Summary
Objectives
The objectives of this study were to assess the prevalence and factors associated with hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)-infected and -uninfected Thai pregnant women and the rate of HCV transmission to their infants.
Patients and methods
Study subjects included 1435 HIV-infected pregnant women and their infants, enrolled in a perinatal HIV prevention trial, and a control group of 448 HIV-uninfected pregnant women. Women were screened for HCV antibodies with an enzyme immunoassay. Positive results were confirmed by recombinant immunoblot and HCV RNA quantification. Infants were tested for HCV antibodies at 18 months or for HCV RNA at between 6 weeks and 6 months.
Results
Of the HIV-infected women, 2.9% were HCV-infected compared to 0.5% of HIV-uninfected women (p = 0.001). Only history of intravenous drug use was associated with HCV infection in HIV-infected women. Ten percent of infants born to co-infected mothers acquired HCV. The risk of transmission was associated with a high maternal HCV RNA (p = 0.012), but not with HIV-1 load or CD4 count.
Conclusions
Acquisition of HCV through intravenous drug use partially explains the higher rate of HCV infection in HIV-infected Thai women than in HIV-uninfected controls. Perinatal transmission occurred in 10% of infants of HIV–HCV-co-infected mothers and was associated with high maternal HCV RNA.
Keywords: HIV, HCV, Risk factors, Perinatal transmission, Intravenous drug use, Thailand
Introduction
The prevalence of hepatitis C virus (HCV) infection worldwide is estimated to be around 2%, representing about 123 million infected persons.1–3 In 2004 the estimated prevalences of HCV in the six World Health Organization (WHO) regions were 5.0% in the Eastern Mediterranean, 3.0% in Africa, 2.1% in the Western Pacific, 1.7% in Europe, 1.3% in the American region, and 1.2% in Southeast Asia.2
Significant differences exist between developed and developing countries regarding major risk factors for HCV infection. In developed countries, with the discovery of hepatitis C virus in 1989 and the introduction of effective screening of donors, infected blood transfusions were replaced by percutaneous exposure through injection drug use as the primary mode of transmission of HCV infection. In the developing world, unsafe medical injections and, in some areas, inadequately screened blood transfusions are the predominant modes of HCV infection.3,4 Sexual transmission of HCV is less frequent and has been associated only with high-risk sexual behavior.5–9 As a result of shared risk factors, HCV is more common in individuals infected with the human immunodeficiency virus (HIV) than in the general population, particularly among injecting drug users, where it reaches prevalences of 30–90%.10–14 In Thailand little is known about the mechanisms of HCV transmission in non-intravenous drug-using (non-IVDU) populations.
The acquisition of HCV infection through perinatal transmission is estimated to occur in about 5% of infants born to HCV-infected mothers.15–20 A high maternal HCV RNA level has been found to be associated with perinatal transmission.15,16,18,21–26 Most studies have reported a higher risk of HCV transmission to infants born to HIV-co-infected mothers,27–32 but the mechanism of this increased risk is unclear. Long duration of membrane rupture24,29 and female sex of infants have also been associated with vertical transmission of HCV.33 Breastfeeding does not appear to pose an additional risk,22,34 and, while there is some disagreement,33,34 cesarean section does not seem to play a role in protection.33,35
In Thailand, where HIV prevalence among pregnant women attending public antenatal care facilities was 1.0% in 2005,36 data on the prevalence of HCV have been limited to a single study in the Bangkok area. In this study conducted between 1992 and 2004, a prevalence of 0.3% in HIV-uninfected women and 3.8% in HIV-infected women was shown. An increase in prevalence was found over this time period.37
The objectives of our study were to estimate the prevalence of HCV infection in a large population of HIV-infected pregnant women and a control group of HIV-uninfected pregnant women in Thailand, and to study risk factors associated with maternal HCV infection. We also describe the risk of perinatal HCV transmission from HIV–HCV-co-infected mothers to their infants, and risk factors associated with HCV transmission from mother to child.
Materials and methods
Population
Between June 1997 and December 1999, 36 738 pregnant women were tested for HIV-1 infection at 27 hospitals in Thailand, and 1435 infected women were enrolled into a clinical trial aimed to define the optimal duration of zidovudine administration for the prevention of perinatal transmission of HIV (Perinatal HIV Prevention Trial-1 or PHPT-1, ClinicalTrials.gov NCT00386230).38 HIV-infected women were instructed not to breastfeed their infants. A control group of 448 HIV-uninfected pregnant women was randomly selected at the same enrolment sites following a ratio of 1:3.
Age, marital status, parity, history of intravenous (IV) drug use, history of sexually transmitted diseases, and history of transfusion and hospitalization were recorded at the first antenatal visit, before 28 weeks of gestation (median 26 weeks). Blood samples were obtained from infants born to HIV-infected mothers at birth, 6 weeks, 4, 6, 9, 12, 15 and 18 months of age. Blood samples from HIV-uninfected women and their infants were not available after screening because they were not followed in this trial.
Virological and other biological evaluations
Methods used for HIV diagnosis in mothers and infants in this cohort were as previously published.38 To determine HCV infection status in mothers, HCV antibodies were assessed at a median 26 weeks of gestation using a third generation enzyme-linked immunosorbent assay (EIA; Murex anti-HCV kit, version 4.0, Abbott Murex, Kyalami, South Africa). Women testing HCV EIA-negative were considered HCV-uninfected. Samples from women testing EIA-positive were retested for HCV RNA quantification by PCR (COBAS Amplicor HCV monitor™ test, version 2.0, Roche Molecular Systems, Inc., Branchburg, NJ, USA) and by recombinant immunoblot assay (Bioblot HCV, Biokit S.A., Spain). Women with an undetectable HCV RNA level and positive immunoblot were considered to have been HCV-infected but to have recovered. Women with a detectable HCV RNA level were considered to have active HCV infection. If their HCV RNA was undetectable and immunoblot was negative, women were considered to be HCV-uninfected. Women with undetectable HCV RNA and an isolated band by immunoblot were considered to have an indeterminate HCV status.39
To determine the HCV infection status in children, the EIA was performed on samples drawn at 18 months of age and the same algorithm was used. If the 18 months of age sample was missing, HCV RNA was quantified in samples drawn at between 6 weeks and 6 months of age. Infants with detectable HCV RNA at any time during this age interval were considered positive.40
Plasma HCV RNA and HIV-1 RNA were quantitated using the Cobas Amplicor HCV Monitor test v. 2.0 and the Cobas Amplicor HIV Monitor test v. 1.5, respectively (Roche Molecular Systems, Inc, Branchburg, New Jersey, USA). The lower limit of RNA detection was 600 IU/ml for HCV and 400 copies/ml for HIV. HCV genotype was determined using a line probe assay (Versant® HCV genotype 2.0, Siemens Medical Solutions Diagnostics, Belgium) according to the manufacturer’s instructions.
Women had CD4 enumeration and serum alanine aminotransferase (ALT) measurement during pregnancy and children a serum ALT measurement at birth.
Data analysis
The baseline characteristics of HIV-infected and -uninfected pregnant women were analyzed according to their HCV status. We used the Fisher’s exact test to compare distributions of categorical data and the Wilcoxon rank-sum test for continuous variables. Statistical analyses were performed using Stata 10.1 (StataCorp LP, Texas, USA).
Ethical considerations
Written informed consent for this study was provided by women on entry to the parent study, PHPT-1, and the protocol was approved by the ethics committees at the Harvard School of Public Health and the Thai Ministry of Public Health.
Results
Prevalence and risk factors of HCV infection in pregnant women
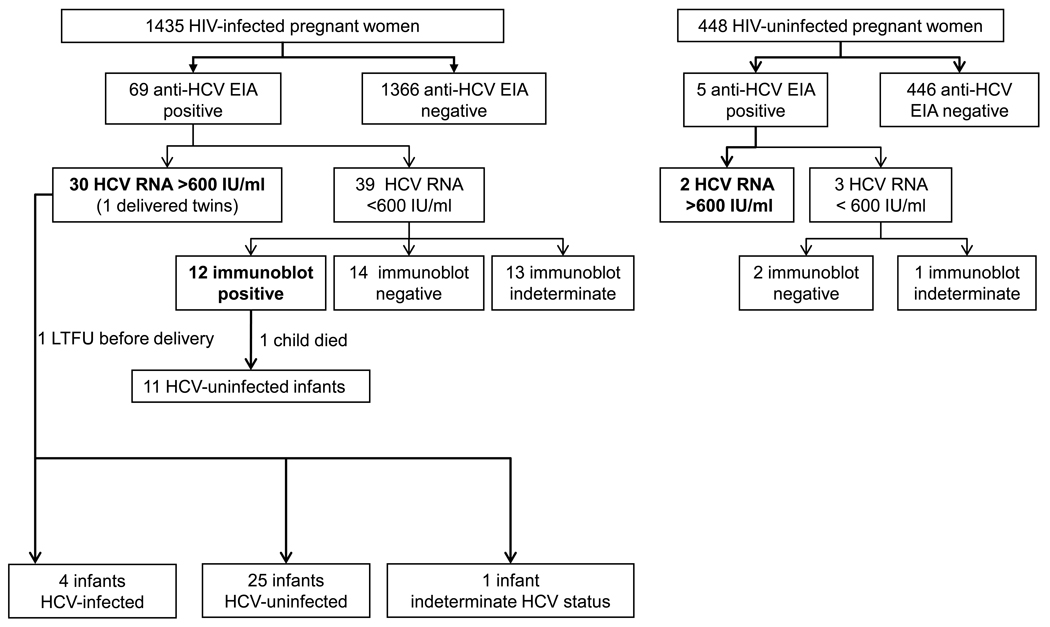
Among the 69 HIV-infected and five HIV-uninfected women with a positive EIA, HCV seropositivity was confirmed by HCV RNA and/or recombinant immunoblot in 42 and two women, respectively (Figure 1). Thirteen women had an indeterminate immunoblot and undetectable HCV RNA and were thus considered HCV indeterminate.39 The prevalence of HCV was 2.9% (42/1435) among HIV-1-infected women compared to 0.5% (2/448) among HIV-uninfected pregnant women (p = 0.001) (Figure 1). The women’s characteristics are presented in Table 1. Among HIV–HCV-co-infected women, 7% (3/41) self-reported a history of IV drug use vs. 0.5% (6/1306) of women infected with HIV only (p = 0.002). The other baseline characteristics studied were not significantly different between these two groups.
Figure 1.
HCV infection status of HIV-infected and HIV-uninfected pregnant women and HCV infection status for children born to HIV–HCV-co-infected women. Among the 30 HIV–HCV-co-infected women with HCV viremia, one woman delivered twins. Two children could not be assessed for HCV infection since no blood samples were available.
Table 1.
Baseline characteristics according to HCV status in HIV-infected cases and controls
| HIV-infected | Controls | |||||
|---|---|---|---|---|---|---|
| HCV-infected | HCV-uninfected | p-Value | HCV-infected | HCV-uninfected | p-Value | |
| n | 42 | 1393 | 2 | 446 | ||
| Age, median years (IQR) | 25.7 (21.6–28.8) | 24.8 (21.9–28.3) | 0.68 | 18.5 and 25.3 | 25.9 (21.6–30.7) | 0.28 |
| Education at least primary school | 11/41 (27%) | 454/1378 (33%) | 0.41 | 2/2 | 163/441 (37%) | 0.066 |
| Married or cohabitating | 40/40 (100%) | 1346/1348 (99.9%) | 1.00 | 2/2 | 441/441 | 1.00 |
| Primiparity | 21/42 (50%) | 770/1367 (56%) | 0.43 | 1/2 (50%) | 266/439 (61%) | 1.00 |
| History of hospitalization(s) since 1989 | 13/41 (32%) | 376/1306 (29%) | 0.73 | 1/2 (50%) | 103/392 (26%) | 0.46 |
| History of blood transfusion(s) since 1989 |
2/41 (5%) | 38/1301 (3%) | 0.35 | 0/2 | 7/392 (2%) | 1.00 |
| History of intravenous drug use | 3/41 (7%) | 6/1306 (0.5%) | 0.002 | 0/2 | 1/392 (0.3%) | 1.00 |
| History of sexually transmitted diseases during the past 3 months |
3/41 (7%) | 69/1299 (5%) | 0.48 | 0/2 | 3/392 (1%) | 1.00 |
| HIV RNA log10/ml, median (IQR) | 3.85 (3.27–4.46) | 3.92 (3.34–4.48) | 0.94 | ND | ||
| CD4 count /mm3, median (IQR) | 400 (240–534) | 360 (240–510) | 0.54 | ND | ||
HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; ND, not determined.
There was a disparity in the distribution of HCV prevalence in HIV-infected women according to the region and the type of regional economic activity, with higher prevalence (that is at or above 2.9%, the median for the entire country) found more often in regions with predominant commercial or industrial activities. In the Bangkok area and the east, the prevalence of HCV infection was found at or above the median at five of six sites (median 3.8%; range 1.5–7.9%) and six of eight sites (median 3.3%; range 0–5.9%), respectively. In the north, where economic activity is predominantly agricultural, the HCV prevalence was at or above the median in only four of 12 sites (median 2.3%; range 0–6.9%). There was no HCV infection case in the single site located in the south.
Among the 42 HIV–HCV-co-infected women, 71% had a detectable HCV viremia (3.61–6.12 log10 IU/ml). However, only three co-infected women had elevated serum ALT levels, and these abnormalities were modest (64, 66, and 75 IU).
Among HIV-infected women, the median HIV RNA was not different between HCV-infected women (n = 42) and HCV-uninfected women (n = 1393); 3.85 log10 copies/ml (95% confidence interval (CI) 3.49–4.28) vs. 3.92 (95% CI 3.86–3.99) (p = 0.46) suggesting that HCV had no impact on HIV replication. Moreover, in HCV-viremic women the level of HCV RNA was not significantly associated with either HIV RNA (p = 0.94) or CD4 (p = 0.54).
HCV genotype could be determined for the 32 women with detectable HCV RNA. Genotype 3a was the most prevalent genotype, accounting for 38% of HCV infection. Genotype 1a was identified in six women (19%) and genotype 1b in seven women (22%). Genotypes 6c to 6i were identified in six women (19%).
Perinatal transmission of HCV
HCV status could be assessed in 41 infants (Figure 1); one of the 42 HIV–HCV-co-infected women was lost to follow-up before delivery, one child died shortly after birth, and one mother delivered twins. Twenty-eight infants had an 18-month blood sample available and were tested for HCV antibodies. For thirteen children, HCV RNA was quantitated in plasma obtained at between 6 weeks and 6 months. Two children were positive for HCV antibody at 18 months of age and two others had measurable HCV RNA, one at 6 weeks of age and the other at 6 months of age.
Among HIV–HCV-co-infected women, the risk of perinatal HCV transmission was 10% (4/40) (95% CI 3– 20%) overall, or 13% (4/29) (95% CI 4–27%) when restricted to mothers with HCV viremia. HCV RNA was found in the plasma of all four children, with titers ranging from 4.8 to 6 log10 HCV IU/ml. Serum ALT results were available at birth for three HCV-infected babies and were normal.
Risk factors for perinatal HCV transmission
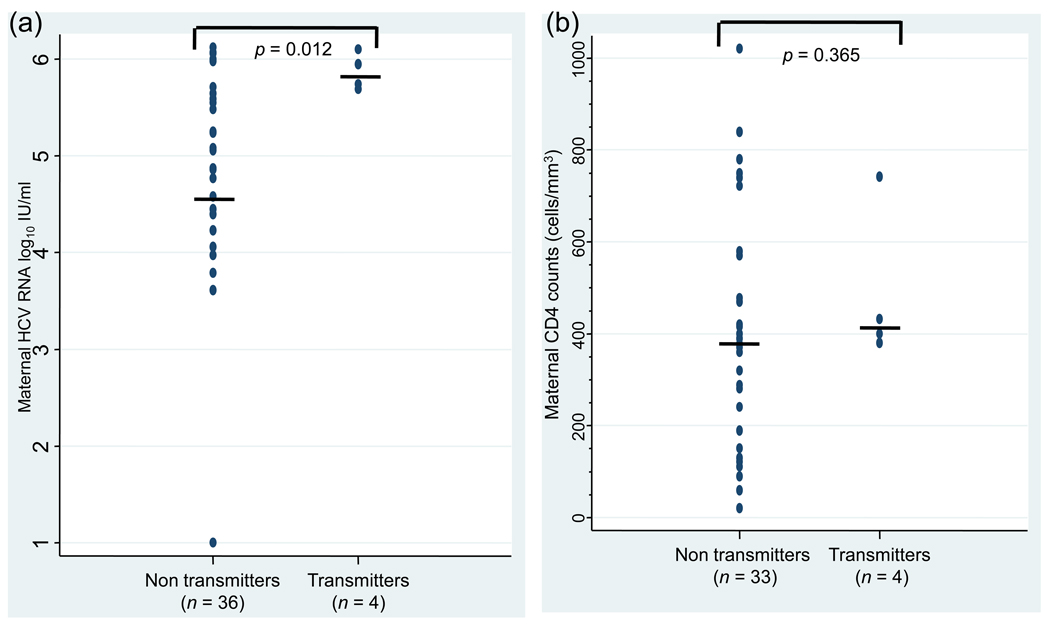
Perinatal HCV transmission was associated with a high maternal HCV viral load (p = 0.012): all HCV transmitting mothers had an HCV viral load above 5.5 log10 IU/ml (Figure 2a). Perinatal HCV transmission was not associated with maternal HIV viral load or absolute CD4 lymphocyte count (p = 0.365; Figure 2b). Delivery of the four HCV-infected children occurred vaginally with a time from labor onset to delivery ranging from 5.5 to 7.9 h. Among the 36 women who did not transmit HCV infection, 31 women delivered vaginally and six delivered through cesarean section, including three prior to onset of labor and rupture of membranes. The children were infected with different HCV genotypes (1a, 1b, 3, and 3a).
Figure 2.
Distribution of plasma HCV RNA concentrations (panel a) and CD4 lymphocyte counts (panel b) among HIV–HCV-co-infected mothers who transmitted (transmitters) or did not transmit (non-transmitters) HCV to their infants. Middle horizontal lines indicate medians.
Relationship between HIV and HCV perinatal transmission
Two of 39 HCV-co-infected mothers (5.1%) transmitted HIV to their children compared to 95 of 1342 (7.1%) HIV-only-infected mothers (p = 0.48). There were no cases of co-transmission.
Discussion
Our study showed that the prevalence of HCV infection was significantly higher in HIV-infected pregnant women than in HIV-uninfected pregnant women in Thailand, 2.9% vs. 0.5%. A history of IV drug use was the only risk factor significantly associated with HCV infection among the group of HIV-infected pregnant women. Only 0.7% of HIV-infected pregnant women self-reported a history of IV drug use – 7% (4/41) of HCV-infected vs. 0.5% (6/1306) of HCV-uninfected women; this suggests that other risk factors for HCV infection were present that were not identified. Although some women may not have reported prior IV drug use because of fears of disclosure, it is unlikely that this alone explains all HCV infections found in this population. In a study conducted among pregnant women in the Bangkok area, IV drug use in women’s sexual partners was found to be associated with the risk of HCV infection. We could not evaluate this factor since women were not specifically asked about the IV drug use of their sexual partners. We observed prevalences of HCV in the two groups of women similar to those described in the Bangkok study, with comparable ranges of HIV RNA and CD4 counts.37 However, because we included women from several regions of Thailand we were able to show a trend towards higher rates of HCV infection in women from industrial areas around Bangkok and in the east.
The 0.5% rate of HCV infection among HIV-uninfected women was lower than the 2.8% rate of HCV reported among persons screened as potential blood donors in northern Thailand around the same period.41 Other studies in volunteer blood donors in Thailand have reported rates of HCV prevalence of between 1.3% and 5.6%.42–44 Because it was randomly selected, our sample of HIV-uninfected women was likely unbiased, and the low prevalence observed could be attributable to the higher specificity of our HCV serology screening algorithm, the sex of our study subjects, their age, and the lack of specific risk factors for HCV infection. Indeed, the prevalence of HCV has been shown to be higher in males than in females,43–45 and to increase with age43 and with risk factors such as a history of IVDU,10–14,44 blood transfusion3,4,44 or a high-risk sexual behavior.5–9,44
Among HIV-infected women, the rate of perinatal transmission of HCV was associated with a high maternal HCV RNA level, consistent with findings reported in studies conducted in other settings.15,16,18,21–26 Transmission of HCV from mother to infant occurred in 10% of co-infected women overall. While transmission was not observed in the women with undetectable viremia, it was seen in 13% of those with measurable HCV viremia, and only in women with the highest viral load, above 5.5 log IU/ml. These rates are higher than the point estimate found in the only previous study of HIV–HCV co-infection and vertical transmission in Thailand, and closer to those reported in European and American studies. The rate of perinatal transmission of HIV was not different in HIV–HCV-co-infected women and in women with HIV infection alone. It has been proposed that the higher rate of vertical HCV transmission in HIV-infected pregnant women results from higher HCV viral loads in mothers as a result of immunosuppression secondary to HIV infection,46 and one large study from the USA has shown an inverse correlation between maternal percent CD4 and HCV transmission.18 On the other hand, this study did not report absolute CD4 concentrations. We did not observe any association between either maternal plasma HCV RNA level or perinatal HCV transmission and maternal absolute CD4 concentration, nor was there a correlation between HIV and HCV viral loads.
In conclusion, we have shown in this first nationwide study of HCV infection in Thai pregnant women that the prevalence of HCV infection among HIV-uninfected women was very low (0.5%), in contrast to a moderately high prevalence in HIV-infected women (2.9%). Only 7% of HIV–HCV-infected women self-reported a history of IV drug use, while 38% were infected with the genotype 3a, known to be associated with IV drug use in Thailand. Although other risk factors for HCV likely remain unidentified, some of which may be common with risk factors for HIV, IV drug use was likely underreported, highlighting the profound stigma attached to this in the female population. Perinatal transmission of HCV was significantly associated with a high maternal HCV RNA (p = 0.012). Surprisingly, HCV infection did not appear to be associated with the level of maternal HIV replication or the perinatal transmission of HIV.
This study confirms and extends data on the prevalence and risks factors of HCV infection in pregnant women in Thailand, as well as rates and risk factors of mother-to-child transmission in HIV–HCV-co-infected women.
Acknowledgements
This study was supported by grants from the National Institutes of Health NICHD (R01 HD33326 and R03 TW01346) and the Global Fund to Fight Aids, Tuberculosis and Malaria.
Members of the PHPT-1 study team (Perinatal HIV Prevention Trial (Thailand) Principal Investigators at 27 sites): Rayong: S. Lorenz Weerawatgoompa, V. Karnchanamayul, C. Tantiyaworawongse, S. Ariyadej; Chiang Rai Provincial: R. Hansudewechakul, J. Achalapong, R. Srismith, P. Wattanaporn (deceased); Chantaburi: S. Phongpanich, P. Yuthavisuthi, C. Ngampiyasakul, S. Sooksengchai; Phayao: S. Bhakeecheep, J. Hemvuttiphan, S. Bhakeecheep, V. Lattiwongsakorn; Banglamung: J. Ithisukanan, S. Siritathandon, K. Boonrod, S. Piyaman, P. Pinchan; Chonburi: N. Chotivanich, S. Hongsiriwan, P. Kittikoon; Bhumibhol Adulyadej: S. Prommat, S. Nimkarn, S. Charnivises, V. Suraseranivong, P. Layangool, B. Gulakirt; Chiang Kham: C. Putiyanun, S. Limsuwan, S. Charkrit, C. Mano; Mae Sai: P. Jindaapilukkul, S. Kunkongkapan; Hat Yai: S. Lamlertkittikul, B. Warachit, K. Veerapradist; Chachoengsao: A. Kanjanasing, R. Keawsonthi, C. Jirawison; Klaeng: S. Hotrawarikarn, S. Techapalokul, A. Palasudhi; Bamrasnaradura: P. Tunthanathip, S. Sirikawin, A. Chaovavanich; Mae Chan: S. Tantanarat, S. Piyaworawong; Phan: P. Lersruangpanya, S. Jeungphichanwanit, T. Jhangjit; Health Promotion Center Region I: I. Tangtitawong, S. Sirinontakan, V. Japikanond, B. Ngamsiriudom; Somdej Prapinklao: S. Suphanich, S. Maitrisathit, N. Tawornpanit, N. Kalawantavanich; Nopparat Rajathanee: T. Chanpoo, S. Ruangsirinusorn, N. Thamanavat, P. Hotrarapavanond; Phranangklao: S. Mokkamakkul, S. Wanwaisart, S. Hongyok; Banchang: S. Tragarngool, S. Chutimanukul, G. Kunawudhi; Somdej Pranangchao Sirikit: W. Pornkijprasarn, T. Hinjiranandana, S. Na Nakorn; Nakornping: V. Gomutbutra, P. Leelanitkul, K. Kunsuikmengrai, S. Kanjanavanit, S. Kahintapongs; Lamphun: W. Matanasaravoot, R. Somsamai, W. Chavengchaiyong; Buddhachinaraj: P. Thanomrat, W. Wannapira, W. Artong, C. Chaipat; Chiang Khong Royal Crown Prince: C. Taiyaitiang, S. Parinya; Phayamengrai: P. Tantiwattanakul, T. Onchomjum (deceased); McCormick: C. Tangchaitrong, S. Suwansarakul, C. Phimphilai.
We thank all mothers who participated in this study and the PHPT team, in particular P. Tungyai, W. Boonprasit, P. Sukrakanchana, S. Tanasri, P. Chailert, N. Chaiboonruang and K. Than-in-at. We also thank C. Gaudy, University F. Rabelais, Tours, for her advice on HCV genotype.
Funding sources: The funding sources of this study had no role in the study design, collection, analysis, data, or interpretation of data, in the writing, or in the decision to submit the manuscript for publication.
Ethical considerations: Written informed consent for this study was provided by women on entry to the parent study, PHPT-1, and the protocol was approved by the ethics committees at the Harvard School of Public Health and the Thai Ministry of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the 5th Dominique Dormont International Conference, Paris, France, March 26–28, 2009.
Conflict of interest: All authors have declared that no conflict of interest exists.
References
- 1.Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Farrington LA, Pecorado C, Hutin YJ, Armstrong GL. Estimated global prevalence of hepatitis C virus infection. 42nd Annual Meeting of the Infectious Diseases Society of America; September 30–October 3; Boston, USA. 2004. [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 5.Feldman JG, Minkoff H, Landesman S, Dehovitz J. Heterosexual transmission of hepatitis C, hepatitis B, and HIV-1 in a sample of inner city women. Sex Transm Dis. 2000;27:338–342. doi: 10.1097/00007435-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hammer GP, Kellogg TA, McFarland WC, Wong E, Louie B, Williams I, et al. Low incidence and prevalence of hepatitis C virus infection among sexually active non-intravenous drug-using adults, San Francisco, 1997–2000. Sex Transm Dis. 2003;30:919–924. doi: 10.1097/01.OLQ.0000091152.31366.E6. [DOI] [PubMed] [Google Scholar]
- 7.Neaigus A, Miller M, Friedman SR, Des Jarlais DC. Sexual transmission risk among noninjecting heroin users infected with human immunodeficiency virus or hepatitis C virus. J Infect Dis. 2001;184:359–363. doi: 10.1086/322020. [DOI] [PubMed] [Google Scholar]
- 8.Stroffolini T, Lorenzoni U, Menniti-Ippolito F, Infantolino D, Chiaramonte M. Hepatitis C virus infection in spouses: sexual transmission or common exposure to the same risk factors? Am J Gastroenterol. 2001;96:3138–3141. doi: 10.1111/j.1572-0241.2001.05267.x. [DOI] [PubMed] [Google Scholar]
- 9.Wyld R, Robertson JR, Brettle RP, Mellor J, Prescott L, Simmonds P. Absence of hepatitis C virus transmission but frequent transmission of HIV-1 from sexual contact with doubly-infected individuals. J Infect. 1997;35:163–166. doi: 10.1016/s0163-4453(97)91677-7. [DOI] [PubMed] [Google Scholar]
- 10.Eicher AD, Crofts N, Benjamin S, Deutschmann P, Rodger AJ. A certain fate: spread of HIV among young injecting drug users in Manipur, north-east India. AIDS Care. 2000;12:497–504. doi: 10.1080/09540120050123891. [DOI] [PubMed] [Google Scholar]
- 11.Hansurabhanon T, Jiraphongsa C, Tunsakun P, Sukbunsung R, Bunyamanee B, Kuirat P, et al. Infection with hepatitis C virus among intravenous-drug users: prevalence, genotypes and risk-factor-associated behaviour patterns in Thailand. Ann Trop Med Parasitol. 2002;96:615–625. doi: 10.1179/000349802125001465. [DOI] [PubMed] [Google Scholar]
- 12.Jittiwutikarn J, Thongsawat S, Suriyanon V, Maneekarn N, Celentano D, Razak MH, et al. Hepatitis C infection among drug users in northern Thailand. Am J Trop Med Hyg. 2006;74:1111–1116. [PubMed] [Google Scholar]
- 13.Murrill CS, Weeks H, Castrucci BC, Weinstock HS, Bell BP, Spruill C, et al. Age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus infection among injection drug users admitted to drug treatment in 6 US cities. Am J Public Health. 2002;92:385–387. doi: 10.2105/ajph.92.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Yang R, Xia X, Qin S, Dai J, Zhang Z, et al. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. J Acquir Immune Defic Syndr. 2002;29:191–196. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Dal Molin G, D’Agaro P, Ansaldi F, Ciana G, Fertz C, Alberico S, et al. Mother-to-infant transmission of hepatitis C virus: rate of infection and assessment of viral load and IgM anti-HCV as risk factors. J Med Virol. 2002;67:137–142. doi: 10.1002/jmv.2202. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero S, Lungaro P, Bruzzone BM, Gotta C, Bentivoglio G, Ragni N. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990–2000) Acta Obstet Gynecol Scand. 2003;82:229–234. doi: 10.1034/j.1600-0412.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 17.Polis CB, Shah SN, Johnson KE, Gupta A. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clin Infect Dis. 2007;44:1123–1131. doi: 10.1086/512815. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DL, Villano SA, Riester KA, Hershow R, Mofenson LM, Landesman SH, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480–1488. doi: 10.1086/515315. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SL, Newell ML, Peckham CS, Ades AE, Hall AJ. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. Int J Epidemiol. 1998;27:108–117. doi: 10.1093/ije/27.1.108. [DOI] [PubMed] [Google Scholar]
- 20.Granovsky MO, Minkoff HL, Tess BH, Waters D, Hatzakis A, Devoid DE, et al. Hepatitis C virus infection in the mothers and infants cohort study. Pediatrics. 1998;102:355–359. doi: 10.1542/peds.102.2.355. [DOI] [PubMed] [Google Scholar]
- 21.Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, et al. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570–575. doi: 10.1097/00005176-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Moriya T, Sasaki F, Mizui M, Ohno N, Mohri H, Mishiro S, et al. Transmission of hepatitis C virus from mothers to infants: its frequency and risk factors revisited. Biomed Pharmacother. 1995;49:59–64. doi: 10.1016/0753-3322(96)82587-x. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Extremera A, Salmeron J, Torres C, De Rueda PM, Gimenez F, Robles C, et al. Follow-up of transmission of hepatitis C to babies of human immunodeficiency virus-negative women: the role of breast-feeding in transmission. Pediatr Infect Dis J. 2000;19:511–516. doi: 10.1097/00006454-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Spencer JD, Latt N, Beeby PJ, Collins E, Saunders JB, McCaughan GW, et al. Transmission of hepatitis C virus to infants of human immunodeficiency virus-negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. J Viral Hepat. 1997;4:395–409. doi: 10.1046/j.1365-2893.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 25.Steininger C, Kundi M, Jatzko G, Kiss H, Lischka A, Holzmann H. Increased risk of mother-to-infant transmission of hepatitis C virus by intrapartum infantile exposure to maternal blood. J Infect Dis. 2003;187:345–351. doi: 10.1086/367704. [DOI] [PubMed] [Google Scholar]
- 26.Xiong SK, Okajima Y, Ishikawa K, Watanabe H, Inaba N. Vertical transmission of hepatitis C virus: risk factors and infantile prognosis. J Obstet Gynaecol Res. 1998;24:57–61. doi: 10.1111/j.1447-0756.1998.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang MH. Mother-to-infant transmission of hepatitis C virus. Clin Invest Med. 1996;19:368–372. [PubMed] [Google Scholar]
- 28.Hershow RC, Riester KA, Lew J, Quinn TC, Mofenson LM, Davenny K, et al. Increased vertical transmission of human immunodeficiency virus from hepatitis C virus-coinfected mothers. Women and Infants Transmission Study. J Infect Dis. 1997;176:414–420. doi: 10.1086/514058. [DOI] [PubMed] [Google Scholar]
- 29.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 30.Papaevangelou V, Pollack H, Rochford G, Kokka R, Hou Z, Chernoff D, et al. Increased transmission of vertical hepatitis C virus (HCV) infection to human immunodeficiency virus (HIV)-infected infants of HIV- and HCV-coinfected women. J Infect Dis. 1998;178:1047–1052. doi: 10.1086/515668. [DOI] [PubMed] [Google Scholar]
- 31.Pappalardo BL. Influence of maternal human immunodeficiency virus (HIV) coinfection on vertical transmission of hepatitis C virus (HCV): a meta-analysis. Int J Epidemiol. 2003;32:727–734. doi: 10.1093/ije/dyg107. [DOI] [PubMed] [Google Scholar]
- 32.Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis. 2005;37:350–353. doi: 10.1080/00365540510032105. [DOI] [PubMed] [Google Scholar]
- 33.European Paediatric Hepatitis C Virus Network. A significant sex – but not elective cesarean section – effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872–1879. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- 34.Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904–907. doi: 10.1016/s0140-6736(00)02681-7. [DOI] [PubMed] [Google Scholar]
- 35.Marine-Barjoan E, Berrebi A, Giordanengo V, Favre SF, Haas H, Moreigne M, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811–1815. doi: 10.1097/QAD.0b013e3282703810. [DOI] [PubMed] [Google Scholar]
- 36.Ministry of Public Health of the Kingdom of Thailand. HIV sero-surveillance in Thailand: result of the 23rd round, June 2005. Nonthaburi: Ministry of Public Health of the Kingdom of Thailand; 2005. [Google Scholar]
- 37.Jamieson DJ, Skunodom N, Chaowanachan T, Roongpisuthipong A, Bower WA, Chotpitayasunondh T, et al. Infection with hepatitis C virus among HIV-infected pregnant women in Thailand. Infect Dis Obstet Gynecol. 2008;2008:840948. doi: 10.1155/2008/840948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343:982–991. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- 39.Lemaire JM, Courouce AM, Defer C, Bouchardeau F, Coste J, Agulles O, et al. HCV RNA in blood donors with isolated reactivities by third-generation RIBA. Transfusion. 2000;40:867–870. doi: 10.1046/j.1537-2995.2000.40070867.x. [DOI] [PubMed] [Google Scholar]
- 40.Dunn DT, Gibb DM, Healy M, Goodall RL, Butler K, Cafferkey M, et al. Timing and interpretation of tests for diagnosing perinatally acquired hepatitis C virus infection. Pediatr Infect Dis J. 2001;20:715–716. doi: 10.1097/00006454-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Nantachit N, Robison V, Wongthanee A, Kamtorn N, Suriyanon V, Nelson KE. Temporal trends in the prevalence of HIV and other transfusion-transmissible infections among blood donors in northern Thailand, 1990 through 2001. Transfusion. 2003;43:730–735. doi: 10.1046/j.1537-2995.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 42.Luengrojanakul P, Vareesangthip K, Chainuvati T, Murata K, Tsuda F, Tokita H, et al. Hepatitis C virus infection in patients with chronic liver disease or chronic renal failure and blood donors in Thailand. J Med Virol. 1994;44:287–292. doi: 10.1002/jmv.1890440313. [DOI] [PubMed] [Google Scholar]
- 43.Songsivilai S, Jinathongthai S, Wongsena W, Tiangpitayakorn C, Dharakul T. High prevalence of hepatitis C infection among blood donors in northeastern Thailand. Am J Trop Med Hyg. 1977;57:66–69. doi: 10.4269/ajtmh.1997.57.66. [DOI] [PubMed] [Google Scholar]
- 44.Thaikruea L, Thongsawat S, Maneekarn N, Netski D, Thomas DL, Nelson KE. Risk factors for hepatitis C virus infection among blood donors in northern Thailand. Transfusion. 2004;44:1433–1440. doi: 10.1111/j.1537-2995.2004.04073.x. [DOI] [PubMed] [Google Scholar]
- 45.Paris R, Sirisopana N, Benenson M, Amphaiphis R, Tuntichaivanich C, Myint KS, et al. The association between hepatitis C virus and HIV-1 in preparatory cohorts for HIV vaccine trials in Thailand. AIDS. 2003;17:1363–1367. doi: 10.1097/00002030-200306130-00010. [DOI] [PubMed] [Google Scholar]
- 46.Zanetti AR, Tanzi E, Newell ML. Mother-to-infant transmission of hepatitis C virus. J Hepatol. 1999;31 Suppl 1:96–100. doi: 10.1016/s0168-8278(99)80383-3. [DOI] [PubMed] [Google Scholar]