol is reduced there is an inverse relationship between β- and αsecretase activities, with β-secretase cleavage decreasing and α-secretase cleavage increasing [31]. This leads to a reduction in Aβ40 and Aβ42.
While the different lipid classes are distributed asymmetrically in the leaflets of the plasma membrane they are not always assigned randomly. Even though cholesterol is an integral part of the plasma membrane, it is not evenly distributed in and between the two leaflets of the bilayer [32;33]. There is a marked asymmetry in cholesterol distribution which strongly favors the cytofacial leaflet, with approximately 85% of total membrane cholesterol residing in this region [32;34]. The importance of transbilayer distribution of cholesterol is not fully understood, but modifications to this distribution are associated with aging, statin use, alcohol and activity of membrane proteins [15;32-37]. The aging mouse membrane is particularly relevant for studies of AD, which is primarily a disease of aging. In synaptosomal plasma membranes, the normal cholesterol levels in the exofacial leaflet of a young male mouse are ~15% of total membrane cholesterol; by 15 months this has increased to ~25%; and by 24 months of age to ~32% [32]. The fluidity profile of the membrane was also altered, with decreased fluidity of the membrane in the older mice [32]. Membrane fluidity is important for basic membrane functions, such as endocytosis of the plasma membrane. Changes in exofacial cholesterol have been demonstrated with the cholesterol modifying agents methyl-β-cyclodextrin, apoE and statins [15;32-37]. All of these compounds are known to impact Aβ production in vivo and in vitro, and we have shown that the changes in neuronal plasma membrane cholesterol distribution caused by statins directly correlate with Aβ levels in vivo [15].
Within the membrane itself, specific lipid domains exist such as annular lipids that closely border integral proteins [38;39]; fast, slow and non-exchangeable cholesterol pools [40]; and dynamic assemblies of cholesterol and sphingolipids into moving platforms in the exoplasmic leaflet (lipid rafts) [41]. Lipid rafts are thought to be a more static, liquid-ordered phase within the phospholipid-rich liquid disordered phase of the membrane. Sphingolipids laterally associate with each other with cholesterol filling any remaining voids. The observance that cholesterol-sphingolipid rafts are insoluble in detergents led to the observation that these rafts associate with many membrane bound proteins. Glycosylphosphatidylinositol (GPI)-anchored proteins found in rafts suggest that lipid rafts are important entities in membrane signaling [42;43]. Rafts are not the dominating lipid phase in the exoplasmic leaflet of the membrane except in the case of myelin in the oligodendrocytes where concentrations of cholesterol and sphingolipids are much higher than in other cells [44]. Rafts are also implicated in membrane trafficking of proteins involved in biosynthetic and endocytic pathways [41].
The discovery that APP, β-secretase, PS-1, and Aβ are all present in lipid rafts [45;46] has led to speculation that the lipid raft fraction is a putative site of membranous APP cleavage. Depletion of membrane cholesterol affects the association of APP with rafts [30], and studies that used antibody cross linking to isolate β-secretase from lipid rafts succeeded in reducing Aβ production [47]. This led to the suggestion that APP in lipid rafts is primarily processed via the β-secretase pathway, and APP outside of rafts is processed via the α-secretase pathway [47]. Further studies have implicated γ-secretase activity in lipid rafts [48]. The localization of these proteolytic proteins suggests that rafts are a prime target for Aβ reducing compounds, and as cholesterol is a key component of these rafts, membrane cholesterol is an early pathway target for reducing Aβ production.
Interestingly, we have found that cytoplasmic APP interacting proteins show differential distribution within cells, sometimes localizing to the regions of lipid rafts. While FE65 and Mint/X11, two APP adaptor proteins [49], are found outside of lipid rafts, Disabled-1 (Dab1) partially fractionates with lipid rafts. Fyn tyrosine kinase, which phosphorylates both APP and Dab1 [50], is found exclusively in lipid rafts [51], as are the tyrosine phosphorylated forms of APP and Dab1 (unpublished data). We further found that phosphorylation of Dab1 promotes its association with APP, which increases its presence on the cell surface and its cleavage by αsecretase [50;52]. Thus, the APP found in lipid rafts differs in its phosphorylation state, its interaction with adaptor proteins, and its proteolytic processing.
Intracellular Cholesterol Transport – the endosomal – lysosomal – ER pathway
The endosomal-lysosomal pathway is involved in the proteolytic processing of APP to Aβ [53-58]. Endosomal abnormalities have been found in AD, where they precede amyloid and tau pathology in the neocortex. Enlarged neuronal endosomes have also been recorded in Down's syndrome (Trisomy 16) prior to dementia symptoms [59], and are thought to be caused by the excess production of APP β-CTF [60]. Thus, factors affecting APP trafficking in endosomal compartments are important for understanding AD pathogenesis.
One disorder that is valuable in determining a role for intracellular cholesterol trafficking in neurodegenerative disease is Niemann-Pick Type C (NPC) disease. The defect in NPC disease can be caused by mutations in either the NPC1 or the NPC2 gene, however NPC1 mutations account for 95% of all cases. NPC1 is a membrane protein found in late endosomes/lysosomes [61]. NPC2 is smaller soluble protein found in the lysosomal lumen [62]. They are both involved in the transport of cholesterol from late endosomes / lysosomes to the endoplasmic reticulum (ER), and are thought to work in tandem with NPC2 facilitating the egress of cholesterol from lysosomes [63]. This explains why mutations in either gene cause a similar phenotype. NPC1 shares sequence homology with the cholesterol-sensing domains of several other proteins, including 3-hydroxy-methylglutaryl-CoA (HMG-CoA), which are implicated in cholesterol homeostatic mechanisms. NPC disease is an autosomal recessive disorder caused by a mutation in the NPC1 gene. It is characterized by a fatal build up of endocytosed, unesterified cholesterol and sphingolipids in late endocytic organelles, leading to demyelination, progressive neurodegeneration and death [61;64]. The Balb/c npcnih mouse [65] synthesizes abnormal NPC1 protein due to an insertion in the NPC1 gene; it develops progressive neurodegeneration and dies at 8-10 weeks of age.
Using this mouse, as well as in vitro models, we and others have shown that the movement of cholesterol through the cell has profound effects on how APP is processed. In vivo, we have shown that mutated NPC1 in mice causes an accumulation of β-CTF, Aβ40 and Aβ42. This coincided with an accumulation of presenilins in early endocytic compartments [66]. Very similar accumulations of Aβ and β-CTF were also found in human NPC1 brain, with accumulations again occurring in early endosomes [67]. In CHO cells deficient in NPC1 protein, and in cells treated with U18666A (a drug that prevents the translocation of cholesterol from lysosomes to the ER), Aβ and presenilin accumulations were found in late endosomes [68].
Cholesterol at the ER and Golgi
Cholesterol that enters the cell via the endocytic pathway is transported to the endoplasmic reticulum (ER) for processing. The ER is a cholesterol-poor environment where regulation of the cells cholesterol balance is maintained. Within the ER resides the sterol regulatory element binding protein (SREBP). Under cholesterol poor conditions, SREBP interacts with SREBP cleavage activating protein (Scap). It binds to CopII proteins, which cluster the Scap/SREBP complex into vesicles for transport to the Golgi apparatus [69]. Once in the Golgi, SREBP undergoes proteolytic cleavage and the N-terminus is released, acting as a nuclear transcription factor, with a consequential increase in cholesterol production. Recent studies demonstrate the delicate balance of ER cholesterol in regulating the cholesterol homeostatic pathway. When ER cholesterol levels make up less than 5% of total ER lipids, it causes translocation of the sterol regulatory binding element to the Golgi; once levels increase past 5%, the Insig protein binds to Scap and vesicle budding from the ER is blocked [70]. A role for SREBP in brain cholesterol homeostasis can be appreciated by the dramatic decrease in SREBP2 cleavage observed after traumatic brain injury [71] when the brain is exposed to high levels of cholesterol from degenerating cells [72].
Excess intracellular cholesterol is either stored as unesterified cholesterol (UC) in cell membranes, or as cholesterol ester (CE) in cytoplasmic lipid droplets. The balance between UC and CE pools is regulated by an ER enzyme called acyl-coA:cholesterol acyltransferase (ACAT). Intracellular concentrations of ACAT are tightly linked to UC levels. Increased UC results in ACAT activation and increased cholesterol esterification. When UC levels decrease, CE hydrolysis increases and UC pools are renewed. Mutant cell lines with inactive SREBP (M19 cells), Scap (25RA cells) or ACAT (AC29 cells) have been used to examine the role of intracellular cholesterol compartmentalization on Aβ production [73]. M19 cells, in which SREBP never becomes active, had significantly decreased UC and unaltered CE. Aβ levels were unaltered in M19 cells. 25RA cells, in which SREBP is constitutively active, had normal UC and increased CE. These cells had elevated Aβ levels. Finally in AC29 cells, in which ACAT is inactive and CE cannot be formed, there is a fourfold increase of UC, but an almost complete lack of CE. AC29 cells produced almost no detectable Aβ [73]. This work suggested that not only did the distribution of cholesterol within the cell matter, but so did the ratio of UC and CE within the cell. The importance of ACAT was confirmed in animal studies that showed that pharmacological inhibition of ACAT can reduce amyloid deposition and reduce cognitive deficits in APP overexpressing mice [74]. However, when cholesterol modulation occurs at the cell surface there is an inverse relationship between α- and β- secretase activity [31]. An intriguing aspect of this ACAT work is that both α- and β- cleaved APP products are simultaneously reduced [73;74]. However, the Kovacs group has recently found that ACAT inhibition causes the delayed maturation of full length APP in the early secretory pathway, both in vivo and in vitro, reducing the availability of APP at the cell surface for processing by the amyloidogenic or non-amyloidogenic pathways [75].
The cholesterol biosynthetic pathway
Following cleavage of SREBP in the Golgi, the N-terminus is released and acts as a transcription factor, entering the nucleus and inducing mRNA for HMG-CoA reductase and low density lipoprotein (LDL) receptors. The LDL receptors will allow more exogenous cholesterol to enter the cell, and HMG-CoA reductase production will induce more intracellular cholesterol production.
The cholesterol biosynthetic pathway has five major stages (Figure 2). Acetyl-CoA is converted to HMG-CoA and mevalonate. Mevalonate is phosphorylated to isopentenyl pyrophosphate and other active isoprenoid units, which condense and combine to form squalene. Squalene is converted to lanosterol, which is finally converted to cholesterol. Enzymes dictate the rate of each of these stages, with HMG-CoA reductase being the rate-limiting enzyme for the entire process. The commonly used statin drugs target this enzyme leading to inhibition of de novo synthesis of cholesterol.
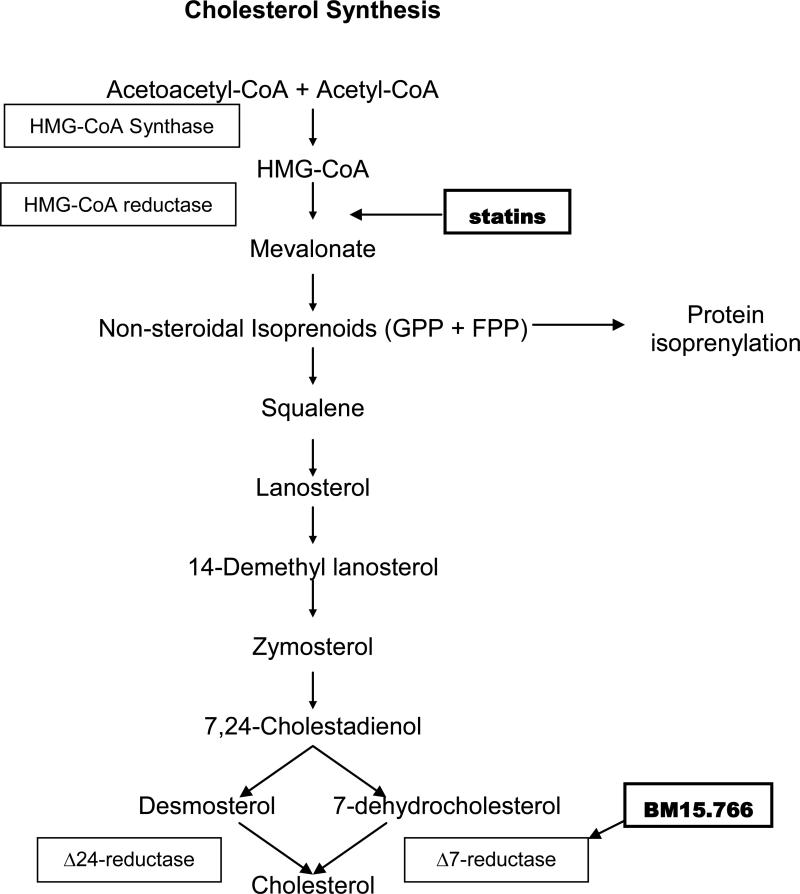
Fig 2.
Schematic diagram displaying cholesterol synthesis in human cells showing target enzymes for statins and BM15.766. GPP = geranyl pyrophosphate. FPP = farnesyl pyrophosphate.
As mentioned earlier, epidemiological studies have indicated a role for statins in preventing the incidence of AD [17-19], and studies on transgenic and non-transgenic mice have demonstrated that statin treatment can reduce levels of Aβ in mice [14-16]. Statins reduce both intracellular and extracellular levels of Aβ40 and Aβ42 peptides in primary cultures of hippocampal neurons transfected with human APP [14]. Studies on PS/APP transgenic mice using atorvastatin have shown that when administered to mice at an early stage in their disease progression, the statin can significantly reduce the levels of Aβ40 and Aβ42 [16]. However, as statins impact an early point on the cholesterol synthetic pathway, it is possible that inhibition of the dependent mevalonate pathway may be also be responsible for the effect on APP processing, rather than cholesterol itself.
The mevalonate pathway is responsible for production of many nonsteroidal isoprenoids, which are responsible for isoprenylation of many vital proteins within the cell, including small GTPases (Ras, Rho and Rab), playing an important role in protein signaling and transport. In fact this pathway has been shown to be important in diverse effects such as activation of microglia [76] and production of nitric oxide by vascular smooth muscle cells [77]. It has been shown that atorvastatin and simvastatin can increase sAPPα production via inhibition of the Rho-associated protein kinases [78]. However, another study has shown that inhibition of the mevalonate pathway will cause increased production of sAPPβ and accumulation of Aβ [79].
Despite this, the cholesterol lowering impact of these compounds is probably the more relevant factor in lowering Aβ, and a study examining the effects of a 7–dehydrocholesterol-Δ7-reductase inhibitor (BM15.766) on PS/APP transgenic mice, showed that Aβ and plaque formation can be reduced by treatment with these types of drugs [80]. BM15.766 inhibits the final step of the cholesterol biosynthesic pathway, and does not alter protein prenylation as statins do. This demonstrates directly that cholesterol production is vital for APP processing via the β-secretase pathway.
Cholesterol Efflux
Once cholesterol has been synthesized at the ER, it is transported to the plasma membrane within a short time frame (half-life of ~10 min) [81]. Cholesterol within the membrane is redistributed throughout the cell, with any excess removed by efflux to extracellular acceptors. The major energy dependent mechanism for cholesterol efflux is via sterol ATP binding cassette (ABC) transporters on the cell surface. There are a number of ABC transporters known to be important for cholesterol efflux found in the CNS, including ABCA1, ABCG1 and ABCG4 [82;83]. Although all three transporters are required to move cholesterol from the cell to extracellular acceptors, there is an important distinction between them. ABCA1 can deliver cholesterol directly to lipid free / lipid poor apolipoproteins such as apoAI and apoE, whereas the ABCG transporters are involved in the delivery of partially lipidated particles generated by the action of ABCA1 [84;85].
In vitro experiments using primary or secondary human neuronal cell lines showed that the majority of radiolabeled cholesterol that is excreted from a neuron to the media does so as unaltered cholesterol, with only a small fraction (<25%) being secreted as a modified polar product such as 24S-hydroxycholesterol [86]. This is in contrast to in vivo calculations in mice that estimate that the conversion of cholesterol to 24S-hydroxycholesterol by the CYP46 enzyme may account for as much as 2/3 of all cholesterol efflux from the brain, much more than any other single mechanism [87;88]. The oxysterol family members (consisting of several hydroxycholesterol molecules) act as endogenous Liver X Receptor (LXR) agonists. LXR are ligand-activated transcription factors that regulate a large number of metabolic and developmental pathways, including cholesterol homeostasis. LXR agonists induce both genes [89;90] and protein levels [91] of ABCA1, ABCG1 and apoE in the CNS. LXR regulate gene expression by forming heterodimers with the 9-cis-retinoic acid receptors (RXR) and binding to LXR-responsive elements on DNA [92]. There are two known LXR isoforms, LXR-α and LXR-β, which occur in mammals. LXRα expression is mainly limited to the liver, adrenals, intestine and spleen, while LXRβ is expressed in all tissue types, including the brain [93-97]. As such, the brains of LXRβ knockout display developmental problems with late neuronal migration [98].
Due to their integral role in modulating cholesterol efflux from the cell, LXR have become a target of interest for AD. In vivo, induction of LXR leads to increased production of ABCA1 and ABCG1, increased cholesterol efflux, and a reduction of synaptosomal plasma membrane cholesterol [91]. As such it is expected that LXR agonists should lower Aβ levels; results from in vitro studies using the LXR agonist T0901317, however, have been inconsistent. While some reports show that T0901317 does indeed decrease Aβ [83;99;100], others have shown that T0901317 selectively increases Aβ42 without changing Aβ40 levels [31;101]. This appears to be partially due to the fact that T0901317 acts as a gamma-secretase modulator in vitro, selectively raising Aβ42 levels at the expense of Aβ38 [31]. Interestingly, this does not appear to be an issue in vivo, with studies in mice showing that T0901317 decreases Aβ and may only selectively decrease Aβ42 [83;99;102]. APP transgenic mice treated with LXR agonists display improved spatial memory in a Morris water maze [103], and contextual memory in a fear conditioning paradigm [102;104]. Furthermore, LXRα or LXRβ knockout mice crossed with PS/APP transgenic mice both have elevated amyloid deposition than PS/APP mice alone [105], and despite the preferential expression of LXRβ in the brain, LXRα knockout mice had a similar increase in Aβ deposition to LXRβ mice.
In 2005, three independent groups concurrently published studies on the effects of ABCA1 knockout in APP transgenic mice [106-108]. Using four different model types, each of the groups found that soluble apoE levels were diminished by 75-85% in the ABCA1 knockout mice. They also found that despite no evidence of changes in total or cleaved APP products, the amount of amyloid deposited in each case was increased in the knockout mice [106-108]. Conversely, overexpression of ABCA1 in APP transgenic mouse has the opposite effects, with increased lipidation of apoE particles and a dramatic reduction in the amount of amyloid deposited in the mice [109]. These studies led to the hypothesis that the lipidation status of apoE was important in the clearance and deposition of Aβ in vivo. This hypothesis was advanced with a recent publication that demonstrated that apoE enhanced the degradation of Aβ by neprilysin and insulin-degrading enzyme [110]. This enhancement was dependent on the lipidation status of apoE [110]. Thus, the importance of cholesterol in AD pathogenesis is not limited to the cholesterol only in the cell membranes, but extends to its presence in brain-specific lipoproteins.
Conclusions
The importance of cellular cholesterol in APP processing relies not simply on the levels of cholesterol in the cell, but also on the distribution of cholesterol in the cell. This distribution can be affected by disease processes (e.g., Down syndrome or Niemann Pick Type C disease), by drug treatments (statins, ACAT inhibitors), or by normal aging. Although an understanding of the normal distribution of cholesterol in the CNS is still just underway, we also need to appreciate the changes that may occur under conditions of acute damage (such as traumatic brain injury or stroke) or chronic damage (such as AD). The redistribution of cholesterol under these conditions could contribute to altered APP trafficking and processing, and to the rate of amyloid deposition.
Acknowledgements
This work was supported by support from the NIH (R01 AG014473 to GWR) and from a Wright Family funded award from the Memory Disorder's Program at Georgetown University (MPB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Reference List
- 1.Hutton M, Perez-tur J, Hardy J. Genetics of Alzheimer's disease. Essays Biochem. 1998;33:117–131. doi: 10.1042/bse0330117. [DOI] [PubMed] [Google Scholar]
- 2.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J. Neurol. Neurosurg. Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 5.Edmond J, Korsak RA, Morrow JW, Torok-Both G, Catlin DH. Dietary cholesterol and the origin of cholesterol in the brain of developing rats. J. Nutr. 1991;121:1323–1330. doi: 10.1093/jn/121.9.1323. [DOI] [PubMed] [Google Scholar]
- 6.Jansen PJ, Lutjohann D, Thelen KM, von Bergmann K, van Leuven F, Ramaekers FC, Monique M. Absence of ApoE upregulates murine brain ApoD and ABCA1 levels, but does not affect brain sterol levels, while human ApoE3 and human ApoE4 upregulate brain cholesterol precursor levels. J. Alzheimers. Dis. 2009;18:319–329. doi: 10.3233/JAD-2009-1150. [DOI] [PubMed] [Google Scholar]
- 7.Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 8.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 9.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- 12.Levin-Allerhand JA, Lominska CE, Smith JD. Increased amyloid- levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J. Nutr. Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- 13.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 14.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns MP, Igbavboa U, Wang L, Wood WG, Duff K. Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. Neuromolecular. Med. 2006;8:319–328. doi: 10.1385/nmm:8:3:319. [DOI] [PubMed] [Google Scholar]
- 16.Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T, Duff K, Pappolla M, Refolo LM. Statin therapy for Alzheimer's disease: will it work? J. Mol. Neurosci. 2002;19:155–161. doi: 10.1007/s12031-002-0026-2. [DOI] [PubMed] [Google Scholar]
- 17.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R, Wolfson C, McDowell I. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch. Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 19.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 20.Friedhoff LT, Cullen EI, Geoghagen NS, Buxbaum JD. Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int. J. Neuropsychopharmacol. 2001;4:127–130. doi: 10.1017/S1461145701002310. [DOI] [PubMed] [Google Scholar]
- 21.Sjogren M, Gustafsson K, Syversen S, Olsson A, Edman A, Davidsson P, Wallin A, Blennow K. Treatment with Simvastatin in Patients with Alzheimer's Disease Lowers Both alpha- and beta-Cleaved Amyloid Precursor Protein. Dement. Geriatr. Cogn Disord. 2003;16:25–30. doi: 10.1159/000069989. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Higdon R, Kukull WA, Peskind E, Van Valen MK, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 23.Rea TD, Breitner JC, Psaty BM, Fitzpatrick AL, Lopez OL, Newman AB, Hazzard WR, Zandi PP, Burke GL, Lyketsos CG, Bernick C, Kuller LH. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch. Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 24.Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M, Welsh-Bohmer KA, Breitner JC. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch. Gen. Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson CM, Gleason CE, Hess TM, Moreland KA, Blazel HM, Koscik RL, Schreiber NT, Johnson SC, Atwood CS, Puglielli L, Hermann BP, McBride PE, Stein JH, Sager MA, Asthana S. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer's disease. J. Alzheimers. Dis. 2008;13:187–197. doi: 10.3233/jad-2008-13209. [DOI] [PubMed] [Google Scholar]
- 26.Hoglund K, Thelen KM, Syversen S, Sjogren M, von Bergmann K, Wallin A, Vanmechelen E, Vanderstichele H, Lutjohann D, Blennow K. The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer's disease. Dement. Geriatr. Cogn Disord. 2005;19:256–265. doi: 10.1159/000084550. [DOI] [PubMed] [Google Scholar]
- 27.Riekse RG, Li G, Petrie EC, Leverenz JB, Vavrek D, Vuletic S, Albers JJ, Montine TJ, Lee VM, Lee M, Seubert P, Galasko D, Schellenberg GD, Hazzard WR, Peskind ER. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J. Alzheimers. Dis. 2006;10:399–406. doi: 10.3233/jad-2006-10408. [DOI] [PubMed] [Google Scholar]
- 28.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 29.Irie T, Fukunaga K, Pitha J. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. J. Pharm. Sci. 1992;81:521–523. doi: 10.1002/jps.2600810609. [DOI] [PubMed] [Google Scholar]
- 30.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czech C, Burns MP, Vardanian L, Augustin A, Jacobsen H, Baumann K, Rebeck GW. Cholesterol independent effect of LXR agonist TO-901317 on gamma-secretase. J. Neurochem. 2007;101:929–936. doi: 10.1111/j.1471-4159.2007.04467.x. [DOI] [PubMed] [Google Scholar]
- 32.Igbavboa U, Avdulov NA, Schroeder F, Wood WG. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J. Neurochem. 1996;66:1717–1725. doi: 10.1046/j.1471-4159.1996.66041717.x. [DOI] [PubMed] [Google Scholar]
- 33.Wood WG, Schroeder F, Hogy L, Rao AM, Nemecz G. Asymmetric distribution of a fluorescent sterol in synaptic plasma membranes: effects of chronic ethanol consumption. Biochim. Biophys. Acta. 1990;1025:243–246. doi: 10.1016/0005-2736(90)90103-u. [DOI] [PubMed] [Google Scholar]
- 34.Igbavboa U, Avdulov NA, Chochina SV, Wood WG. Transbilayer distribution of cholesterol is modified in brain synaptic plasma membranes of knockout mice deficient in the low-density lipoprotein receptor, apolipoprotein E, or both proteins. J. Neurochem. 1997;69:1661–1667. doi: 10.1046/j.1471-4159.1997.69041661.x. [DOI] [PubMed] [Google Scholar]
- 35.Igbavboa U, Eckert GP, Malo TM, Studniski AE, Johnson LN, Yamamoto N, Kobayashi M, Fujita SC, Appel TR, Muller WE, Wood WG, Yanagisawa K. Murine synaptosomal lipid raft protein and lipid composition are altered by expression of human apoE 3 and 4 and by increasing age. J. Neurol. Sci. 2005;229-230:225–232. doi: 10.1016/j.jns.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 36.Kirsch C, Eckert GP, Mueller WE. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem. Pharmacol. 2003;65:843–856. doi: 10.1016/s0006-2952(02)01654-4. [DOI] [PubMed] [Google Scholar]
- 37.Wood WG, Gorka C, Schroeder F. Acute and chronic effects of ethanol on transbilayer membrane domains. J. Neurochem. 1989;52:1925–1930. doi: 10.1111/j.1471-4159.1989.tb07278.x. [DOI] [PubMed] [Google Scholar]
- 38.Jost PC, Griffith OH, Capaldi RA, Vanderkooi G. Evidence for boundary lipid in membranes. Proc. Natl. Acad. Sci. U. S. A. 1973;70:480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jost PC, Griffith OH. Lipid-lipid and lipid-protein interactions in membranes. Pharmacol. Biochem. Behav. 1980;13(Suppl 1):155–165. doi: 10.1016/s0091-3057(80)80025-6. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder F, Wood WG, Kier AB. Lipid domains and biological membrane function. In: Sperelakis N, editor. Cekk Physiology Sourcebook, A Molecular Approach. 3 ed. Academic Press; San Diego, CA: 2001. pp. 81–94. [Google Scholar]
- 41.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 42.Danielsen EM, van Deurs B. A transferrin-like GPI-linked iron-binding protein in detergent-insoluble noncaveolar microdomains at the apical surface of fetal intestinal epithelial cells. J. Cell Biol. 1995;131:939–950. doi: 10.1083/jcb.131.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 44.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 45.Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT, Jr., Kosik KS. A detergent-insoluble membrane compartment contains A beta in vivo. Nat. Med. 1998;4:730–734. doi: 10.1038/nm0698-730. [DOI] [PubMed] [Google Scholar]
- 46.Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr. Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 47.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada S, Morishima-Kawashima M, Qi Y, Misono H, Shimada Y, Ohno-Iwashita Y, Ihara Y. Gamma-secretase activity is present in rafts but is not cholesterol-dependent. Biochemistry. 2003;42:13977–13986. doi: 10.1021/bi034904j. [DOI] [PubMed] [Google Scholar]
- 49.King GD, Scott TR. Adaptor protein interactions: modulators of amyloid precursor protein metabolism and Alzheimer's disease risk? Exp. Neurol. 2004;185:208–219. doi: 10.1016/j.expneurol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Hoe HS, Minami SS, Makarova A, Lee J, Hyman BT, Matsuoka Y, Rebeck GW. Fyn modulation of Dab1 effects on amyloid precursor protein and ApoE receptor 2 processing. J. Biol. Chem. 2008;283:6288–6299. doi: 10.1074/jbc.M704140200. [DOI] [PubMed] [Google Scholar]
- 51.Yasuda K, Nagafuku M, Shima T, Okada M, Yagi T, Yamada T, Minaki Y, Kato A, Tani-Ichi S, Hamaoka T, Kosugi A. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 2002;169:2813–2817. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- 52.Hoe HS, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J. Biol. Chem. 2006;281:35176–35185. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- 53.Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC. Cell Biol. 2005;6:30. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cescato R, Dumermuth E, Spiess M, Paganetti PA. Increased generation of alternatively cleaved beta-amyloid peptides in cells expressing mutants of the amyloid precursor protein defective in endocytosis. J. Neurochem. 2000;74:1131–1139. doi: 10.1046/j.1471-4159.2000.741131.x. [DOI] [PubMed] [Google Scholar]
- 55.Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, Cataldo AM. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J. Biol. Chem. 2003;278:31261–31268. doi: 10.1074/jbc.M304122200. [DOI] [PubMed] [Google Scholar]
- 56.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 57.Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J. Biol. Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- 58.Soriano S, Chyung AS, Chen X, Stokin GB, Lee VM, Koo EH. Expression of beta-amyloid precursor protein-CD3gamma chimeras to demonstrate the selective generation of amyloid beta(1-40) and amyloid beta(1-42) peptides within secretory and endocytic compartments. J. Biol. Chem. 1999;274:32295–32300. doi: 10.1074/jbc.274.45.32295. [DOI] [PubMed] [Google Scholar]
- 59.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer's-related endosome dysfunction in Down syndrome is A{beta}-independent but requires APP and is reversed by BACE-1 inhibition. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 62.Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 63.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson MC, Vanier M, Suzuki K, Morris JA, Carstea ED, Neufeld EB, Blanchette-Mackie EJ, Pentchev PG. Niemann-Pick disease, type C: A Lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler K, Vogelstein B, editors. Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw Hill; New York: 2002. [Google Scholar]
- 65.Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 66.Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L, Mathews PM, Noble W, Matsuoka Y, Duff K. Presenilin redistribution associated with aberrant cholesterol transport enhances {beta}-amyloid production in vivo. J. Neurosci. 2003;23:5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am. J. Pathol. 2004;164:975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J. Neurosci. 2002;22:1679–1689. doi: 10.1523/JNEUROSCI.22-05-01679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cartagena CM, Burns MP, Rebeck GW. 24S-hydroxycholesterol effects on lipid metabolism genes are modeled in traumatic brain injury. Brain Res. 2010 doi: 10.1016/j.brainres.2009.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kay AD, Day SP, Kerr M, Nicoll JA, Packard CJ, Caslake MJ. Remodeling of cerebrospinal fluid lipoprotein particles after human traumatic brain injury. J. Neurotrauma. 2003;20:717–723. doi: 10.1089/089771503767869953. [DOI] [PubMed] [Google Scholar]
- 73.Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 74.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 75.Huttunen HJ, Peach C, Bhattacharyya R, Barren C, Pettingell W, Hutter-Paier B, Windisch M, Berezovska O, Kovacs DM. Inhibition of acyl-coenzyme A: cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway. FASEB J. 2009;23:3819–3828. doi: 10.1096/fj.09-134999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bi X, Baudry M, Liu J, Yao Y, Fu L, Brucher F, Lynch G. Inhibition of geranylgeranylation mediates the effects of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors on microglia. J. Biol. Chem. 2004;279:48238–48245. doi: 10.1074/jbc.M405442200. [DOI] [PubMed] [Google Scholar]
- 77.Kato T, Hashikabe H, Iwata C, Akimoto K, Hattori Y. Statin blocks Rho/Rho-kinase signalling and disrupts the actin cytoskeleton: relationship to enhancement of LPS-mediated nitric oxide synthesis in vascular smooth muscle cells. Biochim. Biophys. Acta. 2004;1689:267–272. doi: 10.1016/j.bbadis.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S. Modulation of Statin-Activated Shedding of Alzheimer APP Ectodomain by ROCK. PLoS. Med. 2005;2:e18. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J. Biol. Chem. 2005;280:18755–18770. doi: 10.1074/jbc.M413895200. [DOI] [PubMed] [Google Scholar]
- 80.Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 81.DeGrella RF, Simoni RD. Intracellular transport of cholesterol to the plasma membrane. J. Biol. Chem. 1982;257:14256–14262. [PubMed] [Google Scholar]
- 82.Bojanic DD, Tarr PT, Gale GD, Smith DJ, Bok D, Chen B, Nusinowitz S, Lovgren-Sandblom A, Bjorkhem I, Edwards PA. Differential expression and function of ABCG1 and ABCG4 during development and aging. J. Lipid Res. 2010;51:169–181. doi: 10.1194/jlr.M900250-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burns MP, Vardanian L, Pajoohesh-Ganji A, Wang L, Cooper M, Harris DC, Duff K, Rebeck GW. The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo. J. Neurochem. 2006;98:792–800. doi: 10.1111/j.1471-4159.2006.03925.x. [DOI] [PubMed] [Google Scholar]
- 84.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 86.Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W, Hill AF, Garner B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J. Biol. Chem. 2007;282:2851–2861. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- 87.Xie C, Lund EG, Turley SD, Russell DW, Dietschy JM. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 89.Muscat GE, Wagner BL, Hou J, Tangirala RK, Bischoff ED, Rohde P, Petrowski M, Li J, Shao G, Macondray G, Schulman IG. Regulation of cholesterol homeostasis and lipid metabolism in skeletal muscle by liver X receptors. J. Biol. Chem. 2002;277:40722–40728. doi: 10.1074/jbc.M206681200. [DOI] [PubMed] [Google Scholar]
- 90.Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, Tippin TK, Wilson JG, Winegar DA, Kliewer SA. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol. Endocrinol. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 91.Eckert GP, Vardanian L, Rebeck GW, Burns MP. Regulation of central nervous system cholesterol homeostasis by the liver X receptor agonist TO-901317. Neurosci. Lett. 2007;423:47–52. doi: 10.1016/j.neulet.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 92.Lala DS. The liver X receptors. Curr. Opin. Investig. Drugs. 2005;6:934–943. [PubMed] [Google Scholar]
- 93.Song C, Hiipakka RA, Kokontis JM, Liao S. Ubiquitous receptor: structures, immunocytochemical localization, and modulation of gene activation by receptors for retinoic acids and thyroid hormones. Ann. N. Y. Acad. Sci. 1995;761:38–49. doi: 10.1111/j.1749-6632.1995.tb31367.x. [DOI] [PubMed] [Google Scholar]
- 94.Kainu T, Kononen J, Enmark E, Gustafsson JA, Pelto-Huikko M. Localization and ontogeny of the orphan receptor OR-1 in the rat brain. J. Mol. Neurosci. 1996;7:29–39. doi: 10.1007/BF02736846. [DOI] [PubMed] [Google Scholar]
- 95.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 97.Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 98.Fan X, Kim HJ, Bouton D, Warner M, Gustafsson JA. Expression of liver X receptor beta is essential for formation of superficial cortical layers and migration of later-born neurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13445–13450. doi: 10.1073/pnas.0806974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J. Biol. Chem. 2005;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- 100.Sun Y, Yao J, Kim TW, Tall AR. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J. Biol. Chem. 2003;278:27688–27694. doi: 10.1074/jbc.M300760200. [DOI] [PubMed] [Google Scholar]
- 101.Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Abeta levels. J. Biol. Chem. 2002;277:48508–48513. doi: 10.1074/jbc.M209085200. [DOI] [PubMed] [Google Scholar]
- 102.Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol. Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Vanmierlo T, Rutten K, Dederen J, Bloks VW, van Vark-van der Zee LC, Kuipers F, Kiliaan A, Blokland A, Sijbrands EJ, Steinbusch H, Prickaerts J, Lutjohann D, Mulder M. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, Poirier J, Van Nostrand W, Wellington CL. The Absence of ABCA1 Decreases Soluble ApoE Levels but Does Not Diminish Amyloid Deposition in Two Murine Models of Alzheimer Disease. J. Biol. Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- 107.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 Considerably Decreases Brain ApoE Level and Increases Amyloid Deposition in APP23 Mice. J. Biol. Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 108.Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 Increases A{beta} Deposition in the PDAPP Transgenic Mouse Model of Alzheimer Disease. J. Biol. Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- 109.Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, Paul SM, Holtzman DM. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J. Immunol. 1994;152:5050–5059. [PubMed] [Google Scholar]