Abstract
Understanding the role of intracellular signaling pathways in ingestive behavior is a challenging problem in behavioral neuroscience. This review summarizes work conducted on two systems with the aim of identifying intracellular events that relate to food and fluid intake. The first set of experiments focused on melanocortin receptors and their ability to signal through members of the mitogen-activated protein (MAP) kinase family. The second set of experiments focused on the role of intracellular signaling pathways in water and saline intakes that are stimulated by angiotensin II (AngII). The initial findings in each line of research have been extended by subsequent research that is discussed in turn.
Keywords: angiotensin, body fluid homeostasis, cAMP, drinking, food intake, inositol trisphosphate, MAP kinase, melanocortin, salt intake, water intake
1. Introduction
I hope there is little need to convince anybody reading this article that studying ingestive behavior has provided countless benefits, yet still offers virtually infinite promise for the future. It is clear that when we study food intake, we gain understanding of processes that underlie obesity. When we study fluid intake, we provide a more complete understanding of body fluid homeostasis and the cardiovascular diseases related to its perturbations. In addition to these most obvious benefits, ingestive behaviors have served as critical model systems in studies of basic behavioral phenomena including motivation and reward, neuro-hormonal interface, steroid-peptide interactions, and learning and memory.
In the recent history of these fields, it is reasonable to highlight two discoveries that each catalyzed outbreaks of scientific progress and achievement within their respective subfields. These discoveries are the identification of the involvement of the peptide hormones angiotensin II (AngII) in the regulation of fluid intake [1] and the discovery of the involvement of leptin in the regulation of food intake and obesity [2]. These two findings played similar roles in advancing our understanding of the basic circuits regulating ingestive behaviors and provided precise targets to guide future studies. In the case of AngII, the discovery offered a unique (at the time) opportunity to advance the understanding of how a peripherally derived peptide could interface with the brain to affect behavior. This line of research identified the circumventricular organs as key sites of AngII action, providing a novel mechanism by which a peripheral peptide could affect the brain. These findings stimulated a series of elegant neuroanatomical studies mapping afferent and efferent connections between the structures and other brain areas (for examples of relevant review articles, see [3–5]).
Decades after the groundbreaking work on angiotensin, the discovery of leptin brought a similar, although arguably more robust, flurry of investigation. The identification of leptin and its receptor provided a perfect target to identify brain circuits with potential relevance in the regulation of feeding and energy balance. These circuits and the behavioral roles of these peptides have been reviewed many times and a complete description seems unnecessary here, where it should suffice to highlight that melanocortins are widely accepted as part of a central leptin-responsive circuit.
This review will describe experiments performed on two ingestive behavior-relevant signals, melanocortins and angiotensin, each having a relatively narrow focus, but each keeping in mind a broader goal of understanding the molecular events associated with ingestive behaviors. Many of these experiments were performed in the laboratory of Steven Fluharty at the University of Pennsylvania, where I was a postdoctoral fellow, and subsequently extended by several independent groups, including our group at the University at Buffalo. The influence of Alan Epstein is clear, particularly with respect to the work described that relates to thirst and salt intake. Thus, the topic seemed especially appropriate for the Alan N. Epstein Research Award symposium upon which this review is based. Although I came to the field after his untimely death, I would like to think that he would appreciate the ways we have tried to build upon his legacy.
2. Melanocortins and Mitogen-Activated Protein (MAP) Kinase
The melanocortin system comprises several ligands and at least five receptor subtypes. Even before components of this system became implicated in the regulation of food intake, much had been discovered about the relevant receptors. Originally identified as the adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormone (MSH) receptors [6, 7], the family that would come to be known as melanocortin receptors quickly grew to include five receptor subtypes [8–10]. The initial studies that isolated and cloned these receptors supported earlier suppositions that they were prototypical seven-transmembrane receptors specifically coupled to Gs and, therefore, stimulated production of cAMP when activated [7–17]. Although a connection between the receptors and G protein-signaling was firmly established, little was known about links between melanocortin receptors and other intracellular signals. A growing body of literature, however, suggested that other seven-transmembrane receptors of initiating G protein-independent signaling cascades, largely through the actions of arrestins [18]. Accordingly, it seemed plausible, if not likely, that melanocortin receptors would show similar additional signaling capabilities.
The location of the melanocortin receptor subtypes provided strong hints regarding their specific functions in melanocortin-related events. Of the five receptor subtypes indentified, the melanocortin type 3 (MC3) and type 4 (MC4) receptors were the only species found at significant expression levels in brain tissue and their location in hypothalamus and caudal brainstem regions associated with ingestive behaviors and energy homeostasis provided a useful clue about their function [19]. Indeed, direct evidence for a role of melanocortins in food intake was demonstrated later by a number of independent laboratories [20–23], each showing that central application of melanocortin receptor agonists decreased food intake. The melanocortin system is unusual because it includes an endogenous antagonist and application of this ligand or its analogs increased feeding when given alone and blocked the intake suppressive effects of melanocortin agonists or leptin [20, 22–25].
The relevant findings of Grill et al. [22] were particularly important to my entry into this field of research, undoubtedly because the experiments were performed in the same department in which I completed my Ph.D. and because I had collaborated with the Grill group on an unrelated project [26]. In addition to these personal and professional links to Grill‘s group, we were especially intrigued by the remarkable long-term effects of a single injection of a melanocortin ligand. We were obviously not alone when we viewed this long-term effect as strongly suggestive of some receptor mediated event beyond that achieved by cAMP formation. Certainly the changes in gene expression could have involved a transcription factor more directly associated with cAMP, such as CREB, but we felt that it was equally possible that an additional regulator of transcription could play a role. To identify a relevant signal with known links to changes in gene expression, we examined the potential coupling of melanocortin receptors to MAP kinase family members. As is true for many scientific discoveries, context is critical and in this case the work of other researchers in my postdoctoral lab provided that context. Indeed, there is no doubt that we chose to start with p44/42MAP kinase (also known as ERK1/2) because members of the lab were investigating its activation in other receptor systems, including work with angiotensin receptors [27] that would influence my scientific career a second time in later years. Given this other work in the lab and the growing literature related to arrestin-related activation of MAP kinase family members by other seven-transmembrane receptors [18]it was straightforward enough to test if melanocortin receptor activation increased phosphorylation of p44/42MAP kinase.
The first experiments, performed with Jonathan Roth, examined these responses in N1E-115 cells. The lab had been exploring this cell line with the hopes that it would provide a useful in vitro model for the actions of a number of feeding-related peptide and receptor systems. These cells were found to express a number of feeding-relevant peptides, receptors, and signaling molecules, including MC3 and MC4 receptors [28] and, therefore, offered a potentially strong model system, but have yet to become a widely used in vitro model for ingestive behavior. Although we were not able to show reliable effects of melanocortin agonists on p44/42MAP kinase phosphorylation in the N1E-115 cells, when we compiled data from multiple experiments we found a hint of an effect, but one that was variable and failed to achieve statistical significance. In spite of these negative results, we continued to explore other means of testing if melanocortins activated MAP kinase.
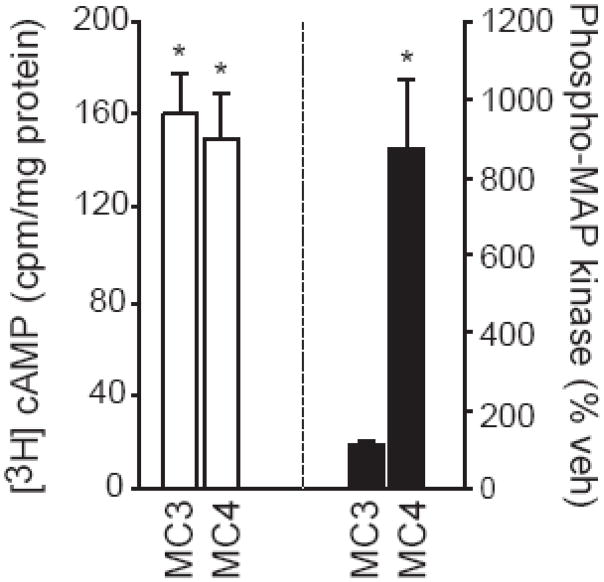
One possibility for the lack of a statistically significant result was that something in the N1E-115 system masked our ability to observe melanocortin-induced MAP kinase phosphorylation. In an effort to circumvent this, we opted for a potentially –cleaner model using transient transfection of COS-1 cells [29]. Using this approach, we were able to show that the synthetic melanocortin agonist, MTII (AcNle4-c[Asp5, d-Phe7, Lys10]α-MSH-(4–10)-NH2), increased cAMP in cells transfected with MC3 or MC4 receptors, but had no effect in untransfected cells or in cells that were transfected with the expression vector alone (Figure 1). In contrast to the similar effect on cAMP in cells transfected with either MC3 or MC4 receptor, we found stark differences in the activation of MAP kinase that depended on which receptor subtype was expressed. Specifically, MTII produced a dose-responsive increase in phosphorylated p44/42MAP kinase when cells were transfected with MC4 receptor, but not when they were transfected with MC3 receptor (Figure 1). To the best of our knowledge, this was the first demonstration of a melanocortin receptor stimulating phosphorylation of a MAP kinase family member and the first finding of different signaling mechanisms engaged by melanocortin receptor subtypes. These experiments became the basis of the thesis work of Caroline Patten, who replicated and extended these studies as described later in this review.
Figure 1.
cAMP and MAP kinase signaling through the melanocortin receptors. COS-1 cells were transfected with MC3 or MC4 receptor and treated with MTII. Formation of cAMP is shown on the left (white bars) and activated (phosphorylated) p44/42MAP kinase is shown on the right (black bars). Asterisks are used to indicate differences from cells in the same transfection condition that were treated with vehicle. Based on Daniels et al. [29].
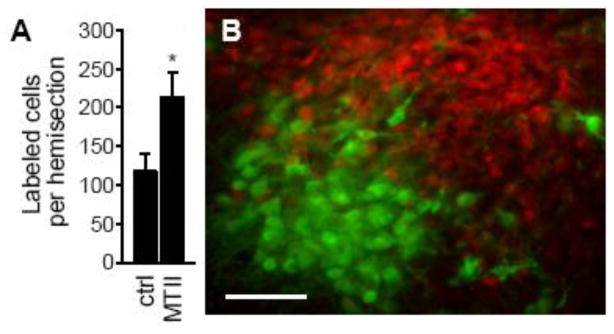
While the in vitro studies were being conducted, parallel efforts were testing if melanocortin receptor activation stimulated MAP kinase in vivo. To this end, we counted the number of cells in the hypothalamus that were immunoreactive for phosphorylated p44/42MAP kinase after rats were injected with MTII into the lateral ventricle. MTII-injected rats had a greater number of cells with activated MAP kinase than were found in control rats (vehicle or no injection; Figure 2A). Our analysis indicated that virtually all of the activated MAP kinase within the hypothalamus was in the paraventricular nucleus (PVN). We also showed an almost complete segregation of cells with activated MAP kinase and cells with oxytocin (Figure 2B). The staining for oxytocin, however, provided an excellent landmark to which we compared the distribution of activated MAP kinase, allowing us to conclude, with reasonable confidence, that the majority of the activation was within the dorsal portion of the medial parvocellular subdivision of the nucleus. We also noted a portion of the PVN that was reliably devoid of MAP kinase staining and, with the aid of the oxytocin as a landmark, were able to determine that it was the ventral part of the medial parvocellular subdivision of the PVN. As described below, a number of subsequent studies built upon these findings while my attention shifted to a different research question.
Figure 2.
Melanocortin-induced p44/42MAP kinase activation in rat PVN. (A) The number of cells immunohistochemically labeled for phosphorylated p44/42MAP kinase in brains from rats treated with vehicle or MTII is shown as the mean (±SEM) cells per PVN hemisection). An asterisk indicates p<0.05. (B) Double-labeling for phosphorylated p44/42MAP kinase (red) and oxytocin (green) in the brain of a rat injected with MTII. The scale bar is 50 μm. Data are from Daniels et al. [29].
3. Divergent Signaling Hypothesis of Angiotensin-Induced Water and Saline Intake
Although I joined the Fluharty lab as part of an effort to expand its focus on body fluid homeostasis to include studies on energy homeostasis, and therefore initially studied melanocortin receptors, I was consistently intrigued by the work of others in the lab studying angiotensin receptor-related events. The laboratory had a rich history in the field of thirst and salt appetite and the lasting influence of Alan Epstein was notable. I was often tempted to move toward the fluid balance side of the lab and was even more tempted after helping author a review paper on salt intake [30]. I eventually succumbed and started an offshoot of a project that had been spearheaded by Daniel Yee, a member of the Fluharty lab. Yee and others in the lab had performed a series of in vitro mutational studies suggesting that the structural elements of the angiotensin type 1 (AT1) receptor that mediate its G protein signaling differed from those that activated p44/42MAP kinase [27]. The AT1 receptor is well known to stimulate the G protein Gq, which initiates a biochemical cascade leading to the activation of protein kinase C (PKC) and formation of inositol trisphosphate (IP3). The additional ability of AngII to stimulate activation of MAP kinase family members was already recognized [31, 32] and appeared involve β-arrestins [18]. Studies in the Fluharty lab extended this work by demonstrating that the activation of p44/42MAP kinase could occur, at least in mutated receptors, independent of the more traditionally ascribed G protein-mediated signaling pathways. Thus, it seemed clear that this so-called G protein-coupled receptor, like other members of the receptor superfamily, could do more than simply stimulate G proteins.
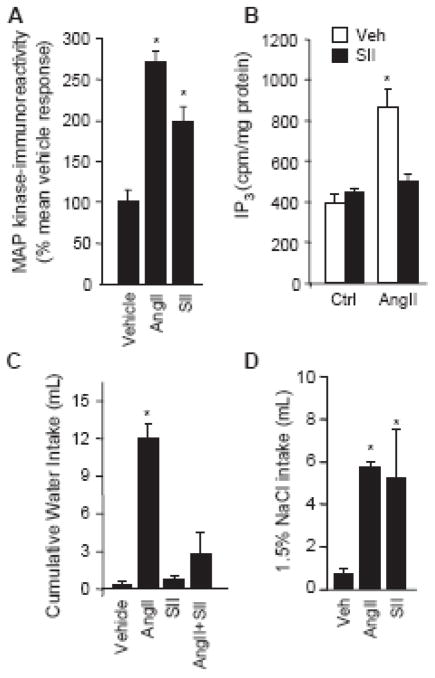
Although the mutagenesis strategies were at the forefront of the research program, similar questions were exploiting advances in peptide development to address some of the prevailing questions. One specific set of experiments took advantage of the unusual intracellular signaling response to a peptide that was similar to AngII, but with three substituted amino acids (Sar1,Ile4,Ile8-angiotensin II; SII). The ligand, developed by Miura and Karnik [33], was initially thought to be an antagonist because it bound, but failed to activate, the AT1 receptor [33]. Interestingly, when activated MAP kinase, rather than IP3 formation, was used as the dependent variable, it became clear that this analog acted as an agonist at the AT1 receptor, in spite of its inability to activate G protein-mediated events [34–36], making it seem more appropriate to refer to the compound as a –signaling selective agonist rather than an agonist or antagonist [37]. Colleagues in the lab had replicated many of these in initial in vitro findings and extended them to show that in addition to failing to generate IP3 formation, SII blocked activation of this pathway in the presence of AngII (Figure 3A–B). This finding alone was a small step forward, but it offered what we perceived to be a unique opportunity to test the ingestive responses that might occur from AT1 receptor activation with or without the full complement of intracellular responses.
Figure 3.
The angiotensin analog, SII, stimulates MAP kinase and saline intake, but antagonizes AngII- formation and water intake. (A) AngII and SII similarly increase activated p44/42MAP kinase induced IP3 in COS-1 cells transfected with the AT1 receptor. (B) COS-1 cells were transfected with AT1 receptor and subsequently treated with vehicle (ctrl) or AngII in the presence or absence of SII. SII failed to stimulate IP3 formation and prevented AngII-induced IP3 formation. (C) Water intake in a one-bottle test was stimulated by AngII injected into the forebrain ventricle of male rats; however, rats injected with SII did not drink more water than controls and AngII-induced intake was prevented by SII. (D) Rats injected with AngII or SII drank similar volumes of 1.5% saline in a two-bottle test. Asterisks indicate p<0.05 compared to controls. Data are from Daniels et al. [38].
In our first experiments, we injected AngII, SII, or vehicle into the forebrain ventricles of rats and measured the resultant water intake. Not surprisingly, rats that were injected with AngII drank a considerable amount of water and those injected with vehicle drank almost no water. Although we started the experiment with no real prediction of what would happen, it would be disingenuous to say that we were not a bit disappointed to find that the rats injected with SII drank virtually no water and closely resembled those injected with vehicle. We conducted additional experiments to determine if SII prevented water intake stimulated by AngII, in addition to the lack of response when SII was given alone, and found that it reliably antagonized the dipsogenic effect of AngII (Figure 3). The real surprise came later, when we examined the effect of SII in rats that were given access to both water and saline. At this point, we were quite convinced that the experiment was going to produce another set of negative results, but recognized that it was worth doing, if only for the sake of completeness. To our surprise, we found that SII- and AngII-treated rats drank markedly different amounts of water, but both groups drank similar amounts of saline, each greater than that consumed by the control group (Figure 3). We subsequently confirmed that SII activated p44/42MAP kinase in rat brain, published the findings [38], and expanded upon our ideas in a presentation at the International Congress on Neuroendocrinology [39] to conceptualize the present working hypothesis. This hypothesis, which I think of as the –divergent signaling hypothesis of AngII-induced water and saline intake, predicts that the divergent limbs of AngII intracellular signaling pathways each have different relevance for the separable water and saline intakes stimulated by AngII. More specifically, we hypothesized that the G protein-mediated limb is more relevant for water intake than for saline intake, and that activated MAP kinase is more relevant for saline intake than it is for water intake, at least with respect to the response to AngII.
4. Building upon the foundations
Both of these lines of research have received considerable development since the initial studies. The melanocortin studies have been expanded upon mostly by other researchers whereas my laboratory at the University at Buffalo has continued the work on angiotensin-mediated ingestive responses, specifically testing the hypothesis emerging from the studies using SII. The studies related to these lines of research will be discussed in turn.
4.1 Further studies related to melanocortin stimulation of MAP kinase signaling pathways
Since the time we found that the MC4 receptor stimulates phosphorylation of p44/42MAP kinase, a number of quite elegant studies have supported and extended our findings. Vongs et al. [40] extended our findings in rodent receptors to show that the human MC4 receptor also activates p44/42MAP kinase. These studies also showed that the response was blocked by PI3 kinase inhibitors and, interestingly, unaffected by PKA inhibitors. In subsequent studies, we also found that a PKA inhibitor failed to block melanocortin-induced p44/42MAP kinase activation [41]. Thus, the relevant signal to activate MAP kinase appears upstream of PKA.
The precise mechanism responsible for coupling the MC4 receptor to MAP kinase family members remains elusive, but subsequent mutational and inhibitor studies provide useful clues for those attempting to elucidate the specifics in this system. Although we and others failed to prevent melanocortin-induced activation of p44/42MAP kinase by PKA inhibition [40, 41], we found that inhibiting adenylyl cyclase prevented p44/42MAP kinase phosphorylation [41], suggesting the requirement of cAMP. It is important to note that cAMP does not, however, appear sufficient for p44/42MAP kinase activation because increased cAMP after MC3 receptor activation was not reliably associated with increased phosphorylation of p44/42MAP kinase [29, 41]. Nevertheless, these experiments represent a significant step toward understanding the putative connection between the MC4 receptor and MAP kinase family members.
Establishing a role for MAP kinase in the behavioral effects of melanocortins is a considerably more difficult problem, but one that has been the subject of studies by several groups, including the groups led by Berthoud and by Schwartz. Studies by Berthoud‘s group demonstrated melanocortin-induced activation of p44/42MAP kinase in the caudal brainstem, another site of MC4 receptor expression [42]. Taken together with previous studies from the Berthoud lab [43], the data support an interesting convergence of melanocortins and cholecystokinin (CCK). More specifically, peripheral CCK administration increased activated p44/42MAP kinase in the caudal brainstem [43], but this effect was prevented by injection of a melanocortin receptor antagonist into the fourth ventricle [42]. Moreover, hindbrain application of U0126, a potent inhibitor of p44/42MAP kinase, prevented the intake suppressive effect of MTII [42] or CCK [43]. The requirement of this interaction for the intake-suppressive effect of CCK has been both supported and questioned by studies using MC4 receptor knock-out models with one group showing that mice without MC4 receptors fail to respond to CCK [44] and another showing a complete, if not an enhanced, response in these mice [45]. Although this issue seems to remain unresolved, an intact CCK response in these mice suggests that mechanisms other than melanocortin receptors can account for the actions of CCK, at least in the genetically disrupted model.
The behavioral relevance of melanocortin-relevant MAP kinase family members in the hypothalamus has received less attention, even though this was the site in which the first in vivo association between melanocortins and MAP kinase was observed [29]. Recent studies and ongoing work by Schwartz and colleagues, however, may offer some clues about the role of melanocortin-relevant MAP kinase signaling in this brain area. Recently, Blouet et al. [46] reported that leucine administration activates melanocortin-producing neurons in the medial basal hypothalamus and that the anorexic effect of leucine is attenuated by a melanocortin antagonist. Although it is difficult to establish a direct link between MAP kinase family members and this response in vivo, it is particularly intriguing that leucine administration activated p44/42MAP kinase in the PVN and that the anorexic effect of leucine was prevented by the MAP kinase inhibitor U0126 [46]. In spite of this strong suggestion that melanocortin-mediated MAP kinase is involved in the response to leucine, immunohistochemical data in the study reveal a subtle discrepancy that remains to be reconciled. Specifically, when we activated forebrain melanocortin receptors by injecting MTII into the lateral ventricle, we found that the resultant phosphorylated MAP kinase was almost completely segregated from oxytocin-producing neurons in the PVN [29]. In contrast, the MAP kinase activation in the PVN that Blouet et al. found after leucine injections into the mediobasal hypothalamus was co-localized with oxytocin [46]. Thus, it appears that the amount of overlap depends on the context, raising a number of open and interesting questions.
4.1 Further studies related to the divergent signaling hypothesis of angiotensin-induced water and saline intake
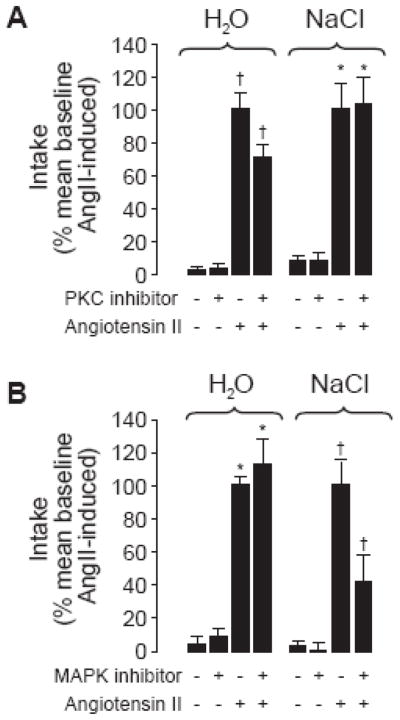
Unlike the work with melanocortins, which was extended mostly by other research groups, the studies on angiotensin signaling and behavior have been built upon almost exclusively by my lab at the University at Buffalo. The first and most obvious experiment was designed as a direct test of the hypothesis generated by our earlier studies. Toward this end, we adopted a strategy used by Fleegal and Sumners [47] and used a PKC inhibitor to test the requirement of PKC in water intake stimulated by AngII. Building upon these earlier studies, we replicated their finding that the PKC inhibitor, chelerythrine, attenuated water intake after AngII treatment and extended it to show that chelerythrine had no effect on saline intake (Figure 4A). This finding is consistent with our hypothesis that G protein-mediated pathways are more relevant to water intake than they are to salt intake [48]. In a separate set of experiments, we used a similar experimental design, but instead of a chelerythrine pretreatment, rats were given the MAP kinase inhibitor, U0126, before injection of AngII. Rats given the MAP kinase inhibitor drank markedly less saline than rats given only AngII, but the groups drank similar amounts of water (Figure 4B). Thus, our first direct test supported the hypotheses generated from our earlier SII experiments.
Figure 4.
The effect of PKC or MAP kinase inhibition on 60-minute water and saline intakes in response to AngII. Data are shown as the mean SEM intake relative to the average response to AngII alone. PKC inhibition attenuated water, but not saline intake (A), whereas MAP kinase inhibition reduced saline, but not water intake (B). Asterisks are used to intake differences from vehicle-treated or inhibitor-only groups and crosses intake differences from all other treatment groups (p<0.05) using the raw intake data before transformation to percent of mean baseline intakes. Based on Daniels et al. [48].
Additional work in the laboratory has tried to build upon the clear separability of angiotensin-induced water and salt intakes, while remaining cognizant of the potential roles of divergent intracellular signaling pathways. The idea that water and salt intakes are separately regulated is not novel by any means. Indeed, the classic double-depletion hypothesis [49, 50] predicts that depletion of the intracellular fluid compartment stimulates water intake whereas dehydration of the extracellular compartment is a stimulus for intake of both water and salt. Given that AngII, at least of peripheral origin, is a key signal of extracellular dehydration, it is not surprising that responses to AngII include both water and salt intake. The natures of these two intakes, however, are largely considered separate events with different properties. Many studies suggest differences in the temporal properties and sensitivity of the intakes, specifically that water intake is more sensitive and occurs faster than salt intake and that salt intake likely is more proximally dependent on sodium loss stimulated by AngII. Our studies and studies of others, however, suggest that this is an oversimplified perspective based almost exclusively on experiments with concentrated saline solutions. Indeed, a dose-response analysis of water and saline intakes after AngII in our lab showed that stimulation of 1.5% saline intake occurred at a lower dose of AngII than was needed to stimulate water intake [48]. Based on this finding, on other experiments from our lab [38, 51, 52], and on experiments from other labs [53, 54] showing that AngII-induced salt intake can occur independent from sodium loss, it seems important to differentiate between the nature of the ingestive response and, perhaps, consider salt –appetite a select form of salt intake in which concentrations of saline that are normally aversive are readily consumed, but recognize that rapid and sensitive initiation of salt intake can be observed when more dilute saline is offered. Even when more concentrated saline (e.g., 3.0%) was offered, separate phases of salt intake were observed in earlier studies of the effects of AngII on intake. In 1983, Fluharty and Manaker [55] noted two separate phases of AngII-induced intake, the first occurring early and preceding any pressor-induced sodium excretion, and the second occurring 8–12 hr after onset of AngII, when a sodium deficit was established. Separating AngII-induced water intake from the second phase of salt intake, or salt –appetite, is common, but demonstrating separability between water intake and the early phase of salt intake has received far less attention. Our laboratory has focused on understanding the differences in this early phase of salt intake, which appears to occur simultaneously with the rapid onset of water intake after administration of AngII.
One line of research in the laboratory has focused on this early phase of salt intake and the potential for separable regulation of water and salt intake by studying the effects of ghrelin on fluid intake. Recent work from our lab [56] and from another group [57] indicate that ghrelin, although more recognized for its orexigenic property, decreases water intake when given with a dipsogenic stimulus. Preliminary studies in our laboratory, conducted primarily by Elizabeth Mietlicki, suggest that ghrelin has different effects on water and saline intakes, affecting saline intake, but not water intake, in some experiments, while affecting both intakes, but in opposite directions, in others. These experiments are still in preliminary form and require a great deal of further study, but we hope that they will help us understand what appears to be a paradoxical response to ghrelin, at least with respect to water intake. More specifically, it remains unclear what the adaptive value could be for a peptide to increase food intake (thereby adding solute to the body and concentrating body fluid) while decreasing water intake. Thus, gaining a more complete picture of the ingestive responses to ghrelin may provide a more complete context to help address the current problem. Nevertheless, our initial experiments appear to provide an example of separable regulation of water and saline intakes.
A separate line of research, conducted primarily by Peter Vento, has used ingestive behavior as an endpoint to examine AT1 receptor desensitization that has been shown in vitro [58–61] and is likely responsible for the decreased behavior observed after short-term, repeated AngII injections [62, 63]. To this end, we have attempted to replicate previous findings [62] showing behavioral desensitization after repeated AngII. We were not able to demonstrate tachyphylaxis using the administration protocols in previous reports, but optimized a procedure that reliably decreased water intake after a challenge injection of AngII in our laboratory. Specifically, administration of AngII in three successive lateral ventricle injections, each 20 min apart (“treatment regimen”) without access to water, followed by a fourth challenge injection (“test”) and the return of water, produced less drinking than a treatment regimen of vehicle followed by the same challenge injection of AngII. In other words, we found that rats given more injections of AngII, spaced out over 60 min, drank less than rats given a single injection of AngII, suggesting a desensitizing property of the earlier doses on the final “test” injection. To date, we have replicated this behavioral desensitization in 4 separate experiments using different challenge injection doses and have shown that the desensitization can be attenuated by pretreatment with the AT1 receptor antagonist, losartan, if the administration of losartan is timed to affect the treatment regimen, but not the test injection. More relevant to the present discussion related to separating water and salt intakes, when we performed a 5th experiment, but provided both water and saline after the test injection, we found that rats given a treatment regimen of AngII again drank less water than their vehicle-treated counterparts, but we found no difference in their saline intakes. Accordingly, these data provide another example of separable effects on water and saline intakes stimulated by AngII. These data were presented in abstract form [51] and are the basis of a manuscript we are preparing for publication.
There are, of course, a number of factors that remain to be addressed with respect to these data (issues that will be more thoroughly discussed when the data are available in manuscript form), but it is tempting to speculate that the difference in water and saline intakes we observed relate to our earlier findings that these intakes are regulated by separate intracellular signaling pathways [38, 39, 48]. In this regard, it is noteworthy that manipulating the G protein-mediated pathway by inhibiting PKC reduced water intake without affecting saline intake [47, 48] because G protein uncoupling is a well-recognized event associated with AT1 receptor desensitization [64–66]. If the G protein-mediated signaling pathways are indeed more relevant to AngII-induced water intake, but not saline intake, and desensitization involves G protein uncoupling, the predicted behavioral result of desensitization would be exactly as we observed in our experiments. Thus, these findings are consistent with, and indirectly supportive of, our hypothesis that different intracellular signaling pathways regulate water and saline intakes stimulated by AngII. If, however, our subsequent experiments indicate that MAP kinase and G protein-mediated signaling are equally affected by the treatment regimen, we will be forced to reconsider this logic. Experiments addressing this and other open questions are currently underway.
5. Conclusion
As indicated earlier, this article is based upon and describes data presented in the Society for the Study of Ingestive Behavior‘s 2009 Alan N. Epstein Award lecture. It is difficult to think of an award that would have more personal meaning to me because of the influence that Alan Epstein has had on my professional development. Indeed, many of the numerous official and unofficial mentors I have accumulated in my short scientific career trained or collaborated with Epstein (e.g, Richard Miselis, Steven Fluharty, and Harvey Grill) and his teachings certainly affected the lessons they passed down to me. From my perspective, the name of the award is equally meaningful as the awarding society. The Society for the Study of Ingestive Behavior has become a home to me since early in my postdoctoral fellowship and continues to be an ideal place to exchange ideas and refine my thinking. The many members who I look forward to seeing at each meeting have greatly influenced my career and I owe a debt of gratitude to each of them. In Epstein‘s obituary, Stellar [67] wrote of a scientific meeting that “he [Epstein] called his natal meeting,‘ for it was his first meeting and his first love. The annual meeting of the Society for the Study of Ingestive Behavior was not my first meeting, but, with respect to scientific meetings, it is my first love and I believe I understand fully what Epstein meant.
The data described here are simply a small step forward in a line of research beginning with the discovery that the renin-angiotensin system stimulates drinking [1, 68]. Whether or not data from other groups or our own continue to support our hypotheses, I hope that the knowledge gained by asking the relevant questions will help move the field forward in at least some small way. Indeed, much remains to be understood about the mechanisms involved in what Alan Epstein called the “neurohormonal control of salt intake in the rat” [69].
Acknowledgments
I am especially grateful to all those who supported my nomination for this award and the many mentors who have influenced my scientific and personal development. Original, unpublished research described in this manuscript was supported by NIH awards DK073800 and HL091911. Elizabeth Mietlicki provided helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzsimons JT, Simons BJ. The effect on drinking in the rat of intravenous infusion of angiotensin, given alone or in combination with other stimuli of thirst. J Physiol. 1969;203:45–57. doi: 10.1113/jphysiol.1969.sp008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:1–127. doi: 10.1007/978-3-642-55532-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AK. The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exerc. 2007;39:1388–400. doi: 10.1249/mss.0b013e3180686de8. [DOI] [PubMed] [Google Scholar]
- 5.Daniels D, Fluharty SJ. Neuroendocrinology of Body Fluid Homeostasis. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, Brain and Behavior, Second Edition. San Diego: Academic Press; 2009. pp. 259–288. [Google Scholar]
- 6.Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann N Y Acad Sci. 1993;680:342–63. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- 7.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–51. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 8.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–50. [PubMed] [Google Scholar]
- 10.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–9. [PubMed] [Google Scholar]
- 11.Chhajlani V, Muceniece R, Wikberg JE. Molecular cloning of a novel human melanocortin receptor. Biochem Biophys Res Commun. 1993;195:866–73. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- 12.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1214–20. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 13.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1007–14. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 14.Labbe O, Desarnaud F, Eggerickx D, Vassart G, Parmentier M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. 1994;33:4543–9. doi: 10.1021/bi00181a015. [DOI] [PubMed] [Google Scholar]
- 15.Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–20. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 16.Barrett P, MacDonald A, Helliwell R, Davidson G, Morgan P. Cloning and expression of a new member of the melanocyte-stimulating hormone receptor family. J Mol Endocrinol. 1994;12:203–13. doi: 10.1677/jme.0.0120203. [DOI] [PubMed] [Google Scholar]
- 17.Fathi Z, Iben LG, Parker EM. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem Res. 1995;20:107–13. doi: 10.1007/BF00995160. [DOI] [PubMed] [Google Scholar]
- 18.Perry SJ, Lefkowitz RJ. Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol. 2002;12:130–8. doi: 10.1016/s0962-8924(01)02239-5. [DOI] [PubMed] [Google Scholar]
- 19.Low MJ, Simerly RB, Cone RD. Receptors for the melanocortin peptides in the central nervous system. Curr Opin Endocrinol Diab. 1994;1:79–88. [Google Scholar]
- 20.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 21.Poggioli R, Vergoni AV, Bertolini A. ACTH-(1–24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7:843–8. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- 22.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–35. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 24.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 25.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–7. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 26.Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci. 2004;24:11457–62. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hines J, Fluharty SJ, Yee DK. Structural determinants for the activation mechanism of the angiotensin II type 1 receptor differ for phosphoinositide hydrolysis and mitogen-activated protein kinase pathways. Biochem Pharmacol. 2003;66:251–62. doi: 10.1016/s0006-2952(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 28.Roth JD, Yee DK, Kisley LR, Fluharty SJ. Modeling the pathways of energy balance using the N1E- 115 murine neuroblastoma cell line. Brain Res Mol Brain Res. 2002;103:146–50. doi: 10.1016/s0169-328x(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 29.Daniels D, Patten CS, Roth JD, Yee DK, Fluharty SJ. Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res. 2003;986:1–11. doi: 10.1016/s0006-8993(03)03162-7. [DOI] [PubMed] [Google Scholar]
- 30.Daniels D, Fluharty SJ. Salt appetite: a neurohormonal viewpoint. Physiol Behav. 2004;81:319–37. doi: 10.1016/j.physbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Sadoshima J, Qiu Z, Morgan JP, Izumo S. Angiotensin II and other hypertrophic stimuli mediated by G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca(2+)-dependent signaling. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Huang XC, Richards EM, Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem. 1996;271:15635–41. doi: 10.1074/jbc.271.26.15635. [DOI] [PubMed] [Google Scholar]
- 33.Miura S, Karnik SS. Angiotensin II type 1 and type 2 receptors bind angiotensin II through different types of epitope recognition. J Hypertens. 1999;17:397–404. doi: 10.1097/00004872-199917030-00013. [DOI] [PubMed] [Google Scholar]
- 34.Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol. 2002;61:768–77. doi: 10.1124/mol.61.4.768. [DOI] [PubMed] [Google Scholar]
- 35.Miura S, Zhang J, Matsuo Y, Saku K, Karnik SS. Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens Res. 2004;27:765–70. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A. 2003;100:10782–7. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yee DK, Suzuki A, Luo L, Fluharty SJ. Identification of structural determinants for G protein-independent activation of mitogen-activated protein kinases in the seventh transmembrane domain of the angiotensin II type 1 receptor. Mol Endocrinol. 2006;20:1924–34. doi: 10.1210/me.2006-0018. [DOI] [PubMed] [Google Scholar]
- 38.Daniels D, Yee DK, Faulconbridge LF, Fluharty SJ. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology. 2005;146:5552–60. doi: 10.1210/en.2005-0774. [DOI] [PubMed] [Google Scholar]
- 39.Daniels D, Yee DK, Fluharty SJ. Angiotensin II receptor signalling. Exp Physiol. 2007;92:523–527. doi: 10.1113/expphysiol.2006.036897. [DOI] [PubMed] [Google Scholar]
- 40.Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept. 2004;120:113–8. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Patten CS, Daniels D, Suzuki A, Fluharty SJ, Yee DK. Structural and signaling requirements of the human melanocortin 4 receptor for MAP kinase activation. Regul Pept. 2007 doi: 10.1016/j.regpep.2007.02.005. in press. [DOI] [PubMed] [Google Scholar]
- 42.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–47. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 43.Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci. 2004;24:10240–7. doi: 10.1523/JNEUROSCI.2764-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–6. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 45.Vaughan CH, Haskell-Luevano C, Andreasen A, Rowland NE. Effects of oral preload, CCK or bombesin administration on short term food intake of melanocortin 4-receptor knockout (MC4RKO) mice. Peptides. 2006;27:3226–33. doi: 10.1016/j.peptides.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–11. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleegal MA, Sumners C. Drinking behavior elicited by central injection of angiotensin II: roles for protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Regul Integr Comp Physiol. 2003;285:R632–40. doi: 10.1152/ajpregu.00151.2003. [DOI] [PubMed] [Google Scholar]
- 48.Daniels D, Mietlicki EG, Nowak EL, Fluharty SJ. Angiotensin II stimulates water and NaCl intake through separate cell signalling pathways in rats. Exp Physiol. 2009;94:130–7. doi: 10.1113/expphysiol.2008.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzsimons JT. Some historical perspectives in the physiology of thirst. In: Epstein AN, Kissileff HR, Stellar E, editors. The Neuropsychology of Thirst: New Findings and Advances in Concepts. Washington, DC: V.H. Winston & Sons; 1973. pp. 3–33. [Google Scholar]
- 50.Epstein AN. Epilogue: Retrospect and prognosis. In: Epstein AN, Kissileff HR, Stellar E, editors. The Neuropsychology of Thirst: New Findings and Advances in Concepts. Washington, DC: V.H. Winston & Sons; 1973. pp. 315–32. [Google Scholar]
- 51.Vento PJ, Daniels D. Desensitization of dipsogenic activity after repeated angiotensin II administration. Appetite. 2009;52:863–863. [Google Scholar]
- 52.Daniels D, Mietlicki EG, Vento PJ. Divergent regulation of water and saline intake. Appetite. 2009;52:825–825. [Google Scholar]
- 53.Avrith DB, Fitzsimons JT. Increased sodium appetite in the rat induced by intracranial administration of components of the renin-angiotensin system. J Physiol. 1980;301:349–64. doi: 10.1113/jphysiol.1980.sp013210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitts DA, Thunhorst RL, Simpson JB. Modulation of salt appetite by lateral ventricular infusions of angiotensin II and carbachol during sodium depletion. Brain Res. 1985;346:273–80. doi: 10.1016/0006-8993(85)90860-1. [DOI] [PubMed] [Google Scholar]
- 55.Fluharty SJ, Manaker S. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: I. Relation to urinary sodium excretion. Behav Neurosci. 1983;97:738–45. doi: 10.1037//0735-7044.97.5.738. [DOI] [PubMed] [Google Scholar]
- 56.Mietlicki EG, Nowak EL, Daniels D. The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav. 2009;96:37–43. doi: 10.1016/j.physbeh.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, Takei Y, Ueta Y. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148:1638–47. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
- 58.Thomas WG. Regulation of angiotensin II type 1 (AT1) receptor function. Regul Pept. 1999;79:9–23. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]
- 59.Thomas WG, Thekkumkara TJ, Baker KM. Cardiac effects of AII. AT1A receptor signaling, desensitization, and internalization. Adv Exp Med Biol. 1996;396:59–69. [PubMed] [Google Scholar]
- 60.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–80. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 61.Gebke E, Muller AR, Jurzak M, Gerstberger R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience. 1998;85:509–20. doi: 10.1016/s0306-4522(97)00601-5. [DOI] [PubMed] [Google Scholar]
- 62.Torsoni MA, Carvalheira JB, Calegari VC, Bezerra RM, Saad MJ, Gontijo JA, Velloso LA. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–28. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]
- 63.Quirk WS, Wright JW, Harding JW. Tachyphylaxis of dipsogenic activity to intracerebroventricular administration of angiotensins. Brain Res. 1988;452:73–8. doi: 10.1016/0006-8993(88)90010-8. [DOI] [PubMed] [Google Scholar]
- 64.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein- coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein- coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 66.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 67.Stellar E, Alan N. Epstein (1932–1992): Obituary. American Psychologist. 1993;48:688–688. [Google Scholar]
- 68.Fitzsimons JT. The role of a renal thirst factor in drinking induced by extracellular stimuli. J Physiol. 1969;201:349–68. doi: 10.1113/jphysiol.1969.sp008760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epstein AN. Neurohormonal control of salt intake in the rat. Brain Res Bull. 1991;27:315–20. doi: 10.1016/0361-9230(91)90118-4. [DOI] [PubMed] [Google Scholar]