Abstract
The Wnt gene family encodes a set of highly conserved secreted signaling proteins that have major roles in embryogenesis and tissue homeostasis. Yet the expression of this family of important mediators in psoriasis, a disease characterized by marked changes in keratinocyte growth and differentiation, is incompletely understood. We subjected 58 paired biopsies from lesional and uninvolved psoriatic skin and 64 biopsies from normal skin to global gene expression profiling. WNT5A transcripts were up-regulated 5-fold in lesional skin, accompanied by increased Wnt-5a protein levels. Notably, WNT5A mRNA was markedly induced by IL-1α, TNF-α, IFN-γ and TGF-α in cultured keratinocytes. FZD2 and FZD5, which encode receptors for Wnt5A, were also increased in lesional psoriatic skin. In contrast, expression of WIF1 mRNA, encoding a secreted antagonist of the Wnt proteins, was down-regulated >10-fold in lesional skin, along with decreased WIF-1 immunostaining. Interestingly, pathway analysis along with reduced AXIN2 expression and lack of nuclear translocation of beta-catenin indicated a suppression of canonical Wnt signaling in lesional skin.
Our results suggest a shift away from canonical Wnt signaling towards non-canonical pathways driven by interactions between Wnt-5a and its cognate receptors in psoriasis, accompanied by impaired homeostatic inhibition of Wnt signaling by WIF-1 and Dkk.
Keywords: Wnt-signaling, psoriasis, WIF-1, Wnt proteins, keratinocytes
Introduction
The Wnt family of signaling proteins are highly conserved, lipid-modified, secreted molecules that participate in multiple developmental events during embryogenesis (Logan and Nusse, 2004). The Wnt proteins have also been shown to have fundamental roles in controlling cell proliferation, cell-fate determination, and differentiation during adult homeostasis (van Amerongen et al., 2008). Classically, Wnt signaling has been divided into two major pathways. First, the canonical signaling pathway involves the use of Frizzled (Fz) receptors paired with low-density lipoprotein receptor-related proteins (LRP) 5 and 6 as co-receptors (Wehrli et al., 2000). This leads to the activation and nuclear translocation of β-catenin and is typically linked to cell fate determination and stem cell maintenance. Alternatively, Wnt signaling occurs via the non-canonical pathway involving Fz receptors, independent of the β-catenin activation cascade (Kuhl et al., 2000). In mammals 19 Wnt proteins and 10 Fz transmembrane receptors are known (van Amerongen et al., 2008). To date, several non-canonical pathways have been described, involving the receptor tyrosine kinase Ror2 (Oishi et al., 2003), the atypical tyrosine kinase Ryk (Lu et al., 2004), and Wnt-Ca2+ signaling pathways (Kohn and Moon, 2005), all signaling modes associated with controlling cell adhesion and movement. Based on this concept, Wnt proteins have been divided into two main categories depending on which pathway they activate (Sen and Ghosh, 2008). Wnt-1, Wnt-3A and Wnt-8 have been classified as canonical Wnts whereas others such as Wnt-5a and Wnt-11 have been classified as non-canonical Wnts (van Amerongen et al., 2008). However, it has recently become evident that this is probably an over-simplification, as typical non-canonical Wnt ligands such as Wnt-5a (Liu et al., 2005) and Wnt-11 (Tao et al., 2005) have been shown to be able to activate β-catenin signaling. Thus, it is likely that individual Wnt proteins may activate multiple pathways, depending upon which Fz receptors are expressed on the cell surface (van Amerongen et al., 2008).
Three families of secreted proteins are known to inhibit Wnt signaling activity. These are the secreted Frizzled-related protein family (sFRPs) which bind Wnt proteins and prevent them from binding to the Fz receptors (Kawano and Kypta, 2003); the Dickkopf (Dkk) protein family that promote the internalization of LRP making it unavailable for Wnt binding (Logan and Nusse, 2004); and finally WIF-1, a secreted protein that binds to Wnt proteins and inhibits their activity (Hsieh et al., 1999). As is evident from the description above, this combination of multiple ligands along with multiple receptors and soluble inhibitors creates an extremely complex system.
Psoriasis is a disease characterized by chronic inflammation and altered differentiation and hyperproliferation of keratinocytes. In normal skin the fraction of proliferating keratinocytes is probably around 20% (Wright and Camplejohn, 1983), whereas in psoriasis it is almost 100%, and the mean cell cycle time is reduced from 13 days to 36 h (Weinstein et al., 1985). Moreover, it has been suggested that this hyperproliferation is not restricted to the basal epidermal layer containing keratinocyte stem cells, but may also involve suprabasal cells (Leigh et al., 1985). Despite the fundamental roles played by Wnt proteins in controlling cell proliferation and differentiation, surprisingly little is known about the state of Wnt signaling in psoriasis. Of the Wnt proteins, only Wnt-5a has been described to be up-regulated in lesional psoriatic skin as determined by gene expression (Reischl et al., 2007) and was recently shown to synergize with type 1 interferons (Romanowska et al., 2009). However, the pathogenic role of this molecule in psoriasis is presently unknown. The aims of this study were to determine the cellular source of the increased expression of Wnt-5a in psoriasis and to characterize the expression of other mediators of canonical vs. non-canonical Wnt signaling.
Materials and Methods
Study Subjects
58 psoriatic cases and 64 normal healthy controls were enrolled for the study. The criteria for entry as case was the manifestation of one or more well demarcated, erythematous, scaly psoriatic plaques that were not limited to the scalp. In instances of only a single psoriatic plaque, a plaque size of at least 1% of total body surface area was required. Study subjects did not use any systemic anti-psoriatic treatments for 2 weeks prior to biopsy. Informed consent was obtained from all subjects, under protocols approved by the Institutional Review Board of the University of Michigan. This study was conducted in compliance with good clinical practice and according to the Declaration of Helsinki Principles. Local anesthesia was obtained with lidocaine HCl 1% with epinephrine 1:100,000 (Hospira, Inc, Lake Forest, IL). Two biopsies were taken from each patient; one 6mm punch biopsy was obtained from lesional skin of patients and the other from uninvolved skin, taken at least 10cm away from any active plaque. One to two biopsies were obtained from healthy controls. Immediately upon removal, biopsies were snap frozen in liquid nitrogen and stored at -80°C.
Microarrays
The biopsy samples were prepared as previously prescribed (Gudjonsson et al., 2009). Data from the most up- or down-regulated probes were used for analysis.
Quantitation of mRNA Levels
Quantitative reverse transcription-polymerase chain reaction (QRT)-PCR was performed on paired lesional and nonlesional samples from 10 psoriatic patients and 10 normal controls. The RNA used was from the same samples used for the gene microarrays. The reverse transcription reaction was performed on 0.5 μg of RNA template and cDNA was synthesized using anchored-oligo(dT)18 primers as instructed by the manufacturer (Roche Diagnostics, Mannheim, Germany). QRT-PCR was carried out using a LightCycler2.0 system (Roche Diagnostics). The reaction profile consisted of an initial denaturation at 95°C for 15 minutes followed by 40 cycles of PCR at 95°C for 10 seconds (denaturation), 58°C for 10 seconds (annealing) and 72°C for 10 seconds (extension). The fluorescence emitted was captured at the end of the extension step of each cycle at 530nm. Primers for the genes WIF1, WNT5A, WNT3A, FZD1, FZD10, DKK2, KRT16, LRP6, RAB5A, RAB27A, GNA15, CCND1 were obtained from Superarray Biosciences (Frederick, MD). Results were normalized to the expression of the housekeeping gene ribosomal protein, large, P0 (RPLP0/36B4) (Laborda, 1991). QRT-PCR of cultured normal human keratinocytes (NHK) was carried out using cDNA prepared as above and primer sets for DEFB4, CXCL1, WNT5A and WIF1 were obtained from Applied Biosystems and run on an Applied Biosystems 7900HT Fast Real Time PCR System with results normalized to RPLP0 expression.
Immunohistochemistry
Immunohistochemistry was performed on 5μm fresh frozen tissue sections from uninvolved, lesional psoriatic and control skin using goat anti-Wnt5a (1 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-WIF-1 (5 μg/ml, R&D Systems, Minneapolis, MN) and goat anti-Fzd5 (2 μg/ml, R&D Systems) antibodies overnight at 4°C, followed by the appropriate biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA), streptavidin-horseradish peroxidase conjugate (Vector) and visualized with 3-amino-9-ethyl-carbazole (BioGenex, San Ramon, CA), followed by hematoxylin counterstaining (Biocare Medical, Concord, CA). Stained sections were examined by light microscopy, and each stained tissue section was subjected to image capture in its entirety via five digital images taken with a 20X objective. All images were subsequently analyzed using Image Pro software (Image-Pro Plus, Media Cybernetics, Silver Spring, MD), to quantify the area stained in epidermis vs. dermis (calculated as density per area).
Paraffin embedded slides from normal controls (n=3), uninvolved psoriatic (n=3) and lesional psoriatic (n=3) were used for β-catenin immunofluorescence staining. Antigen retrieval was performed for 20 minutes (Trizma base/EDTA) and the slides were then incubated with mouse anti-human β-catenin (Sigma, St. Louis, MO, USA,) overnight at 4°C. The slides were then washed three times and incubated with secondary antibody (AF594 chicken anti-mouse (Invitrogen, Carlsbad, CA). For Wnt5a CD31/CD34 counterstaining, paraffin embedded slides were processed as described above and stained with goat anti-mouse Wnt5a (R&D Systems, 1:200 dilution) overnight followed by incubation with biotinylated anti-goat antibodies for 30 minutes and avidin-488 (Vector Laboratories, Burlingame, CA, 1:200 dilution) for 10 minutes. The slides were then washed and counterstained with either mouse anti-human CD34 (Dako North America, Carpinteria, CA, ready for use) for 1 hour, or CD31 (Dako, 1:20) for 1 hour, followed by AF594 chicken-anti-mouse antibodies (Invitrogen, 1:300) for 30 minutes. The slides were washed three times and mounted with Vectashield fluorescent medium containing DAPI (Vector Laboratories, Burlingame, CA).
Keratinocyte Cultures
Normal human keratinocytes (NHK) were obtained from sun-protected adult skin by trypsin flotation and propagated in modified MCDB 153 medium (M154, Cascade Biologics, Portland, OR) as described previously (Stoll et al., 2001), with the calcium concentration set at 0.1mM. To determine the proliferative effects of exogenous Wnt-5a and WIF-1, NHK were seeded at a density of 2000 cells/cm2 on Costar® 12 well polystyrene plates (Corning Life Sciences, Lowell, MA) and allowed to attach for 48 hours. Cells were treated with either M154, Wnt5a-conditioned medium (from L Wnt-5A cell line), or medium conditioned by the parental untransfected cell line (L-M (TK-1) line). The L-M(TK-1) (CCL-1.3) and L Wnt-5A cell lines (CRL-2814) were obtained from American Type Culture Collection (ATCC) (Manassas, VA).
To condition medium for use in experiments with NHK, the L Wnt5a cell line was grown as recommended, then passaged 1:10 and grown in M154 without selection agents for 3 days. After 3 days culture medium was collected and stored at 4°C while a second batch of conditioned medium was obtained from the same cells. On day 6, cells were discarded and the 2 batches of media were combined and stored at -20°C until required,
Before use, conditioned media were diluted 1:4 in fresh M154and subsequently added to the NHK cultures, in the presence or absence of recombinant human WIF-1 (300 ng/ml) (R&D Systems) or Wnt5a-antibody (5ìg/ml; R&D Systems) for variable time periods (24-96h). Cells were trypsinized and counted with a hemacytometer at the times indicated. All experiments were performed in triplicates. Evaluation of growth was also measured by flow cytometry after 96 hours using CFSE-labeled NHK (CellTrace CFSE Detection kit, Invitrogen, Carlsbad, CA). NHK were in a similar manner exposed to recombinant Wnt-3a (200ng/ml) and recombinant Wnt-5a (200ng/ml) (R&D Systems) for variable time periods (24-96h) and manually counted.
To examine the induction of Wnt proteins by pro-inflammatory cytokines, NHK cultures were grown to 40% confluency or maintained to 4 days post-confluence. Cultures were then starved of growth factors in unsupplemented M154 for 24 hours before use. Cultures were stimulated with recombinant human TNF-α (10ng/ml), IL-17A (20ng/ml), IFN-γ (20ng/ml), IL-22 (20ng/ml), IL-1α (10 ng/ml) (R&D Systems) or TGF-α (24ng/ml, R&D Systems) for 24 hours and processed for RNA isolation as described above.
Western Blots
One 6-mm punch biopsy was obtained from normal skin from healthy individuals (n=3) and paired lesional and non-lesional biopsies were obtained from psoriatic patients (n=3) as described above. Biopsies were snap frozen in liquid nitrogen and stored at -80°C. Protein lysates were obtained by pulverizing the biopsies while still frozen and the pulverized tissue was transferred to a 2ml glass tissue grinder with 500ìl of RIPA lysis buffer. The samples were centrifuged at 4,500 rpm for 4 min and supernatants collected. Protein concentrations were measured and all samples diluted to 1mg/ml. Samples were separated on 10% SDS polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Billerica, MA). Western blots were blocked with TBS/0.1% Tween-20 (TBS-T) containing 5% nonfat dry milk for 1 hour at room temperature and incubated with Wnt-5a antibody (Cell Signaling Technology, Danvers, MA, 1:1000) overnight at 4°C. The blots were washed 3 times with TBS-T and then incubated with HRP-conjugated rabbit secondary antibody (GE, 1:2500) for 1.5 hours at room temperature. Blots were washed again and detected by chemiluminescence using ECL (GE) and Kodak X-Omat fil (Kodak). Anti-β-actin (Sigma, 1:2500 dilution) was used as a loading control.
Statistical analyses
Student's t-test was used to analyze differences. A paired t-test was used when uninvolved and lesional psoriatic datasets were compared, unpaired t-test was used for other comparisons. Quantitative immunohistochemistry data were tested for significance using Student's t-test assuming equal variances and p-values = 0.05 were considered significant.
Ingenuity Pathway Analyses
Microarray data were analyzed using Ingenuity Pathway Analysis software (Ingenuity Systems, www.ingenuity.com). For network generation, a data set containing gene identifiers and corresponding expression values for WNT pathway genes was uploaded into the application. Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base. These genes, called focus genes, were overlaid onto a global molecular network developed from information contained in the Ingenuity Pathways Knowledge Base.
Results
Differential expression of Wnt pathway genes in psoriasis lesions
We have previously used a microarray dataset to explore the differences between normal skin and uninvolved psoriatic (Gudjonsson et al., 2009b) skin, to assess the expression of candidate risk genes in psoriasis (Nair et al., 2009) and the activity of the sonic hedgehog pathway in psoriasis (Gudjonsson et al., 2009a). Here we show that this dataset contains a differential expression of Wnt pathway genes in psoriatic lesions. Global gene expression analysis revealed significant changes in several members of the Wnt ligand family and several of the Fz receptors in lesional psoriatic skin compared with both normal and uninvolved psoriatic skin (Figure 1). Of the Wnt ligands, WNT5A showed a 5.0-fold up-regulation (p<0.0001) and WNT10A had a 1.3-fold up-regulation (p<0.0001). In contrast, WNT2, WNT2B, WNT5B, WNT7B were all down-regulated (1.3-, 1.3-, 1.2-, 1.3-fold respectively, all p<0.0001) (Table 1). Of the Fz receptor genes, FZD2 and FZD5, which encode receptors for Wnt-5a, had a 1.2 and 1.3-fold up-regulation, respectively (p<0.0001), whereas FZD1, FZD4, FZD7, FZD8 and FZD10 were decreased (1.3-, 1.5-, 1.7-, 1.4- and 1.5-fold, respectively, p <0.0001, table 1). The Fz homologs act in concert with the low-density lipoprotein receptor-related proteins LRP5 or LRP6. LRP6 demonstrated a 1.6-fold down-regulation and data for LRP5 was inconclusive due to a limited probe set.
Figure 1. Microarray analysis reveals that WNT5A is strikingly upregulated, and WIF1 down-regulated in the vast majority of lesional skin samples.
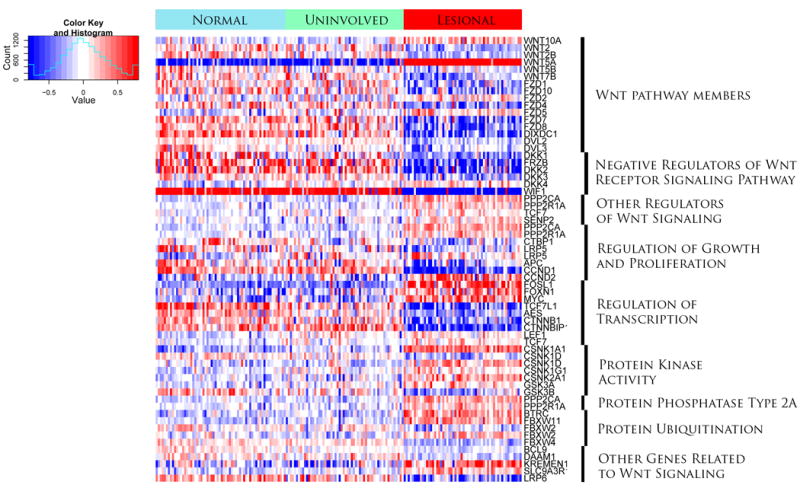
This gene expression heat map image used transcripts from 58 paired lesional and uninvolved psoriasis and 64 normal skin samples and displays Wnt pathway members, regulators of Wnt signaling, and regulators of growth, proliferation and transcription. In addition genes involved in protein kinase activity, protein phosphatases, protein ubiquitination and other genes related to Wnt signaling are shown. Color key: red, increased expression, blue, decreased expression, as indicated in the color key and histogram (inset).
TABLE 1.
Fold changes between lesional psoriatic (PP) and control (NN) skin for Wnt family members and Fz receptors present on the HU133 PLUS 2.0 microarray. P-value is corrected for multiple testing (FDR; false discovery rate).
| Gene | Fold change PP vs. NN | P-value (FDR) | Probe id |
|---|---|---|---|
| WNT1 | 1.03 | ns | 208570_at |
| WNT2 | 0.74 | 2.54E-23 | 205648_at |
| WNT2B | 0.77 | 2.86E-20 | 206458_s_at |
| WNT3 | 1.08 | ns | 221455_s_at |
| WNT4 | 1.01 | ns | 208606_s_at |
| WNT5A | 5.01 | 3.19E-176 | 205990_s_at |
| WNT5B | 0.82 | 7.75E-08 | 221029_s_at |
| WNT6 | 1.05 | ns | 221609_s_at |
| WNT7A | 1.11 | 9.94E-05 | 210248_at |
| WNT7B | 0.774 | 2.80E-15 | 217681_at |
| WNT8A | 1.00 | ns | 224259_at |
| WNT8B | 1.04 | ns | 207612_at |
| WNT9A | 0.94 | ns | 230643_at |
| WNT9B | 1.03 | ns | 1552973_at |
| WNT10A | 1.31 | 4.69E-17 | 223709_s_at |
| WNT11 | 0.92 | ns | 206737_at |
| WNT16 | 0.96 | ns | 221113_s_at |
| FZD1 | 0.74 | 1.50E-14 | 204451_at |
| FZD2 | 1.16 | 4.41E-06 | 210220_at |
| FZD3 | 0.95 | ns | 219683_at |
| FZD4 | 0.66 | 1.66E-19 | 218665_at |
| FZD5 | 1.27 | 4.13E-14 | 206136_at |
| FZD6 | 1.04 | ns | 203987_at |
| FZD7 | 0.58 | 5.19E-26 | 203705_s_at |
| FZD8 | 0.66 | 3.86E-29 | 227405_s_at |
| FZD9 | 1.07 | ns | 207639_at |
| FZD10 | 0.68 | 9.17E-17 | 219764_at |
Among the soluble inhibitors and modulators of Wnt signaling, WIF-1 was most strongly decreased in psoriatic skin, being expressed at 14-fold less than in uninvolved skin (p<0.0001). The secreted frizzled-related protein transcripts SFRP1, SFRP2, SFRP4 and SFRP5 were all down-regulated (1.3-, 1.3-, 1.3- and 1.1-fold, respectively, p<0.001). Additionally, the Dickkopf homolog genes DKK1, DKK2 and DKK3 were down-regulated by 1.6-, 2.2- and 1.3 fold, respectively (p<0.0001), whereas DKK4 showed a modest 1.2 fold up-regulation (p<0.0001).
Downstream members of the Wnt-canonical pathway, such as β-catenin 1 (CTNNB1) and β-catenin interacting protein 1 (CTNNB1) were down-regulated by 1.5 and 1.9 fold, respectively, (p<0.0001). Consistent with previously published studies (Belso et al., 2008), cyclin D1 (CCND1), which is downstream of β-catenin (Prasad et al., 2007), was down-regulated (2.0-fold, p<0.0001), whereas CCND2 was up-regulated (1.5-fold, p<0.0001).
Confirmation of microarray data by QRT-PCR
To validate the microarray results, we performed QRT-PCR for several Wnt-related genes including WIF1, WNT5A, DKK2, and CCND1, along with keratin 16 (KRT16) as a positive control for up-regulation in lesional psoriatic skin (Leigh et al., 1995). This analysis confirmed the up-regulation of WNT5A (2.9 fold, p<0.001) and down-regulation of WIF1 (9.3-fold, p<0.05), DKK2 (8.4-fold, p<0.001), and CCND1 (4.3-fold, p<0.001). As anticipated, KRT16 demonstrated more than 40-fold up-regulation (p<0.001) (Figure 2).
Figure 2. QRT-PCR confirmed the altered expression of several components of the Wnt signaling pathways in psoriasis.
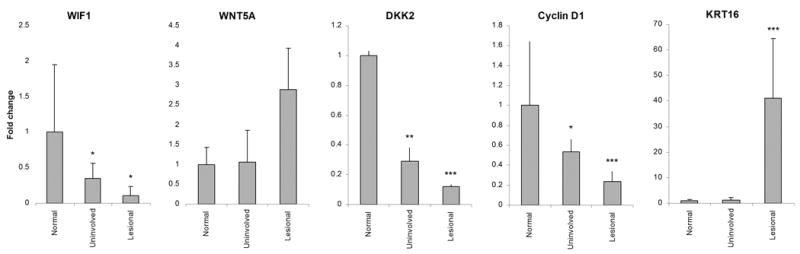
Keratin 16 (KRT16) expression was used as a positive control for lesional psoriasis skin. Data are expressed as fold-change relative to normal skin. Bars indicate mean ± S.D (n=10). Statistical significance denoted * p< 0.05, ** p<0.01, *** p<0.001.
The Wnt canonical pathway is suppressed in lesional skin
To determine the effect of gene expression changes in lesional psoriatic skin on the Wnt-pathway, we used the Ingenuity Pathway Analysis software tool (www.ingenuity.com). Gene expression differences between normal control skin and lesional psoriatic skin were overlaid onto a global molecular network within the Ingenuity Pathway Knowledge Base. This revealed global down-regulation of nearly all members of the canonical signaling pathway in psoriasis (Figure 3A). Decreased activity of the canonical Wnt-pathway was confirmed by QRT-PCR for AXIN2 (p<0.05), a marker of canonical Wnt signaling (Jho et al., 2002) (Figure 3B). Consistent with these results, we found β-catenin to be decreased in lesional psoriatic skin (Figure 3C) and there was decreased nuclear localization of β-catenin in psoriatic skin compared to normal or uninvolved skin (insets in Figure 3C).
Figure 3. Pathway analysis reveals global down-regulation of nearly all members of the canonical Wnt signaling pathway in psoriasis.
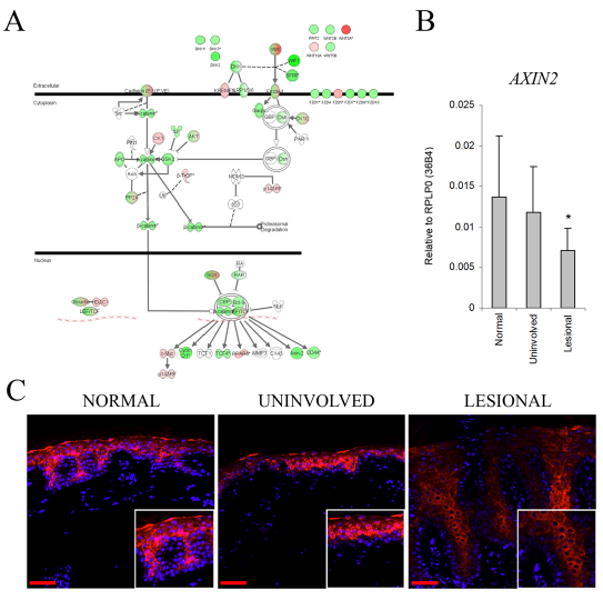
Global gene expression differences between normal and lesional psoriatic skin were overlaid onto a global molecular and pathway network within the Ingenuity Pathway Knowledge Base (A). This revealed near global down-regulation (green) of nearly all members of the canonical signaling pathway. However, several genes, associated with the non-canonical Wnt pathway, were upregulated (pink) including; WNT5A and FZD5. Decreased activity of the canonical Wnt-pathway was confirmed by QRT-PCR for AXIN2, a marker of canonical Wnt signaling (B). Bars indicate mean ± S.D, * p < 0.05. Immunofluorescent microscopy of normal, uninvolved and psoriatic skin (C) (20X, n=3).
Wnt-5a is upregulated in lesional skin whereas WIF-1 is down-regulated
We examined the protein levels of Wnt-5a and WIF-1 proteins in normal, psoriatic, and symptom-free skin from psoriatic patients by semi-quantitative immunohistochemistry. Lesional psoriatic skin demonstrated increased Wnt-5a staining in both the epidermis and dermis compared to control and uninvolved skin (Figure 4A). These findings were confirmed using computer-assisted image quantification (Figure 4C). There were strong foci of Wnt-5a staining in the papillary dermis of lesional skin. Counterstaining with CD34 and CD31 did not show any co-localization (data not shown) indicating that the source of this staining is not from vascular structures. In addition, tissue lysates from normal, uninvolved and lesional psoriatic skin demonstrated increased levels of Wnt-5a protein in lesional psoriatic skin (Figure 4B). In agreement with our gene chip and QRT-PCR data we observed decreased WIF-1 staining in the epidermis of lesional psoriatic skin, although, interestingly, there was a slight increase in the dermis (Figure 4A and C). There was slight nuclear staining of WIF-1 in epidermis of lesional skin (Figure 4A) but similar nuclear staining has been seen in bladder cancer (Urakami et al., 2006) but not in renal cell carcinoma (Kawakami et al., 2009). The significance of this nuclear staining in the psoriatic epidermis is at this time not clear.
Figure 4. Immunohistochemistry of normal, uninvolved and lesional psoriasis skin revealed increased Wnt-5a and decreased WIF-1 tissue expression.
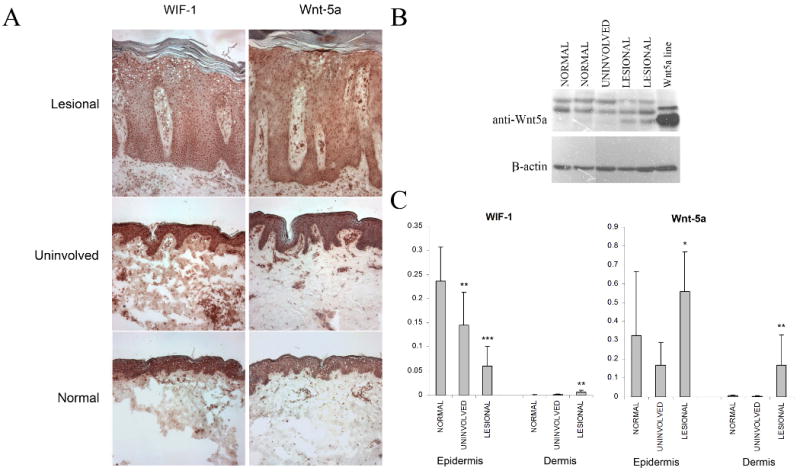
Immunohistochemical staining was performed on fresh frozen sections of skin for Wnt-5a (n=5) and WIF-1 (n=5) (A). Western-blot of normal, uninvolved and lesional psoriasis n=3). Lysate from a Wnt-5a transgenic cell line was used as a positive control (B). Differences in the expression of these proteins were confirmed with computer-assisted image quantification (C). Bars indicate mean ± S.D. Magnifications 100X. Isotype-control antibodies were used and did not show any staining (not shown).
WNT5A expression by keratinocytes is induced by several pro-inflammatory cytokines
Psoriatic skin is replete with pro-inflammatory cytokines and growth factors. To examine the effects of such a cytokine environment on Wnt expression by keratinocytes, NHK were stimulated with TNF-α, IL-17A, IFN-γ, IL-22, TGF-α and IL-1α or a combination of TGF-α and IL-1α for 24 hours. WIF1 expression was undetectable in both proliferating and differentiated NHK (data not shown), whereas WNT5A mRNA was readily detectable in both (Figure 5). We observed an approximately 1.5 to 2-fold increase in WNT5A expression treated with TGF-α, TNF-α, IFN-γ or IL-1α. The combination of both TGF-α and IL-1α had an additive effect resulting in a 2 to 3-fold increase in WNT5A expression (Figure 5). There was no change in WNT5A expression with IL-22 and IL-17A stimulation. Interestingly, the effects of these cytokines were observed only in the more differentiated keratinocytes, suggestive of a role for differentiation in the development of cytokine responsiveness. Baseline expression of WNT5A in both proliferating and differentiated NHKs was comparable to that of control and uninvolved skin. However, the maximum induction of WNT5A expression observed after cytokine or TGF-α stimulation was only about half the level of that observed in lesional psoriatic skin (data not shown), indicating that other additional mediators, or cell types, play a role in WNT5A mRNA induction in lesional psoriatic skin in vivo.
Figure 5. Expression of WNT5A by normal human keratinocytes can be induced by pro-inflammatory cytokines.
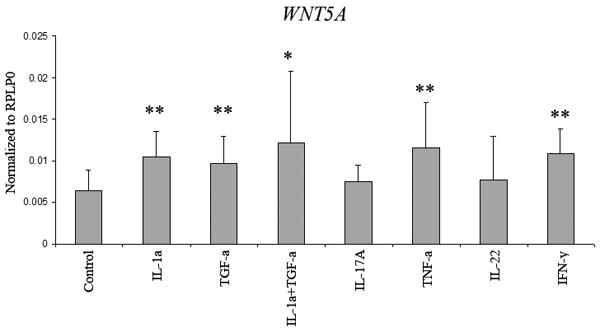
WNT5A expression could be induced by 24hours' treatment with TNF-α (10ng/ml), IFN-γ (20ng/ml), IL-1α (10ng/ml) or TGF-α (24ng/ml) after 24 hours. Expression could be further induced by the additive response to IL-1α and TGF-α. Neither IL-17A (20ng/ml) nor IL-22 (20ng/ml) had any effect on WNT5A expression. Data are expressed as fold-change relative to unstimulated NHK. Bars indicate mean ± S.D (n= 3 in duplicate wells). * p<0.05, ** p<0.01.
Effect of exogenous Wnt-5a and WIF-1 on keratinocytes
We were unable to observe any effect of recombinant Wnt-5a and Wnt-3a on NHK growth or migration (data not shown). As recombinant Wnt proteins lack post-translational modifications that are essential for their full activity we used secreted Wnt-5a from a tranfected cell line to assess the effects of Wnt agonists and antagonists on keratinocyte growth and function, NHK were cultured in the presence of diluted (20% v/v) conditioned medium from Wnt-5a transfected cell line and a control cell line in the presence of WIF-1 and anti-Wnt-5a antibodies for 24, 48, 72 and 96 hours and counted at each time point. Exogenous Wnt-5a had an suppressive effect on keratinocyte growth (p<0.01, Figure 6A), which was prevented by anti-Wnt5a antibodies. There was a minimal to no effect of WIF-1 on the anti-proliferative effect of Wnt5a (Figure 6A and B). These data were confirmed by flow cytometry using CSFE-labeled NHK examined after 96 hours of culture (Figure 6B).
Figure 6. Biological effects of Wnt-5a and WIF-1 on normal human keratinocytes.
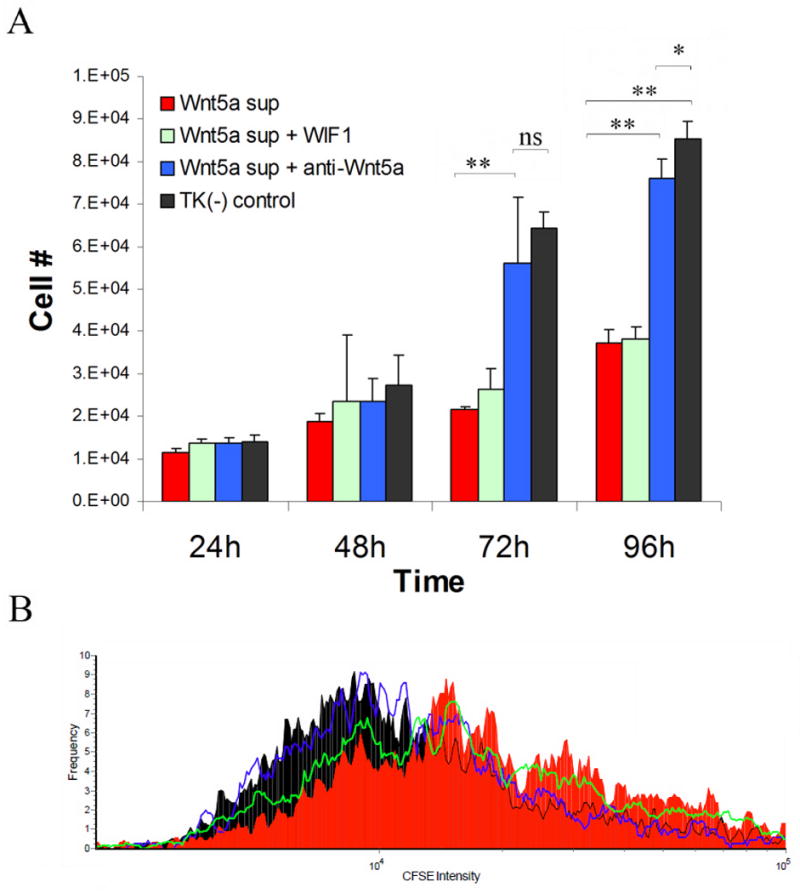
Conditioned medium containing Wnt-5a was obtained from a transfected cell line and diluted 1:4 in fresh M154CF culture medium. Control medium was obtained from the untransfected parental cell line. Wnt5a had growth suppressive effect on NHK that could be neutralized with anti-Wnt-5a antibodies but no effect was seen with exogenous WIF-1. Bars indicate mean ± S.D (n=3 in duplicate wells). * p<0.05, ** p<0.01 (A). CFSE staining by flow cytometry correlated with cell counts (B).
Discussion
Psoriasis is a common chronic inflammatory skin disease characterized by marked changes in keratinocytes growth and differentiation. The basis of this alteration in epidermal growth and differentiation is incompletely understood but has been shown to be dependent on the activity of the immune infiltrate within the psoriatic lesions (Valdimarsson et al., 1995). Several cytokines and growth factors have been implicated in this process based on mouse models (Gudjonsson et al., 2007) and in vitro/ex vivo studies. These include cytokines and growth factors such as interleukin (IL)-1α (Lee et al., 1997), vascular endothelial growth factor (VEGF) (Detmar et al., 1994), the epidermal growth factors TGF-α (Elder et al., 1989), amphiregulin (Cook et al., 1992), HB-EGF (Stoll and Elder, 1998), and several cytokines secreted by the Th17 subset of T lymphocytes, most prominently IL-17 and IL-22 (Zaba et al., 2007). As outlined earlier, the Wnt family of signaling proteins are a set of highly conserved molecules that participate and control processes such as cell proliferation, cell-fate determination and differentiation during adult homeostasis (van Amerongen et al., 2008). The changes that are observed in lesional psoriatic skin have been noted to have many similarities to wound healing (Hertle et al., 1992; Mansbridge et al., 1984), which is of interest as wounding has been shown to activate the Wnt-mediated signaling pathway (Ito et al., 2007). Thus, based on the functions of these signaling proteins and the marked changes that occur within psoriatic lesions it would not be unanticipated that these proteins may play a major role in the pathogenesis of psoriasis.
To date, very few studies have been performed to determine whether and how the Wnt pathway is activated in psoriasis. One of the reasons for this is the complexity of this signaling pathway. To date, 19 Wnt proteins and 10 Fz transmembrane receptors have been described (van Amerongen et al., 2008) which require the LRP5 and LRP6 co-receptors for effective signaling (Wehrli et al., 2000). Classically Wnt signaling has been divided into the canonical pathway which leads to activation and nuclear translocation of membrane-bound β-catenin and the non-canonical pathway which is independent of β-catenin (Logan and Nusse, 2004) but can be mediated by several different signaling cascades (Katoh, 2007; Logan and Nusse, 2004). To complicate matters, several of the Wnt proteins, including Wnt-5a, can activate either pathway depending on receptor context on the surface of the responding cells (van Amerongen et al., 2008). Wnt-5a is typically classified as a non-canonical Wnt as its transcriptional activation has been reported to be β-catenin independent (Sen and Ghosh, 2008). However, Wnt-5a has been shown to be able to either activate or inhibit β-catenin signaling depending on receptor context (Mikels and Nusse, 2006). The activity of the Wnt canonical pathway in psoriasis, as determined by β-catenin activation, has been controversial. A recently published study demonstrated increased nuclear β-catenin staining high in the suprabasal layer in lesional psoriatic skin (Hampton et al., 2007) whereas another study demonstrated only membranous staining in lesional psoriatic skin (Yamazaki et al., 2001), indicating lack of β-catenin activation in lesional psoriatic skin. Thus, based on these two reports it is not clear whether or not β-catenin activity is increased in lesional psoriatic skin.
To date only two studies have tried to directly address whether the Wnt pathway was involved in the pathogenesis of psoriasis (Reischl et al., 2007; Romanowska et al., 2009). One of these studies was based on microarray gene expression in 16 patients screening for 22,283 oligonucleotide probes (Reischl et al., 2007). The authors determined that 10% of the differentially expressed genes in their study were directly or indirectly related to the canonical Wnt/β-catenin or to the non-canonical Wnt/Ca2+ pathways. Of these genes, WNT5A was the one most markedly changed, being 5-fold up-regulated compared to uninvolved psoriatic skin whereas DKK2, an inhibitor of Wnt signaling, was found to be down-regulated (Reischl et al., 2007). Based on decreased expression of cyclin D1 (CCND1) the authors argued that this indicated decreased activity of the canonical Wnt/β-catenin pathway (Reischl et al., 2007). Our study, which is based on a microarray interrogating a much larger probe set (>55,000) and on a larger cohort (64 cases and 58 controls), has extended our knowledge on the status of the Wnt family in psoriasis. We have been able to confirm the findings of Reischl et al. (Reischl et al., 2007) on the up-regulation of WNT5A and down-regulation of DKK2 and several of the Fz receptors. Furthermore, we have been able to extend these findings and show that several members of the β-catenin pathway, including β-catenin itself, are down-regulated in lesional skin. Thus, there was both decreased expression of the beta-catenin gene and protein in lesional skin (Figure 3). In addition there was decrease in nuclear translocation of beta-catenin in psoriatic skin compared to normal and uninvolved skin (Figure 3C). These findings are consistent with the decreased AXIN2 expression observed in lesional psoriatic skin (Figure 3B), but AXIN2 is a reliable marker for canonical pathway activation (Jho et al., 2002), and demonstrate that the activity of the canonical Wnt pathway is suppressed in lesional skin. As most if not all of the Wnt inhibitory genes are down-regulated in lesional psoriatic skin, including WIF1, an interesting question is why the canonical Wnt pathway is still depressed. Of all the Wnt family members, WNT5A was highly upregulated, WNT10A and WNT7A expression were moderately increased, and other Wnt members were either unchanged or showed decreased expression (Table 1). Importantly all the Fz receptors were down-regulated except FZD5, and FZD2, both of which have been demonstrated to be involved in non-canonical Wnt signaling (Ahumada et al., 2002; Slusarski et al., 1997) and both of which can interact with Wnt-5a (He et al., 1997; Slusarski et al., 1997). Thus, this pattern of gene expression in lesional psoriatic skin is consistent with a shift away from the canonical pathway towards the non-canonical signaling mediated by Wnt-5a and its cognate receptors.
The pathogenic role of Wnt-5a in psoriasis, if any, is still unclear. Our data suggests that the main source of Wnt-5a in psoriatic lesions is the epidermis (Figure 4). Wnt-5a has been implicated both in inflammatory responses of human mononuclear cells (Blumenthal et al., 2006) and vascular proliferation (Masckauchan et al., 2006), processes that have been implicated in psoriasis pathogenesis (Lowes et al., 2007). Vascular changes in psoriasis are characterized by capillary elongation, widening and tortuosity predominantly in the dermal papillae (Hern and Mortimer, 2007). WNT5A has been described to be expressed by human endothelial cells and inhibit the canonical Wnt signaling (Masckauchan et al., 2006). Additionally Wnt-5a has been shown to promote angiogenesis (Masckauchan et al., 2006) and proliferation of endothelial cells in a dose dependent manner (Cheng et al., 2008). Wnt-5a can induce expression of a number of genes including Tie-2 (Masckauchan et al., 2006), which is a receptor tyrosine kinase and acts as the receptor for angiopoietins 1 and 2 (Kuroda et al., 2001). Tie-2 has been demonstrated to be upregulated in psoriasis (Kuroda et al., 2001) and transgenic mouse model over-expressing Tie-2 in the skin results in a psoriasis-like phenotype (Voskas et al., 2005). It is well known that vascular changes reflected by redness of lesions take a longer time to resolve than the thickness and scaling. Given these data, it is enticing to speculate given the lack of normalization of Wnt-5a during anti-TNF treatment (unpublished observation) that Wnt-5a may have a role in promoting and/or maintaining vascular changes in lesional skin.
WNT5A has also been shown to be expressed by human antigen-presenting cells through stimulation through Toll-like receptors and directly by TNF-α (Blumenthal et al., 2006). In this context, it is of interest that we observed increased expression of Wnt-5a in lesional dermis although less than that seen for the epidermis (Figure 4). However, we did not determine whether it was the inflammatory infiltrate or the vascular cells that were the main source of dermal Wnt-5a in psoriasis. TNF-α is a pro-inflammatory cytokine that has been shown to have a central role in psoriasis (Gottlieb et al., 2005) and treatments directed against TNF-α are highly effective (Chew et al., 2004; Leonardi et al., 2003). As mentioned above and confirmed in our study, TNF-α has been shown to be able to directly induce the expression of Wnt-5a (Blumenthal et al., 2006). In contrast, WNT5A expression in lesional skin does not decrease during anti-TNF-α treatment (unpublished observation), suggesting, that in psoriasis lesions, Wnt-5a is not acting down-stream of TNF-α. Wnt-5a has also been shown to be induced through stimulation of Toll-like receptor signaling, in which case, neutralizing antibodies against TNF- α did not have any suppressive effect (Blumenthal et al., 2006) indicating that WNT5A expression can also occur independently of TNF-α, which is consistent with the findings presented in our study. Interestingly, neutralization of Wnt-5a led to a decrease in the production of the cytokines IFN-γ and IL-12p40 (the common subunit of IL-12 and IL-23) (Blumenthal et al., 2006). IFN-γ and IL-23 have been shown to have crucial roles in the pathogenesis of psoriasis through the maintenance and effector functions of Th1 and Th17 cells (Cargill et al., 2007; Krueger et al., 2007; Uyemura et al., 1993; Zaba et al., 2007). Recently it was reported that Wnt-5a may synergize with type 1 interferons such as IFN-α and IFN-β (Romanowska et al., 2009), and type I interferons have been implicated in the onset of psoriasis (Nestle et al., 2005). These data support the notion that Wnt-5a has a role in amplifying and/or maintaining the inflammatory processes present in lesional skin.
Interestingly, IL-1α, TNF-α and IFN-γ and TGF-α, a member of the epidermal growth factor ligand family, were able to induce expression of WNT5A by keratinocytes. Although we were not able to detect any activity on keratinocytes with recombinant Wnt-5a or Wnt-3a in vitro as determined by both growth assays and migration assays (data not shown) we observed growth suppressive effect of secreted Wnt-5a on human keratinocytes in vitro (Figure 6). It should be noted that in vivo several of the Wnt proteins including Wnt-1, Wnt-3a and Wnt-5a have significant post-translational modifications (Kurayoshi et al., 2007) that the commercially available recombinant proteins lack. The Wnts are heavily glycosylated, which is essential for folding and secretion (Kurayoshi et al., 2007) and several, including Wnt-3a and Wnt-5a, are additionally conjugated to palmitate. This palmitate conjugation is essential for their biological activity and the lipid-unmodified form of Wnt-5a has been shown to be unable to bind to Fz5 and therefore unable to activate intracellular signal cascades and stimulate cell migration (Kurayoshi et al., 2007). This might be one of the reasons for the observed lack of biological activity in vitro with either recombinant Wnt-3a and Wnt-5a. Taken together our data indicates that Wnt-5a may have a growth suppressive effect on the psoriatic epidermis, a finding that has been observed in other settings (Olson et al., 1998), and may therefore be a part of a regulatory mechanism keeping proliferation under control.
In conclusion, our data indicates that there is a shift in lesional psoriatic skin away from canonical Wnt signaling towards non-canonical pathways likely mediated by the increased expression and production of Wnt-5a and its cognate receptors along with impaired homeostatic inhibition of Wnt signaling by WIF-1 and the Dkk proteins. Our results and previously published studies indicate that Wnt-5a may have a role in inducing the marked vascular changes in lesional skin, influencing epidermal proliferation, and play a role in the amplification of inflammatory responses. Further studies are warranted to elucidate the exact role of the Wnt pathway in psoriasis pathogenesis.
Acknowledgments
The authors express their appreciation to all of the research subjects who participated in this study and the assistance of Lynda Hodges and Kathleen McCarthy. Dr. Frank Wang for suggestions and comments. This work was supported by grants to J.T.E. (National Institute of Arthritis, Musculoskeletal and Skin Diseases, R01-), J.E.G (American Skin Association, Dermatology Foundation).
Abbreviations
- Fzd
Frizzled
- IL
interleukin
- DKK
dickkopf
- LRP
lipoprotein receptor-related proteins
- KRT
Keratin
- NHK
normal human keratinocyte
- PCR
Polymerase chain reaction
- PGA
Physician global assessment
- RMA
robust multi-array average
- SFRP
secreted frizzled-related protein
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- QRT
Quantitative real time PCR
- WIF
WNT inhibitory factor
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–10. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- Belso N, Szell M, Pivarcsi A, Kis K, Kormos B, Kenderessy AS, et al. Differential expression of D-type cyclins in HaCaT keratinocytes and in psoriasis. J Invest Dermatol. 2008;128:634–42. doi: 10.1038/sj.jid.5701059. [DOI] [PubMed] [Google Scholar]
- Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–73. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008;365:285–90. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- Chew AL, Bennett A, Smith CH, Barker J, Kirkham B. Successful treatment of severe psoriasis and psoriatic arthritis with adalimumab. Br J Dermatol. 2004;151:492–6. doi: 10.1111/j.1365-2133.2004.06105.x. [DOI] [PubMed] [Google Scholar]
- Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52:3224–7. [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–6. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Jr, et al. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243:811–4. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–9. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Aphale A, Grachtchouk M, Ding J, Nair RP, Wang T, et al. Lack of evidence for activation of the hedgehog pathway in psoriasis. J Invest Dermatol. 2009a;129:635–40. doi: 10.1038/jid.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, Nair RP, Tejasvi T, Qin ZS, et al. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol. 2009b;129:2795–804. doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- Hampton PJ, Ross OK, Reynolds NJ. Increased nuclear beta-catenin in suprabasal involved psoriatic epidermis. Br J Dermatol. 2007;157:1168–77. doi: 10.1111/j.1365-2133.2007.08195.x. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–4. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Hern S, Mortimer PS. In vivo quantification of microvessels in clinically uninvolved psoriatic skin and in normal skin. Br J Dermatol. 2007;156:1224–9. doi: 10.1111/j.1365-2133.2007.07889.x. [DOI] [PubMed] [Google Scholar]
- Hertle MD, Kubler MD, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992;89:1892–901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–6. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515–23. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116:713–20. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19:3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Briggs WH, Cheng GC, Rossiter HB, Libby P, Kupper T. Mechanical deformation promotes secretion of IL-1 alpha and IL-1 receptor antagonist. J Immunol. 1997;159:5084–8. [PubMed] [Google Scholar]
- Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–11. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Leigh IM, Pulford KA, Ramaekers FC, Lane EB. Psoriasis: maintenance of an intact monolayer basal cell differentiation compartment in spite of hyperproliferation. Br J Dermatol. 1985;113:53–64. doi: 10.1111/j.1365-2133.1985.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–82. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Mansbridge JN, Knapp AM, Strefling AM. Evidence for an alternative pathway of keratinocyte maturation in psoriasis from an antigen found in psoriatic but not normal epidermis. J Invest Dermatol. 1984;83:296–301. doi: 10.1111/1523-1747.ep12340429. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–72. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Oshimura M, Otte AP, Kumar R. Ectopic expression of wnt-5a in human renal cell carcinoma cells suppresses in vitro growth and telomerase activity. Tumour Biol. 1998;19:244–52. doi: 10.1159/000030014. [DOI] [PubMed] [Google Scholar]
- Prasad CP, Gupta SD, Rath G, Ralhan R. Wnt signaling pathway in invasive ductal carcinoma of the breast: relationship between beta-catenin, dishevelled and cyclin D1 expression. Oncology. 2007;73:112–7. doi: 10.1159/000120999. [DOI] [PubMed] [Google Scholar]
- Reischl J, Schwenke S, Beekman JM, Mrowietz U, Sturzebecher S, Heubach JF. Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 2007;127:163–9. doi: 10.1038/sj.jid.5700488. [DOI] [PubMed] [Google Scholar]
- Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS ONE. 2009;4:e5354. doi: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Ghosh G. Transcriptional outcome of wnt-frizzled signal transduction in inflammation: evolving concepts. J Immunol. 2008;181:4441–5. doi: 10.4049/jimmunol.181.7.4441. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–3. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998;7:391–7. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Kansra S, Peshick S, Fry DW, Leopold WR, Wiesen JF, et al. Differential utilization and localization of ErbB receptor tyrosine kinases in skin compared to normal and malignant keratinocytes. Neoplasia. 2001;3:339–50. doi: 10.1038/sj.neo.7900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–71. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol Today. 1995;16:145–9. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Voskas D, Jones N, Van Slyke P, Sturk C, Chang W, Haninec A, et al. A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice. Am J Pathol. 2005;166:843–55. doi: 10.1016/S0002-9440(10)62305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Weinstein GD, McCullough JL, Ross PA. Cell kinetic basis for pathophysiology of psoriasis. J Invest Dermatol. 1985;85:579–83. doi: 10.1111/1523-1747.ep12283594. [DOI] [PubMed] [Google Scholar]
- Wright NA, Camplejohn RS, editors. Psoriasis: cell proliferation. Churchill Livingstone; Edinburgh London Melbourne and New York: 1983. [Google Scholar]
- Yamazaki F, Aragane Y, Kawada A, Tezuka T. Immunohistochemical detection for nuclear beta-catenin in sporadic basal cell carcinoma. Br J Dermatol. 2001;145:771–7. doi: 10.1046/j.1365-2133.2001.04468.x. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–94. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, Nair RP, Tejasvi T, Qin ZS, et al. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol. 2009;129:2795–804. doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Hirata H, Yamamura S, Kikuno N, Saini S, Majid S, et al. Functional significance of Wnt inhibitory factor-1 gene in kidney cancer. Cancer Res. 2009;69:8603–10. doi: 10.1158/0008-5472.CAN-09-2534. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998;7:391–7. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res. 2006;12:383–91. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]


