Summary
Sirtuins comprise a family of NAD+-dependent protein deacetylases and ADP-ribosyltransferases. Mammalian SIRT1 - a homolog of Sir2, the prototypical member of the sirtuin family - is an important regulator of metabolism, cell differentiation and senescence, stress response, and cancer. As a NAD+-dependent enzyme, SIRT1 regulates gene expression programs in response to cellular metabolic status, thereby coordinating metabolic adaptation of the whole organism. Several important mechanisms have emerged for SIRT1-dependent regulation of transcription. First, SIRT1 can modulate chromatin function through direct deacetylation of histones, as well as by promoting alterations in the methylation of histones and DNA, leading to the repression of transcription. The latter is accomplished through the recruitment of other nuclear enzymes to chromatin for histone methylation and DNA CpG methylation, suggesting a broader role of SIRT1 in epigenetic regulation. Second, SIRT1 can interact and deacetylate a broad range of transcription factors and coregulators, thereby regulating target gene expression both positively and negatively. Cellular energy state, specifically NAD+ metabolism, plays a major role in the regulation of SIRT1 activity. Recent studies on the NAD+ biosynthetic enzymes in the salvage pathway, nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase 1 (NMNAT-1), have revealed important functions for these enzymes in SIRT1-dependent transcription regulation. The collective molecular actions of SIRT1 control specific patterns of gene expression that modulate a wide variety of physiological outcomes.
Keywords: SIRT1, NAD+, deacetylation, transcription, chromatin, metabolism
1. Introduction
The regulation of gene expression in response to internal and external signals allows cells to control a wide variety of cellular processes in a coordinated manner to direct physiological, as well as pathophysiological, outcomes. Signal-regulated transcription by RNA polymerase II (Pol II), which controls the expression of protein-coding genes in the chromatin environment of the genome, is a complex process that requires a host of protein factors and multipolypeptide complexes [1-3]. These include sequence-specific DNA binding transcription factors, as well as a variety of enzymes that (1) covalently modify or de-modify histones and other transcription-related proteins, (2) mobilize or structurally alter nucleosomes, or (3) enhance the loading and activity of the Pol II machinery at gene promoters. The concerted actions of these enzymes allow an exquisite level of control of gene expression.
Members of the sirtuin family of NAD+-dependent protein deacetylases, which include the nuclear proteins Sir2 (yeast) and SIRT1 (mammals) (Fig. 1), represent an evolutionarily conserved class of regulatory enzymes [4, 5]. Sirtuins play key roles in a variety of cellular processes from yeast to mammals, including signal-regulated transcription by Pol II [6]. Sir2, the prototypical sirtuin family member, regulates gene silencing at the mating type loci (HML and HMR), the ribosomal DNA (rDNA) locus, and telomeres [4, 7]. These and other functions of Sir2 are dependent on its ability to deacetylate nucleosomal histones [4]. Among mammalian sirtuins, SIRT1 is the family member most closely related to Sir2. SIRT1 controls cell differentiation, metabolism, circadian rhythms, stress responses, and cellular survival [4, 8, 9], and the levels of SIRT1 fluctuate during various cellular processes [10]. As with yeast Sir2, many of the functions of SIRT1 are dependent on its deacetylase activity, which is directed towards nucleosomal histones (e.g., histone H4 lysine 16 or “H4K16”) [11, 12], as well as a wide range of transcription factors and other nuclear regulatory proteins [4, 5]. The unique requirement for the metabolic co-enzyme NAD+ as a substrate for both Sir2 and SIRT1 catalytic activity provides a crucial link between cellular metabolic status and gene regulation. In addition, the deacetylation reaction by sirtuins generates a unique NAD+ metabolite, O-acetyl-ADP-ribose (OAADPR, Fig. 1) [4, 5]. OAADPR plays an essential role in yeast SIR (silent information regulator) complex assembly [13]. It also binds to macrodomains and may modulate the function of macrodomain-containing proteins [14, 15]. Multiple enzymatic activities, including Nudix hydrolases, contribute to OAADPR hydrolysis [16-19], suggesting a role for these enzymes in regulating the cellular functions of Sir2 and SIRT1.
Fig. 1. The SIRT1-catalyzed protein deacetylation reaction.
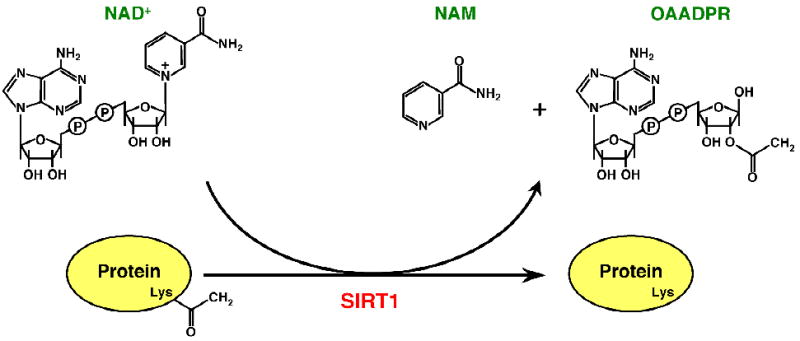
NAD+ is consumed as a substrate for the deacetylation of target proteins, which are acetylated on specific lysine (Lys) residues. The products in the reaction are deacetylated protein, nicotinamide (NAM), and O-acetyl-ADP-ribose (OAADPR).
Many of the functions of Sir2, including the locus-specific gene silencing effects noted above [4, 7], are dependent on its dynamic association with chromatin in response to different environmental or physiological states. For example, as yeast cells age, the Sir proteins relocate from the silent mating type loci to the nucleolus. The concomitant loss of silencing leads to sterility and aging [20-22]. In addition, Sir proteins relocate to DNA breaks in a DNA damage checkpoint-dependent manner [23-25]. Interestingly, a recent study has revealed parallel functions of SIRT1 in mammalian cells. Specifically, Oberdoerffer et al. have shown that DNA damage causes a global redistribution of SIRT1 away from silent repeat sequences and gene promoters to sites of DNA damage [26]. The redistribution of SIRT1 (1) is required for efficient DNA double strand break repair and genome stability, (2) protects mice from irradiation-induced cancer, and (3) causes deregulation of SIRT1 bound genes [26].
In this review, we will examine the available literature describing how the localization and enzymatic activity of Sir2 and SIRT1 at specific sites in the genome regulate gene expression (Fig. 2). The focus will be on SIRT1, but Sir2 will also be discussed when involved in parallel processes.
Fig. 2. Mechanisms and functions of SIRT1-dependent transcription regulation in mammalian cells.
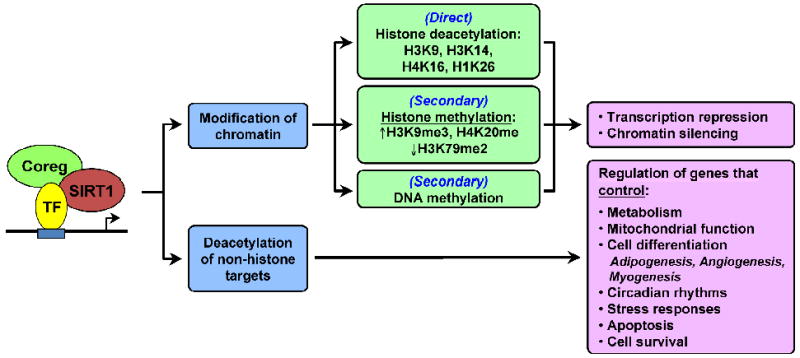
Details are provided throughout the text. TF, transcription factor; Coreg, coregulator.
2. SIRT1-dependent gene regulation through histone modification, DNA methylation, and the modulation of chromatin structure
2.1. Histone modifications
Many of the functions of Sir2 and SIRT1 are dependent on their ability to deacetylate nucleosomal histones at specific residues [4, 5]. For example, Sir2 is essential for establishing and maintaining silent chromatin in yeast, which requires deacetylation of H4K16 [4, 5]. In this regard, age-associated decreases in Sir2 protein are accompanied by an increase in H4K16 acetylation and a loss of histones at specific subtelomeric regions, which results in impaired transcriptional silencing [27].
SIRT1 has been shown to have parallel functions in mammalian cells, promoting chromatin silencing and transcription repression. SIRT1 is recruited to chromatin through interactions with a variety of chromatin-associated proteins, such as transcription factors and coregulators (see the text and Table 1, below). Upon recruitment to chromatin, SIRT1 can deacetylate H4K16, H3 lysine 9 (“H3K9”) and H3 lysine 14 (“H3K14”) [11, 12]. In addition, SIRT1 interacts directly with histone H1 and deacetylates H1 lysine 26 (“H1K26”) [11]. Beyond direct deacetylation of histones, SIRT1 also interacts with histone-modifying enzymes and regulates their activity. For example, SIRT1 binds and deacetylates the histone acetyltransferase p300, which inhibits p300 enzymatic activity [28]. This has the potential to promote hypoacetylation of nucleosomal histones and affect gene expression outcomes.
Table 1. Non-histone substrates and interacting proteins of SIRT1.
| SIRT1 Deacetylation Substrate | Consequence of Deacetylation | Biological Outcome | References |
|---|---|---|---|
| AR | Inhibition | Transcriptional repression of target gene | [48, 49] |
| BMAL1 | Inhibition of BMAL1-CRY1 interaction, leading to transcription activation | Maintenance of circadian clock | [46, 105] |
| E2F1 | Inhibition | Anti-apoptotic, negative feedback regulation of SIRT1 expression | [129] |
| ERα | Inhibition of DNA binding activity | Transcriptional repression of target gene | [47] |
| FOXO1, FOXO3a, FOXO4 | Inhibition or activation, context dependent | Regulation of stress resistance, cell survival, angiogenesis, gluconeogenesis, and adipocyte function | [44, 45, 61, 76, 130-133] |
| Ku70 | Activation | Anti-apoptotic | [58] |
| HIF-2α | Activation | Positive regulation of stress response to hypoxia | [51] |
| LXR | Ubiquitylation and activation of LXR | Regulation of cholesterol homeostasis | [50] |
| MEF2 | Repression of target gene expression | Negative regulation of myogenesis | [134] |
| MyoD | Repression of target gene expression | Negative regulation of myogenesis | [135] |
| p53 | Inhibition | Anti-apoptotic, cell survival | [42, 43, 136, 137] |
| p73 | Inhibition | Anti-apoptotic | [138] |
| p300 | Inhibition of HAT activity | Transcription repression | [28] |
| PCAF | Inhibition of p73 expression | Negative regulation of the E2F1/p73 apoptotic pathway | [139] |
| PCAF and GCN5 | Deacetylation of PCAF and MyoD in a SIRT1/PCAF/MyoD complex, repression of target gene expression | Negative regulation of myogenesis | [135] |
| Per2 | Per2 degradation and activation of target gene transcription | Maintenance of circadian clock | [106] |
| PGC-1a | Activation | Regulation of mitochondrial function and glucose and lipid metabolism | [52-57] |
| RelA/p65 (NF-κB) | Inhibition | Pro-apoptotic in response to TNFα; anti-apoptotic in neuronal cells | [140, 141] |
| Smad7 | Ubiquitylation and degradation of Smad7 | Inhibition of TGFβ-induced apoptosis | [142] |
| Sox9 | Activation | Activation of cartilage-specific gene expression | [103] |
| SUV39H1 | Activation | H3K9 trimethylation, reduced rRNA transcription | [30, 31] |
| TAFI68 | Inhibition of DNA binding activity | Repression of RNA Pol I-dependent rRNA transcription | [143] |
| SIRT1 Interacting Protein | Consequence of interaction | Biological Outcome | References |
| CTIP1 and CTIP2 | H3 and H4 deacetylation | Transcription repression | [144, 145] |
| HES1 and HEY2 | Transcription repression | Regulation of cell differentiation | [146, 147] |
| HIC1 | Recruitment of SIRT1 to its own promoter | Feedback inhibition of SIRT1 expression | [148] |
| NCoR and SMRT | Transcription repression | Inhibition of PPARγ-dependent gene expression in adipocytes | [149] |
| Tat | Recycling of Tat | Activation of HIV transcription | [150] |
Histone acetylation and histone methylation are often coordinately regulated [29]. In this regard, the binding of SIRT1 to chromatin and the concomitant deacetylation of nucleosomal histones can promote alterations in histone methylation. For example, in an integrated reporter gene assay, recruitment and activity of SIRT1 at the promoter induced H4 lysine 20 monomethylation (H4K20me) and H3 lysine 9 trimethylation (H3K9me3), and decreased H3 lysine 79 dimethylation (H3K79me2) [11]. Although in most cases an understanding of the detailed mechanisms is still lacking for SIRT1-induced histone methylation, recent reports suggest that SIRT1 can recruit histone methyltransferases (e.g., suppressor of variegation 3–9 homolog 1 or SUV39H1) to target sites, thereby regulating both histone acetylation and methylation at these sites. SIRT1 interacts with and deacetylates the catalytic domain of SUV39H1, the principle enzyme responsible for catalyzing H3K9me3 [30]. These interactions recruit SUV39H1 to chromatin and increase the methyltransferase activity of SUV39H1, leading to increased levels of H3K9me3 at SIRT1 target sites. Consistent with this observation, loss of SIRT1 greatly inhibits SUV39H1-dependent H3K9me3 and impairs the localization of heterochromatin protein 1 (HP-1), an H3K9me3-binding protein [30].
Both SIRT1 and SUV39H1 are also found in a complex with nucleomethylin, a nuclear protein that binds to H3K9me2 at the rDNA locus [31]. This complex, eNoSC (energy-dependent nucleolar silencing complex), senses cellular energy status and regulates silent chromatin formation at the rDNA locus through H3 deacetylation and H3K9 methylation. These observations suggest that the NAD+-dependent deacetylase activity of SIRT1 in the eNoSC complex provides a critical link between energy status and rRNA transcription [31]. Interestingly, DBC1 (deleted in breast cancer 1) disrupts the SIRT1-SUV39H1 complex. DBC1 directly binds to both SIRT1 and SUV39H1 and inhibits their enzymatic activity [32-34]. This activity of DBC1 may play an important role in the regulation of heterochromatin formation and genomic stability. In addition to its interaction with SUV39H1, SIRT1 has also been found in the PRC4 (Polycomb repressive complex 4) complex with Ezh2 (Enhancer of zeste homolog 2), a histone methyltransferase, and may therefore indirectly regulate H3K27 and H1K26 methylation [35].
Recent advances in mass spectrometry technology, including linear trap quadrupole (LTQ) orbitrap and Fourier transform mass spectrometry (FTMS), have made it possible to directly examine combinatorial post-translational modifications (PTMs) on histone tails [36-39]. One interesting use of these technologies would be to further decipher the role of SIRT1 in coordinating histone modifications, such as acetylation and methylation, and to understand how SIRT1-dependent modification of histones regulates target gene expression.
2.2. DNA methylation
SIRT1 has been shown to associate with the promoters of aberrantly silenced tumor suppressor genes (i.e., genes whose promoter CpG islands are hypermethylated) in cancer cells [40]. Inhibition of SIRT1 leads to re-expression of these genes [40]. Interestingly, in cell lines where these promoters are not hypermethylated and the genes are expressed, no SIRT1 binding is detected [40]. One of the tumor suppressor genes repressed by the actions of SIRT1 is CDH1, the gene encoding E-cadherin. Using an experimental model in which a DNA double strand break was induced in a reporter construct containing the CDH1 promoter, O'Hagan et al. examined the relationship between DNA damage and SIRT1-dependent gene silencing [41]. In their assays, SIRT1 bound to the region surrounding the DNA break in the reporter construct, consistent with the results of genomic localization (i.e., the ChIP-chip) studies showing global redistribution of SIRT1 upon DNA damage [26]. Importantly, SIRT1 binding is required for recruitment of DNA methyltransferase 3B (DNMT3B) and the subsequent heritable methylation of CpG islands at the reporter gene promoter [41]. These observations reveal a critical role for SIRT1 in DNA methylation and epigenetic silencing, although details of the mechanism remain to be elucidated.
2.3. SIRT1-dependent gene regulation through deacetylation of transcription factors and coregulators
SIRT1 interacts with a wide variety of DNA-bound or chromatin-associated factors (i.e., sequence-specific DNA binding transcription factors and coregulators, respectively) and is recruited to their target binding sites in the genome [6]. These include p53 [42, 43], the FOXO transcription factors [44, 45], the circadian clock protein BMAL1 [46], the nuclear receptors LXR, estrogen receptor α, and androgen receptor [47-50], the hypoxia-inducible factor 2α [51], the coregulator PGC1α [52-57], and the DNA repair and anti-apoptotic factor Ku70 [58] (Table 1). Deacetylation of these non-histone targets by SIRT1 is largely responsible for the complexity of transcriptional regulation by SIRT1 since it contributes to both negative and positive regulation of target gene expression [6]. The activity of SIRT1 with these target proteins underlies its function in cell differentiation, metabolism, circadian regulation, stress response, and survival (Table 1). Several excellent reviews of this subject have recently been published [5, 6, 59-62] and the reader is directed to these reviews for a detailed discussion of this subject. In the following section, we describe one example of SIRT1 regulation of a non-histone target as an example.
Here we use peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 alpha (PGC-1α) as an example to discuss in more detail how SIRT1 regulates target gene expression through interactions with specific transcription factors and coregulators. PGC-1α is a transcriptional coactivator that controls mitochondrial biogenesis and respiration, and whole-body energy expenditure [63, 64]. To achieve these functions, PGC-1α serves as a coactivator for a variety of transcription factors, including the PPARs, thyroid hormone receptors, glucocorticoid receptors, estrogen receptors, estrogen-related receptors (ERRs), myocyte enhancer factor-2 (MEF-2) and the FOXO transcription factors [65-71].
The activity of PGC-1α is regulated at both transcriptional and posttranslational levels. Importantly, acetylation by GCN5 and SRC3 negatively regulates PGC-1α activity [72, 73]. Lysine to arginine mutations in PGC-1α, which block acetylation of lysine residues and mimic the deacetylated state, markedly increase the activity of PGC-1α [52, 56]. In vivo, SIRT1 directly interacts with and deacetylates PGC-1α at multiple lysine residues [56], thereby enhancing the activity of PGC-1α. Recent studies indicate that SIRT1-dependent activation of PGC-1α plays a major role in regulating metabolic adaptations in different tissues [52, 54-56]. For example, during fasting, gluconeogenesis in the liver is upregulated to maintain normal blood glucose levels. Both SIRT1 expression and activity are induced in the fasted liver, leading to increased activity of PGC-1α [56].
Once PGC-1α is induced, it regulates gluconeogenic, glycolytic, and fatty acid oxidation genes through co-activation of transcription factors, such as FOXO1 [65, 74, 75]. Additionally, SIRT1 can also directly interact with and potentiate FOXO1 activity [76]. Therefore, increased hepatic SIRT1 activity enhances gluconeogenesis and inhibits glycolysis through activation of both PGC-1α and FOXO1. Similar interactions between SIRT1 and PGC-1α have been reported in skeletal muscle cells under fasting and low glucose conditions, where activated PGC-1α induces expression of genes involved in mitochondrial fatty acid oxidation [55]
Interestingly, in vivo treatment with the SIRT1 activator resveratrol in mice leads to deacetylation and activation of PGC-1α and enhanced mitochondrial function in several tissues [53, 54]. Increased insulin sensitivity, exercise performance and thermogenic activity are observed in these mice, suggesting that SIRT1 is a potential target for improvement of general health and treatment of metabolic diseases [53, 54]. In fact, SIRT1 activators are in clinical trials for Type 2 Diabetes (http://www.sirtrispharma.com/pipeline.html). Other promising therapeutic areas for SIRT1 include mitochondrial, cardiovascular, neurodegenerative, and inflammatory diseases, as well as various forms of cancer [77].
3. Regulation of SIRT1 activity by NAD+ biosynthetic enzymes
The cellular functions of Sir2 and SIRT1 are dependent upon its deacetylase activity, which requires the metabolic coenzyme NAD+. In addition, Sir2 and SIRT1 activity are inhibited by nicotinamide (NAM), which is (1) a by-product of NAD+-dependent protein modifications by sirtuins and poly(ADP-ribose) polymerases (PARPs) and (2) a precursor for NAD+ biosynthesis through a salvage pathway [78-80]. The regulation of Sir2 and SIRT1 activity by NAD+ and its metabolites provides a crucial link between cell metabolism and transcriptional regulation. This was first characterized with respect to calorie restriction (CR)-induced lifespan extension in yeast [81, 82], but has since been expanded to mammalian systems [83-86].
Studies in yeast have shown that the effect of CR on lifespan requires an intact NAD+ salvage pathway, and overexpression of the enzymes in this pathway extends yeast lifespan [81, 82, 87]. Because no overall change in NAD+ levels is observed in the long-lived yeast strains [87], other metabolic parameters regulated by the NAD+ salvage pathway, including NAD+:NADH ratio [88, 89] and NAM levels [80, 82, 90], have been proposed as the actual signal that regulate Sir2 activity during CR. Studies in mammalian cells have focused on the role of two enzymes in the NAD+ salvage pathway, nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase 1 (NMNAT-1), in controlling SIRT1 function in the nucleus. The salvage pathway comprised by these enzymes uses NAM as the precursor for NAD+ synthesis in the nucleus (Fig. 3) and, therefore, can regulate SIRT1 activity through both NAM removal and NAD+ production.
Fig. 3. Mammalian NAD+ biosynthetic pathways.
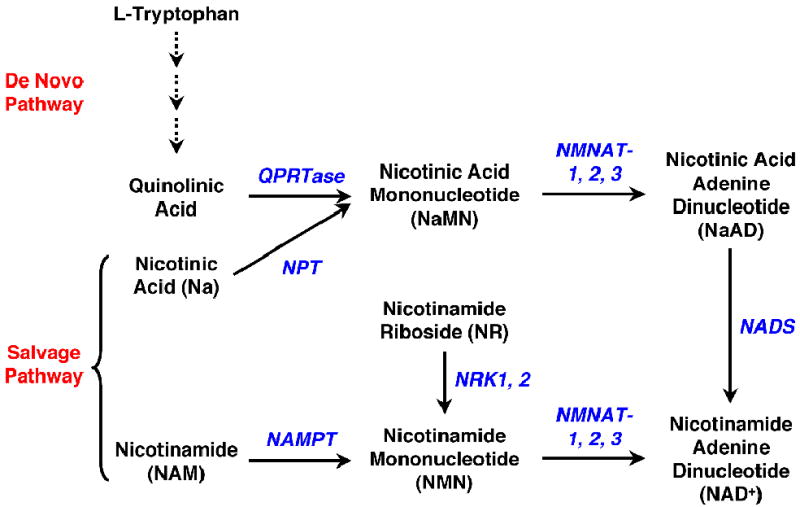
In mammals, NAD+ can be synthesized through two pathways. The de novo pathway uses L-tryptophan as the precursor for NAD+ production in a multi-step pathway. The salvage pathway uses nicotinamide or nicotinic acid (together called niacin or vitamin B3) to make NAD+. In addition, nicotinamide riboside has been identified as a dietary precursor for NAD+ biosynthesis. QPRTase, quinolinic acid phosphoribosyltransferase; NPT, nicotinic acid phosphoribosyltransferase; NMNAT, nicotinamide/nicotinic acid mononucleotide adenylyltransferase; NAMPT, nicotinamide phosphoribosyltransferase; NADS, NAD synthase, NRK, nicotinamide riboside kinase.
3.1. NAMPT
NAMPT, which catalyzes the conversion of NAM to nicotinamide mononucleotide (NMN) (Fig. 3) [91], localizes to both the cytoplasm and nucleus [92, 93]. In addition, an extracellular form of the protein has been reported to act as an NAD+ biosynthetic enzyme (eNAMPT) [94, 95], a cytokine named PBEF (pre-B cell colony-enhancing factor) [96], and a adipocytokine with insulin-mimetic function (visfatin) [97], although the later study has been retracted [98]Biochemical analyses suggest that NAMPT is the rate-limiting enzyme in the NAD+ salvage pathway [99], while in vivo studies demonstrate that NAMPT expression levels closely correlate with cellular NAD+ production [83, 85, 86, 99-102]. The enzymatic activity of NAMPT also directly reduces cellular NAM levels [83]. Both of these actions of NAMPT have the potential to stimulate SIRT1 activity.
The role of NAMPT in the regulation of SIRT1 activity has been explored in a variety of cell types, including skeletal myoblasts, vascular smooth muscle cells (SMCs), and chondrocytes. Restriction of nutrients in skeletal myoblasts stimulates NAMPT expression, leading to the modulation of cellular NAD+/NADH ratio, as well as NAM levels [83]. These changes activate SIRT1, which promotes a SIRT1-dependent impairment of myoblast differentiation [83]. Similarly, in human vascular smooth muscle cells (SMCs), NAMPT is upregulated during cell maturation [84] and declines during cell aging [101]. These changes in NAMPT expression are accompanied by similar changes in cellular NAD+ levels, which regulate SMC function in a SIRT1-dependent pathway [84, 101]. Intriguingly, ectopic expression of NAMPT delays SMC senescence through activation of SIRT1 [101]. Together, these studies illustrate how NAMPT-dependent regulation of SIRT1 plays a critical role in muscle cell differentiation, maturation, and senescence. In human chondrocytes, SIRT1 is recruited to the promoter of a cartilage-specific gene through interactions with SOX9 [103]. At this gene, NAMPT stimulates SIRT1 activity and target gene expression through NAD+ production [103]. These studies illustrate how the gene regulatory activity of SIRT1 is controlled by cellular NAD+ production.
Recent studies have connected the actions of NAMPT and SIRT1 in the regulation of the circadian rhythm. The circadian rhythm in mammals is controlled by internal “clocks” that temporally regulate biological functions in response to environmental and physiological cues in a process that involves negative feedback regulation of gene expression [62]. Many of the core clock proteins are transcription factors, including CLOCK (a protein acetyltransferase [104, 105]) and BMAL1, which form a DNA-binding heterodimer. CLOCK:BMAL1 heterodimers promote the expression of the genes encoding Period (PER) and Cryptochrome 1 (CRY1) through the CLOCK-dependent acetylation of histones and transcription factors [62]. Once synthesized, the PER and CRY1 proteins form heterodimers that inhibit CLOCK:BMAL1-dependent transcription (Fig. 4). A key aspect of this regulatory pathway is the acetyltransferase activity of CLOCK, which can acetylate histones H3 and H4 [104], as well as non-histone substrates including its binding partner BMAL1 [105] and potentially other clock proteins [62]. BMAL1 acetylation facilitates the association of the repressor CRY1 with CLOCK:BMAL1, thereby forming a crucial link in the feedback regulation of circadian rhythm [105] (Fig. 4).
Fig. 4. Feedback regulation of circadian clock gene expression.
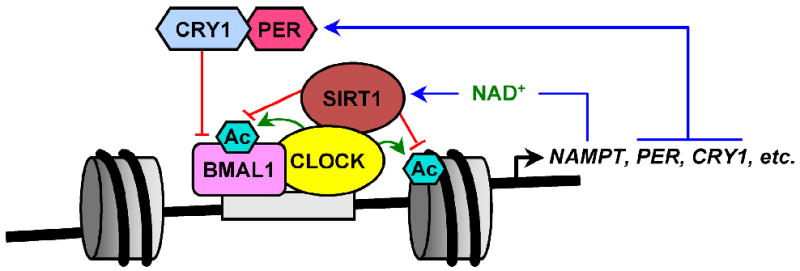
The circadian rhythm in mammals is controlled by internal “clocks” that temporally regulate biological functions in response to environmental and physiological cues in a process that involves negative feedback regulation of gene expression. Many of the core clock proteins are DNA-binding transcription factors, including: CLOCK (a protein acetyltransferase) and BMAL1 (a heterodimerization partner of CLOCK). CLOCK:BMAL1 heterodimers promote the expression of the genes encoding NAMPT, Period (PER) and Cryptochrome 1 (CRY1) through the CLOCK-dependent acetylation of histones and transcription factors (green arrows; Ac, acetyl groups covalently linked to proteins). PER and CRY1 form heterodimers that inhibit CLOCK:BMAL1-dependent transcription. Since the gene encoding NAMPT is a direct target for regulation by CLOCK:BMAL1 heterodimers, NAMPT expression and cellular NAD+ biosynthesis are subject to circadian regulation and exhibit circadian oscillations. Increased NAMPT-dependent NAD+ production enhances SIRT1 deacetylase activity, which acts to inhibit the transcription of NAMPT through interactions with the CLOCK:BMAL1 complex at the NAMPT promoter, thus completing a feedback loop involving NAMPT/NAD+/SIRT1 and CLOCK:BMAL1.
Exciting new developments in this field have revealed the involvement of SIRT1 and NAMPT in circadian regulation. SIRT1 binds to CLOCK and is recruited to the CLOCK:BMAL1 complex at circadian gene promoters [46, 106]. At these sites, SIRT1 deacetylates H3K9 and H3K14 [46], as well as non-histone proteins BMAL1 [46] and Per2 [106]. Deletion or inhibition of SIRT1 leads to alterations in the circadian cycle, as revealed by changes in circadian gene expression profiles [46, 106]. Interestingly, SIRT1 activity is regulated in a circadian manner [46, 106], suggesting that SIRT1 may be a crucial link between cellular metabolism and the circadian clock. Two new reports have provided evidence to support this view. These studies revealed that the gene encoding NAMPT (i.e., NAMPT) is a direct target for regulation by CLOCK:BMAL1 heterodimers [85, 86]. Consequently, NAMPT expression and cellular NAD+ biosynthesis are subject to circadian regulation and exhibit circadian oscillations. The increased NAMPT-dependent NAD+ production leads to higher SIRT1 activity. The activated SIRT1, in turn, acts to inhibit the transcription of NAMPT through interactions with the CLOCK:BMAL1 complex at the NAMPT promoter. This completes a feedback loop involving NAMPT/NAD+/SIRT1 and CLOCK:BMAL1 [85, 86, 107] (Fig. 4).
3.2. NMNAT-1
The NMN produced by NAMPT is further converted into NAD+ by NMNAT (Fig. 3). Three NMNAT isoforms are found in mammals (NMNAT-1, -2, and -3) [108]. Among them, NMNAT-1 localizes exclusively to the nucleus [109] and is likely the key NMNAT family member responsible for regulating SIRT1 activity. Studies using the Wallerian degeneration slow (WldS) mouse model, which contains a gene fusion encoding the N-terminal fragment of ubiquitination factor E4B (Ube4B) linked to full-length NMNAT-1 [110, 111], have revealed a critical function for NMNAT-1 in neuronal protection that may be SIRT1 dependent. Wallerian degeneration is the process by which the distal part of an injured nerve axon undergoes degeneration; this process is slowed in the WldS mouse. Overexpression of the chimeric WldS protein, which retains NMNAT-1 enzymatic activity, significantly delays the axonal degeneration process [110, 111].
Controversy exists over the mechanism of WldS action. In some experimental systems, NMNAT-1 enzymatic activity is required for the neuronal protection phenotype through a SIRT1-dependent pathway [112, 113], while in others, NMNAT-1 enzymatic activity is dispensible[114-117]. In one study using primary dorsal root ganglion neuron explants, the axon protective effect of WldS was mimicked by NMNAT-1 overexpression or addition of exogenous NAD+, and the protective effect of NAD+ required SIRT1 enzymatic activity [112]. Interestingly, the cellular levels of NAD+ were not altered in the NMNAT-1 overexpressing cells [110, 112], suggesting that localized production of NAD+ in the nucleus, rather than total level of cellular NAD+, regulates SIRT1 function. Further studies are required to fully understand if and how NAD+-dependent interplay between NMNAT-1 and SIRT1 contributes to the WldS phenotype.
In a recent study, we uncovered a new aspect of NMNAT-1-dependent regulation of SIRT1 function during gene regulation [118]. Using global gene expression analyses, we identified a group of genes that are (1) commonly regulated by NMNAT-1 and SIRT1 and (2) show SIRT1 binding to their promoters. These genes also show SIRT1-dependent recruitment of NMNAT-1 to their promoters. Knockdown of NMNAT-1 in this system reduced total cellular NAD+ levels and inhibited the deacetylation of H4K16 by SIRT1 at target gene promoters. These results demonstrate that (1) NMNAT-1 regulates SIRT1-dependent transcription through NAD+ production and (2) an NAD+ biosynthetic enzyme can be recruited to target gene promoters as a coregulator to modulate transcriptional outcomes (Fig. 5).
Fig. 5. Regulation of SIRT1 activity at target gene promoters by nuclear NAD+- producing enzymes.
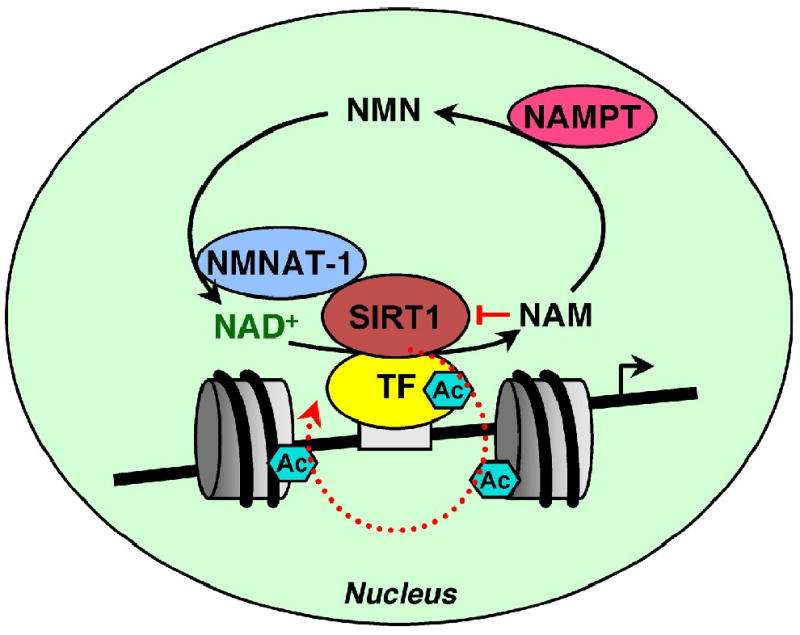
SIRT1 is recruited to target gene promoters and regulates the acetylation state of histones, transcription factors, and other chromatin-associated proteins in an NAD+-dependent manner. Nicotinamide (NAM), a byproduct of SIRT1-catalyzed deacetylation reactions, is a potent inhibitor of SIRT1 activity. NAMPT and NMNAT-1 constitute a nuclear NAD+ recycling pathway that utilizes NAM for NAD+ biosynthesis. NAM removal and NAD+ production by NAMPT and NMNAT-1 stimulate SIRT1 activity in the nucleus. In addition, NMNAT-1 interacts with SIRT1 and is recruited to SIRT1 target gene promoters. SIRT1-dependent recruitment of NMNAT-1 to chromatin may produce NAD+ for direct shuttling to SIRT1 to support its activity. TF, transcription factor. Ac, acetyl group.
Colocalization of NMNAT-1 and SIRT1 at target gene promoters may regulate SIRT1 activity in several ways. For example, close proximity of NMNAT-1 and SIRT1 may facilitate more efficient NAD+ utilization by SIRT1, perhaps through a substrate channeling mechanism [119]. Indeed, such a mechanism has been proposed previously for SIRT1 regulation by NAD+ biosynthetic enzymes [120]. The interaction between NMNAT-1 and SIRT1 at target gene promoters may also promote allosteric regulation that enhances the enzymatic activity of either or both enzymes, as has been shown for NMNAT-1 and poly(ADP-ribose) polymerase 1 (PARP-1) [121]. Finally, cellular signaling inputs may regulate interactions between NMNAT-1 and SIRT1, providing an additional level of regulatory control. Similar regulation has been reported for the interaction between NMNAT-1 and PARP-1 [121]. Overall, colocalization of NMNAT-1 and SIRT1 at target gene promoters suggests that NMNAT-1 plays an important role in modulating SIRT1 activity during transcription regulation.
4. Interplay between SIRT1 and PARP-1
PARP-1 is another chromatin and transcription modulating enzyme that, like SIRT1, requires a source of nuclear NAD+ [122, 123]. SIRT1 and PARP-1 regulate many common pathways, including cellular differentiation, stress response and survival, and the two enzymes share the same intranuclear NAD+ pool. Consequently, they may influence each other's activity through competition for NAD+ [124, 125]. This may have a significant impact on SIRT1 function because PARP-1 is the major NAD+ consumer in the cell during stress responses [125, 126]. In response to genotoxic stress, PARP-1 activation can deplete intracellular NAD+ stores and, at the same time, release high levels of NAM. Both of these actions of PARP-1 can inhibit SIRT1 enzymatic activity.
Interestingly, recent studies have revealed direct regulation of PARP-1 activity by SIRT1, providing a mechanism for feedback control of PARP-1 activation in response to genotoxic stress (Fig. 6). For example, SIRT1 activation can inhibit poly(ADP-ribose) (PAR) synthesis by PARP-1 following H2O2 treatment [127]. On the other hand, deletion of SIRT1 leads to a dramatic increase in PAR synthesis and apoptosis inducing factor (AIF)-mediated cell death following genotoxic insult [127]. Furthermore, studies in cardiomyocytes have shown that PARP-1 is acetylated in response to cellular stress, which leads to the activation of PARP-1 enzymatic activity. [128]. SIRT1 directly binds to and deacetylates PARP-1, therefore inhibiting PARP-1 activity [128]. SIRT1 also directly inhibits the expression of the PARP-1 gene, thereby reducing cellular PARP-1 levels and activity even further. Interestingly, the physical interactions between SIRT1 and PARP-1 are regulated by NAD+, with higher NAD+ levels inhibiting the interaction [128].
Fig. 6. Cross-talk between SIRT1 and PARP-1 in stress responses.
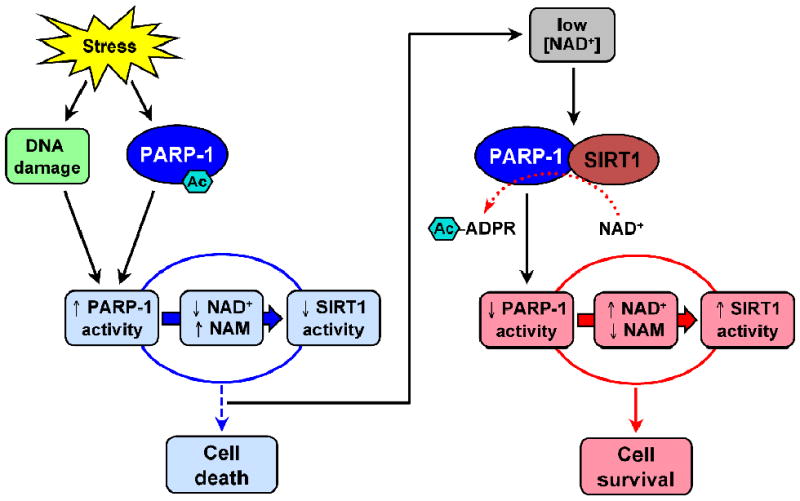
Stress activates PARP-1 through DNA damage and acetylation of PARP-1. Poly(ADP-ribosyl)ation by PARP-1 markedly reduces cellular NAD+ levels and increases NAM levels, leading to inhibition of SIRT1 activity. Together, these changes can lead to apoptotic or necrotic cell death. To counteract the action of PARP-1, SIRT1 interacts with and deacetylates PARP-1 when NAD+ levels are low, thereby inhibiting PARP-1 activity. This function of SIRT1 helps to maintain cellular NAD+ levels and SIRT1 activity, and promotes cell survival in response to stress. Ac-ADPR, acetylated ADP-ribose or OAADPR.
Based on these observations, a detailed mechanism has emerged for cross-talk between SIRT1 and PARP-1 in stress response [128] (Fig. 6). PARP-1 is acetylated and activated following enhanced cellular stress. Poly(ADP-ribosyl)ation by PARP-1 markedly reduces cellular NAD+ levels, leading to inhibition of SIRT1 activity. When NAD+ levels are low, SIRT1 interacts with and deacetylates PARP-1 and inhibits expression of the PARP-1 gene, thereby reducing total cellular PARP-1 activity. These actions of SIRT1 block NAD+ depletion by PARP-1, thus forming a feedback loop to control PARP-1 activity. The interplay between PARP-1 and SIRT1 has been proposed to play an important role in determining cell fate between life and death in response to cellular stress.
5. Summary and perspectives
A host of historical, as well as recent, studies have demonstrated clearly a role for yeast Sir2 and mammalian SIRT1 in NAD+-dependent gene regulation. In this regard, these deacetylases can (1) reduce the acetylation levels of specific lysines in histones and transcription factors to alter their function, (2) modulate the activity of other histone and protein-modifying enzymes (e.g., lysine methyltransferases), (3) collaborate with DNA methyltransferases to regulate the levels of DNA methylation, (4) interact physically and functionally with enzymes in the NAD+ biosynthetic pathway, and (5) interact physically and functionally with PARP-1 to regulate its activity. Although the details of SIRT1-dependent gene regulation are becoming clearer, many unanswered questions still remain. Additional studies are required to resolve the specific roles of nuclear and extranuclear NAD+ production in regulating the activity of SIRT1. In addition, the factors that lend specificity to SIRT1-dependent gene regulation at specific promoters under specific physiological conditions still need to be identified. Finally, a full cataloging of all of the proteins that are deacetylated by SIRT1, which will require a proteomic approach, is needed to fully understand the spectrum of SIRT1 functions in the nucleus.
Acknowledgments
The authors would like to thank members of the Kraus lab for helpful suggestions regarding this manuscript. The authors' research related to the topic of this review is supported by grants from the NIH/NIDDK (R01 DK069710) and the Endocrine Society to W.L.K. and a postdoctoral fellowship from the New York State Health Research Science Board to T.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 2.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 3.Reinberg D, Orphanides G, Ebright R, Akoulitchev S, Carcamo J, Cho H, Cortes P, Drapkin R, Flores O, Ha I, Inostroza JA, Kim S, Kim TK, Kumar P, Lagrange T, LeRoy G, Lu H, Ma DM, Maldonado E, Merino A, Mermelstein F, Olave I, Sheldon M, Shiekhattar R, Zawel L, et al. The RNA polymerase II general transcription factors: past, present, and future. Cold Spring Harb Symp Quant Biol. 1998;63:83–103. doi: 10.1101/sqb.1998.63.83. [DOI] [PubMed] [Google Scholar]
- 4.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 5.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 6.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasser SM, Cockell MM. The molecular biology of the SIR proteins. Gene. 2001;279:1–16. doi: 10.1016/s0378-1119(01)00741-7. [DOI] [PubMed] [Google Scholar]
- 8.Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology (Bethesda) 2006;21:404–410. doi: 10.1152/physiol.00031.2006. [DOI] [PubMed] [Google Scholar]
- 9.Wojcik M, Mac-Marcjanek K, Wozniak LA. Physiological and pathophysiological functions of SIRT1. Mini Rev Med Chem. 2009;9:386–394. doi: 10.2174/1389557510909030386. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 13.Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell. 2005;121:515–527. doi: 10.1016/j.cell.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Kraus WL. New functions for an ancient domain. Nat Struct Mol Biol. 2009;16:904–907. doi: 10.1038/nsmb0909-904. [DOI] [PubMed] [Google Scholar]
- 15.Kustatscher G, Hothorn M, Pugieux C, Scheffzek K, Ladurner AG. Splicing regulates NAD metabolite binding to histone macroH2A. Nat Struct Mol Biol. 2005;12:624–625. doi: 10.1038/nsmb956. [DOI] [PubMed] [Google Scholar]
- 16.McLennan AG. The Nudix hydrolase superfamily. Cell Mol Life Sci. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Tong L, Denu JM. Quantification of endogenous sirtuin metabolite O-acetyl-ADP-ribose. Anal Biochem. 2008;383:174–179. doi: 10.1016/j.ab.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafty LA, Schmidt MT, Perraud AL, Scharenberg AM, Denu JM. Analysis of O-acetyl-ADP-ribose as a target for Nudix ADP-ribose hydrolases. J Biol Chem. 2002;277:47114–47122. doi: 10.1074/jbc.M208997200. [DOI] [PubMed] [Google Scholar]
- 19.Tong L, Lee S, Denu JM. Hydrolase regulates NAD+ metabolites and modulates cellular redox. J Biol Chem. 2009;284:11256–11266. doi: 10.1074/jbc.M809790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeal T, Claus J, Kennedy B, Cole F, Guarente L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell. 1996;84:633–642. doi: 10.1016/s0092-8674(00)81038-7. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, Pak SM, Laroche T, Gasser SM, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 23.Mills KD, Sinclair DA, Guarente L. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell. 1999;97:609–620. doi: 10.1016/s0092-8674(00)80772-2. [DOI] [PubMed] [Google Scholar]
- 24.McAinsh AD, Scott-Drew S, Murray JA, Jackson SP. DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr Biol. 1999;9:963–966. doi: 10.1016/s0960-9822(99)80424-2. [DOI] [PubMed] [Google Scholar]
- 25.Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 26.Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Schones DE, Zhao K. Characterization of human epigenomes. Curr Opin Genet Dev. 2009;19:127–134. doi: 10.1016/j.gde.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- 31.Murayama A, Ohmori K, Fujimura A, Minami H, Yasuzawa-Tanaka K, Kuroda T, Oie S, Daitoku H, Okuwaki M, Nagata K, Fukamizu A, Kimura K, Shimizu T, Yanagisawa J. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133:627–639. doi: 10.1016/j.cell.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Chen L, Kabra N, Wang C, Fang J, Chen J. Inhibition of SUV39H1 methyltransferase activity by DBC1. J Biol Chem. 2009;284:10361–10366. doi: 10.1074/jbc.M900956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzmichev A, Margueron R, Vaquero A, Preissner TS, Scher M, Kirmizis A, Ouyang X, Brockdorff N, Abate-Shen C, Farnham P, Reinberg D. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102:1859–1864. doi: 10.1073/pnas.0409875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nat Methods. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siuti N, Kelleher NL. Efficient readout of posttranslational codes on the 50-residue tail of histone H3 by high-resolution MS/MS. Anal Biochem. 2009 doi: 10.1016/j.ab.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X, Aslanian A, Yates JR., 3rd Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12:483–490. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taverna SD, Ueberheide BM, Liu Y, Tackett AJ, Diaz RL, Shabanowitz J, Chait BT, Hunt DF, Allis CD. Long-distance combinatorial linkage between methylation and acetylation on histone H3 N termini. Proc Natl Acad Sci U S A. 2007;104:2086–2091. doi: 10.1073/pnas.0610993104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN, Herman JG, Baylin SB. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2:e40. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Hagan HM, Mohammad HP, Baylin SB. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 2008;4:e1000155. doi: 10.1371/journal.pgen.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 44.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 45.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 46.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20:1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Y, Ngo D, Forman LW, Qin DC, Jacob J, Faller DV. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol Endocrinol. 2007;21:1807–1821. doi: 10.1210/me.2006-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, Pattabiraman N, Pestell TG, Wang F, Quong AA, Wang C, Pestell RG. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 51.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 52.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 57.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 59.Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008;29:187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41:81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 63.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 64.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 66.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 68.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 69.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 70.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 72.Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O'Malley BW, Auwerx J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1{alpha} Proc Natl Acad Sci U S A. 2008;105:17187–17192. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 77.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 78.Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003;42:9249–9256. doi: 10.1021/bi034959l. [DOI] [PubMed] [Google Scholar]
- 79.Landry J, Slama JT, Sternglanz R. Role of NAD(+) in the deacetylase activity of the SIR2-like proteins. Biochem Biophys Res Commun. 2000;278:685–690. doi: 10.1006/bbrc.2000.3854. [DOI] [PubMed] [Google Scholar]
- 80.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 81.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 82.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 85.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- 88.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 90.Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302:2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitani T, Okuno S, Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 93.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 94.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imai S. The NAD World: a new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 98.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Retraction. Science. 2007;318:565. doi: 10.1126/science.318.5850.565b. [DOI] [PubMed] [Google Scholar]
- 99.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 100.Rongvaux A, Galli M, Denanglaire S, Van Gool F, Dreze PL, Szpirer C, Bureau F, Andris F, Leo O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 101.van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 102.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of Cartilage-specific Gene Expression in Human Chondrocytes by SirT1 and Nicotinamide Phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 105.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 106.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 107.Wijnen H. Circadian rhythms. A circadian loop asSIRTs itself. Science. 2009;324:598–599. doi: 10.1126/science.1174132. [DOI] [PubMed] [Google Scholar]
- 108.Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci. 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 109.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 110.Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 111.Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 113.Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, Schulze KL, Verstreken P, Bellen HJ. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:e416. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, Magni G, Glass JD, Coleman MP. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 117.Fainzilber M, Twiss JL. Tracking in the Wlds--the hunting of the SIRT and the luring of the Draper. Neuron. 2006;50:819–821. doi: 10.1016/j.neuron.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 118.Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, Dumond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009 doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 120.Grubisha O, Smith BC, Denu JM. Small molecule regulation of Sir2 protein deacetylases. FEBS J. 2005;272:4607–4616. doi: 10.1111/j.1742-4658.2005.04862.x. [DOI] [PubMed] [Google Scholar]
- 121.Berger F, Lau C, Ziegler M. Regulation of poly(ADP-ribose) polymerase 1 activity by the phosphorylation state of the nuclear NAD biosynthetic enzyme NMN adenylyl transferase 1. Proc Natl Acad Sci U S A. 2007;104:3765–3770. doi: 10.1073/pnas.0609211104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 123.Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J. Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? Bioessays. 2003;25:808–814. doi: 10.1002/bies.10317. [DOI] [PubMed] [Google Scholar]
- 126.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 127.Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 128.Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, Hottiger MO, Gupta MP. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 130.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 131.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 133.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 136.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dai JM, Wang ZY, Sun DC, Lin RX, Wang SQ. SIRT1 interacts with p73 and suppresses p73-dependent transcriptional activity. J Cell Physiol. 2007;210:161–166. doi: 10.1002/jcp.20831. [DOI] [PubMed] [Google Scholar]
- 139.Pediconi N, Guerrieri F, Vossio S, Bruno T, Belloni L, Schinzari V, Scisciani C, Fanciulli M, Levrero M. hSirT1-dependent regulation of the PCAF-E2F1-p73 apoptotic pathway in response to DNA damage. Mol Cell Biol. 2009;29:1989–1998. doi: 10.1128/MCB.00552-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 142.Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, Isono M, Uzu T, Guarente L, Kashiwagi A, Koya D. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 143.Muth V, Nadaud S, Grummt I, Voit R. Acetylation of TAF(I)68, a subunit of TIF-IB/SL1, activates RNA polymerase I transcription. EMBO J. 2001;20:1353–1362. doi: 10.1093/emboj/20.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senawong T, Peterson VJ, Leid M. BCL11A-dependent recruitment of SIRT1 to a promoter template in mammalian cells results in histone deacetylation and transcriptional repression. Arch Biochem Biophys. 2005;434:316–325. doi: 10.1016/j.abb.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. J Biol Chem. 2003;278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takata T, Ishikawa F. Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun. 2003;301:250–257. doi: 10.1016/s0006-291x(02)03020-6. [DOI] [PubMed] [Google Scholar]
- 147.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 148.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 149.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, Hruby H, Jung M, Verdin E, Ott M. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]


