Abstract
Orexins (also named hypocretins) are recently discovered neuropeptides made exclusively in the hypothalamus. Recent studies have shown that orexin cells located specifically in lateral hypothalamus (LH) are involved in motivated behavior for drugs of abuse as well as natural rewards. Administration of orexin has been shown to stimulate food consumption, and orexin signaling in VTA has been implicated in intake of high-fat food. In self-administration studies, the orexin 1 receptor antagonist SB-334867 (SB) attenuated operant responding for high-fat pellets, sucrose pellets and ethanol, but not cocaine, demonstrating that signaling at orexin receptors is necessary for reinforcement of specific rewards. The orexin system is also implicated in associations between rewards and relevant stimuli. For example, Fos expression in LH orexin neurons varied in proportion to conditioned place preference (CPP) for food, morphine, or cocaine. This Fos expression was altered accordingly for CPP administered during protracted abstinence from morphine or cocaine, when preference for natural rewards was decreased and drug preference was increased. Additionally, orexin has been shown to be involved in reward-stimulus associations in the self-administration paradigm, where SB attenuated cue-induced reinstatement of extinguished sucrose- or cocaine-seeking. Although the specific circuitry mediating the effects of orexin on food reward remains unknown, VTA seems likely to be a critical target for at least some these orexin actions. Thus, recent studies have established a role for orexin in reward-based feeding, and further investigation is warranted for determining whether function/dysfunction of the orexin system may contribute to the overeating associated with obesity.
Keywords: orexin, addiction, obesity, reward-based feeding, palatable food, conditioned stimuli
Introduction
The neuropeptides orexin A and orexin B (also denoted hypocretin 1 and hypocretin 2) were discovered in the late nineties and are synthesized solely in hypothalamic neurons [1,2]. Sakurai et al. (1998) characterized two individual receptors for the orexin system, termed OxR1 and OxR2 (also denoted HcrtR1 and HcrtR2) [2]. OxR1 binds both orexin A and orexin B but has a much lower affinity for orexin B, whereas OxR2 binds both peptides with a similar high affinity. Both receptors couple with G proteins; OxR1 couples exclusively with Gq subunits while OxR2 couples to both Gq and Gi/o [3]. Orexin neurons send dense fiber projections throughout the brain including cerebral cortex, hippocampus, thalamus, midbrain and spinal cord [4,5]. Furthermore, OxR1 and OxR2 are widely distributed throughout the CNS but are largely non-overlapping and are regionally selective [6–9].
Orexin has been implicated in feeding behavior, in keeping with the location of these cells in the area of lateral hypothalamus (LH) specifically associated with feeding. The initial study by Sakurai et al. (1998) demonstrated that central administration of orexin A or orexin B into the lateral ventricle stimulated food consumption, which prompted them to name the new peptides “orexin”, meaning appetite [2]. Furthermore, this study showed that prepro-orexin mRNA is up-regulated following fasting [2]. Subsequent studies confirmed that orexin A stimulated feeding, whereas systemic administration of the OxR1 antagonist SB-334867 (SB) reduced feeding [11–15]. Importantly, it was shown that SB reduced feeding by selectively enhancing the behavioral satiety sequence rather than through aversive mechanisms [11–14]. Other studies showed that the orexin neuronal field has reciprocal connections with areas known to regulate food intake including neuropeptide Y (NPY) neurons in arcuate nucleus [16,17], and that orexin A-induced feeding is partially attenuated by antagonism of the NPY-Y1 receptor [18]. Furthermore, obese mice (ob/ob and db/db) show decreased prepro-orexin gene expression [19], and antagonism of OxR1 produced a greater reduction of high-fat intake in Osborne-Mendel rats, a rat strain susceptible to dietary-induced obesity, as compared to obesity-resistant rats [20]. Taken together, these findings led to the view that the orexin neuropeptides modulate a homeostatic central feedback mechanism regulating feeding.
Further interest in the orexin system was generated when concurrent studies implicated it in another homeostatic function, sleep-wakefulness. Two groups simultaneously reported that dysfunction in the orexin system was linked with narcoleptic symptoms in mice and dogs [21,22]. Later work in humans showed that narcoleptics (with cataplexy) lack orexin in their CSF and lack most orexin neurons in posterior hypothalamus [23–25]. Together these findings led to the view that the orexin system is involved in arousal and maintenance of the waking state. In support of this view, additional findings showed that orexin neurons send dense projections to brain arousal areas including the locus coeruleus, tuberomammillary nucleus, and basal forebrain cholinergic system [4,5,26,27] and that orexin application typically strongly activates these cells [17,28,30]. Together, the findings that the orexin system is involved in both feeding and arousal led to the hypothesis that the primary function of this system was in promoting arousal in response to food deprivation and in a fashion to promote food consumption [1,2]. Recent studies, however, have investigated a possible role for orexins in reward-seeking for food and drugs of abuse independently of deprivation. As discussed below, this reward-associated function of the orexin system may be separated from its role in homeostatic feeding and maintenance of the arousal state, and may be mediated by a separate population of (laterally located) orexin neurons.
Much attention has been focused recently on the role of orexin in drug reward (reviewed in 30–33); however, with the current growing obesity epidemic, several studies have now linked the orexin system to reward-based eating. Dysfunction of the orexin system may be a contributing factor in the overeating associated with obesity. This seems a plausible hypothesis given the fact that hypothalamus is optimally located within the brain to communicate with lower brainstem nuclei controlling homeostatic processes and higher cortical and limbic areas associated with motivation. Furthermore, there is overlapping neural circuitry involved in food and drug reward [34]. We recently reviewed orexin’s role in drug addiction [30,31]. Therefore, this review will focus on orexin’s role in reward-based feeding and will only briefly highlight studies involving drug reward.
Orexin and brain sites involved in reward-based eating
Endogenous opioids in nucleus accumbens (NAc) play an important role in control of appetite and are postulated to mediate the hedonic aspects of food intake [35]. Rats will consume highly palatable foods even when sated, and overriding of homeostatic control mechanisms by rewarding aspects of food has been postulated to contribute to obesity [34,36]. A neural connection between NAc, a critical site for the regulation of reward-related behaviors, and hypothalamic orexin neurons may mediate the rewarding aspects of palatable food intake [37]. In this study, selective intake of high-fat food was induced by injection of the mu opioid receptor agonist D-Ala2-N-Me-Phe4-gly5-ol-enkephalin (DAMGO) into NAc core. DAMGO-induced feeding was inhibited by local injection of SB into VTA but not by injections of SB into arcuate nucleus or paraventricular nucleus of the thalamus (Fig. 1). These findings demonstrate that orexin signaling in VTA is involved in stimulating intake of a rewarding high-fat diet even in sated rats. Additionally, NAc DAMGO increased the proportion of orexin neurons expressing Fos in the perifornical hypothalamus (PeF) [37]. Tract tracing studies revealed that DAMGO-responsive sites of the NAc make close anatomical connections with hypothalamic orexin neurons. Taken together, these findings suggest that the orexin system is involved in DAMGO-induced intake of high fat diet through via a projection from NAc to hypothalamic orexin neurons, which in turn project to VTA. Previous findings also showed that high-fat diet intake induced by inactivation of NAc shell caused increased Fos expression in LH; this high-fat diet intake was suppressed by NMDA antagonism in LH [38,39]. These findings suggest that a neural pathway linking the NAc and LH is involved in reward-based eating in sated rats.
Figure 1.

Attenuation of DAMGO-induced high-fat diet intake following pretreatment with the OxR1 antagonist, SB-334867, in the VTA. Rats were given SB (15 nmol/side) or vehicle in VTA, and DAMGO (250 ng) in NAc, following overnight presentation of high-fat. Bars that do not share the same letter are significantly different from each other (p<0.05), based on ANOVA for high-fat diet. Effects on DAMGO-induced high-fat intake are shown. Adapted from [37].
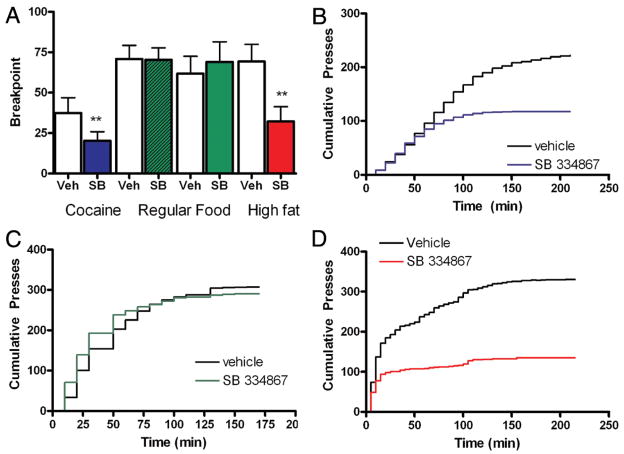
As discussed above central administration of orexin A increases free-feeding [2], and systemic administration of SB decreases feeding [11–14]. More recent studies investigating the role of the orexin system in reward-based eating have utilized a self-administration paradigm, in which an animal performs an operant response to obtain a food reward. Central administration of orexin increased free-feeding of sweet pellets as well as fixed ratio and progressive ratio responding for sweet pellets during self-administration [40]. Studies investigating the effects of systemic administration of SB on fixed ratio responding for sucrose have seen mixed results [41,42]. Initially, Richards et al. (2008) found that systemic injection of 10, 15, or 20 mg/kg SB to rats fed ad libitum did not decrease operant responding for a 5% sucrose solution [41]. More recent findings in our laboratory [42] found that systemic administration of SB at a higher dose (30 mg/kg) attenuated intake of sucrose pellets (Fig. 2). In this study, rats were trained in standard self-administration chambers to perform an operant response (lever press) to obtain a sucrose reward (45 mg sucrose pellet) during daily 1-hour sessions. Each pellet delivery was accompanied by a light + tone cue. Once stable responding for sucrose was established, rats were given injections of vehicle or SB (10, 20, or 30 mg/kg). Two or more days separated injections to allow sucrose responding to return to baseline between injections. Administration of 30 mg/kg, but not 10 or 20 mg/kg, SB significantly decreased the number of sucrose reinforcers obtained during self-administration in both rats fed ad libitum and rats food restricted to 85% of their free-feeding weight (Fig. 2). However, there was no significant effect of SB on active lever pressing in ad libitum fed rats (data not shown), similar to the previous findings using a sucrose solution by Richards et al (2008) [41]. In contrast, both low and high doses of SB decreased active lever pressing in food-restricted rats. The effects of 30 mg/kg SB are not thought to be due to a general motor reduction because this dose causes minimal effects on locomotor activity and no effect on certain operant behaviors [43]. Additional self-administration studies by others investigating high-fat diet have found that 10 or 20 mg/kg SB reduced intake on a fixed ratio schedule [44] and 10 mg/kg SB reduced intake on a progressive ratio schedule with no effect on responding for normal chow (Fig. 3) [45], indicating that OxR1 signaling is important for motivation for highly salient, positive food reinforcement. Contrasting findings have also been reported for intake of drugs of abuse. Systemic SB administration attenuated fixed ratio responding for ethanol [41] but not cocaine (discussed in more detail below) [43]. Taken together, these findings suggest orexin is necessary for reinforcement associated with some natural and drug rewards while it is not necessary for others.
Figure 2.
Attenuation of sucrose self-administration following pretreatment with the OxR1 antagonist SB-334867. Ad libitum fed (A) and food-restricted (B) rats were given SB (10, 20, 30 mg/kg, i.p.) or vehicle prior to self-administration sessions following at least 10 days of previous self-administration sessions. Effects on the number of earned reinforcers are shown (*p<0.05). SB doses were compared to vehicle treatment using a between-subjects design.
Figure 3.
Attenuation of progressive ratio (PR) responding for high-fat chocolate (red bars) or cocaine (blue bars) following pretreatment with the OrxR1 antagonist SB-3348667 (10 mg/kg). There was no effect on PR responding for regular chow (SB 10 mg/kg, green hatched bars; SB 20 mg/kg, green bars). Effects on breakpoint (A) and cumulative presses (B–D) are shown (**p<0.001).
Taken from [45].
Orexin and food-stimulus associations
Orexin neurons in LH also play an active role in stimulus-driven reward-seeking. A strong association was found between Fos activation in orexin neurons and the expression of conditioned place preference (CPP) for food, cocaine, or morphine in rats [46]. Notably, this Fos induction was directly proportional to the degree of preference that animals exhibited on the CPP test day (r=0.72 to 0.90, p < 0.01). Moreover, this correspondence between behavior and Fos induction was selective for orexin neurons in the lateral part of the orexin cell field (in LH) and was not found for more medially located orexin neurons (i.e. perifornical, PeF, or dorsomedial hypothalamus, DMH) nor for non-orexin neurons within LH (Table 1; Fig. 4). Fos activation in orexin neurons has also been observed following exposure to stimuli associated with alcohol [47] or cocaine [48].
Table 1.
Percentages of orexin-positive cells in LH, PeF, and DMH that were also Fos-positive in animals CPP tested for morphine, food, cocaine, or novelty preference, animals CPP tested following no conditioning, or naïve animals. The right column gives correlation coefficients for the comparisons between these percentages and the corresponding preference score in each animal. LH by group ANOVA, F(3,39)=33, p<0.01. The non-orexin Fos-positive neurons in the LH are given as total counts, not percentage. Significantly different from other groups, p<0.05. Orx = orexin-positive neurons. Taken from Harris et al (2005).
| Groups | Cell types | Percentage Fos+ | Correlations R | |
|---|---|---|---|---|
| Morphine-conditioned N= 12 | Orx LH | 48±2* | 0.72 | p<0.01* |
| NonOrx LH | 55±6 | 0.30 | p = 0.34 | |
| Orx PeF | 62±2 | 0.04 | p = 0.91 | |
| Orx PeF | 67±4 | 0.11 | p = 0.71 | |
| Food-conditioned N= 8 | Orx LH | 50±3* | 0.87 | p< 0.01* |
| NonOrx LH | 47±5 | 0.20 | p = 0.64 | |
| Orx PeF | 42±3 | 0.26 | p = 0.54 | |
| Orx DMH | 47±6 | 0.16 | p = 0.71 | |
| Cocaine-conditioned N= 8 | Orx LH | 52±5* | 0.90 | p < 0.01* |
| NonOrx LH | 78±7 | 0.51 | p = 0.20 | |
| Orx PeF | 67±3 | 0.41 | p = 0.32 | |
| Orx DMH | 74±3 | 0.50 | p = 0.20 | |
| Non-conditioned N= 15 | Orx LH | 17±2 | 0.11 | p = 0.81 |
| NonOrx LH | 43±6 | 0.30 | p = 0.53 | |
| Orx PeF | 52±4 | 0.42 | p = 0.36 | |
| Orx DMH | 59±4 | 0.02 | p = 0.96 | |
| Naïve N= 6 | Orx LH | 15±1 | ||
| NonOrx LH | 29±8 | |||
| Orx PeF | 52±3 | |||
| Orx DMH | 57±6 | |||
| Novelty-conditioned N= 6 | Orx LH | 18±2 | 0.09 | p=0.86 |
| NonOrx LH | 50±1 | 0.52 | p = 0.31 | |
| Orx PeF | 56±3 | 0.02 | p =0.97 | |
| Orx DMH | 63±5 | 0.42 | p =0.43 |
Figure 4.
Photomicrograph of divisions of hypothalamic neurons expressing orexin (brown cytoplasm) as described in [46]. All orexin labelled neurons lateral to the fornix (f) are considered lateral hypothalamus (LH). Orexin labelled neurons dorsal and 0.4mm medial to the fornix are considered perifornical hypothalamus (PFA). All remaining orexin labelled neurons from the medial edge of the PFA region to the third ventricle (3V) are considered dorsomedial hypothalamus (DMH). Opt = optic tract
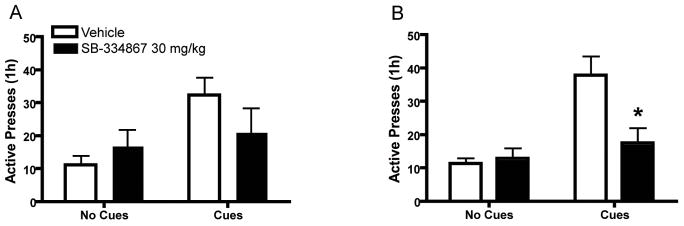
The role of orexin in cue-induced reinstatement of extinguished food-seeking has not previously been explored but has interesting clinical implications given that cue-induced feeding has been linked to obesity in humans [49]. Animal reinstatement models were originally designed to mimic relapse to drug addiction in humans and have more recently been used to explore food-seeking. In the cue-induced reinstatement model, reward-seeking is elicited following extinction training via presentation of discrete cues (i.e., light + tone) that were previously associated with reward delivery. Preliminary findings in our laboratory [42] suggest that systemic injections of SB attenuate cue-induced reinstatement of sucrose-seeking (Fig. 5). In this study, free-feeding and food-restricted rats were trained to self-administer sucrose pellets (45 mg) during daily 1-hour sessions. Each press on an active lever resulted in delivery of a single sucrose pellet accompanied by a discrete light + tone cue. After stable responding for sucrose was acquired, extinction training in the absence of sucrose and cues was conducted. Reinstatement of extinguished sucrose-seeking was then elicited by presentation of the sucrose-associated discrete cues in response to active lever pressing. Initial findings show that systemic administration of SB at 30 mg/kg, but not 10 mg/kg, attenuates active lever pressing during cue-induced reinstatement when compared to vehicle controls, reaching statistical significance in food-restricted rats (Fig. 5) [42]. Although further investigation is needed, these preliminary findings suggest that the orexin system is involved in cue-induced reinstatement of sucrose-seeking.
Figure 5.
Attenuation of sucrose-seeking behavior elicited by conditioned cues following pretreatment with the OxR1 antagonist SB-334867. Ad libitum fed (A) and food restricted (B) rats were given SB (30 mg/kg, i.p.) or vehicle prior to reinstatement of extinguished sucrose-seeking by discrete tone + light cues. Effects on active lever presses are shown (*p<0.05). SB was compared to vehicle treatment in the same animals using a within-subjects design. Note that SB significantly decreased presses in food-restricted, but not in ad lib-fed, rats.
In related studies investigating the role of orexin in other types of reinstatement, it was shown that systemic administration of SB at relatively low doses (5 or 10 mg/kg) attenuated yohimbine-induced reinstatement of extinguished sucrose- or alcohol-seeking (Richards et al., 2008), but that 20 mg/kg SB had no effect on reinstatement of food-seeking for high-fat diet elicited by yohimbine or pellet prime [44]. Again, these findings suggest that orexin’s influence on reinstatement may be sensitive to the type of reward and reinstatement modality being investigated. It is unknown where orexin is acting to influence these behaviors and additional studies are needed to identify orexin’s site of action as well as the neural mechanisms underlying the behavior. Possible sites of action include VTA (as discussed above for reward-based eating), NAc shell, bed nucleus of stria terminalis, medial prefrontal cortex or the basal cholinergic system. Previous studies have shown that presentation of food or food-associated stimuli elicits cortical acetylcholine release [50–52] and that this acetylcholine release requires orexin signaling via the OxR1 receptor [53].
Previous studies have investigated neural sites involved in cue-induced reinstatement of food-seeking but have not looked at the orexin system specifically. McLaughlin and Floresco (2007) demonstrated that temporary inactivation of the caudal portion of the basolateral amygdala (BLA) by bupivacaine potentiated cue-induced reinstatement of food-seeking [54]. Reinstatement was unaffected by inactivation of the rostral BLA. However, as bupivacaine also inactivates fibers of passage as well as somata, it is unknown whether the effects on reinstatement are due to inactivation of the cell bodies within the amygdala or inactivation of fibers of passage that travel through this region. In a similar experiment, Floresco et al. (2008) found that temporary inactivation of the NAc core by muscimol + baclofen (GABAA and GABAB agonists) attenuated cue-induced reinstatement of food-seeking whereas inactivation of the Nac shell potentiated reinstatement [55]. Petrovich et al (2002) proposed that an amygdalo-hypothalamic circuit allows learned cues (i.e., discrete cues) previously paired with food delivery to override satiety and promote eating [56]. In their study, a bilateral disconnection technique was used to demonstrate that BLA and LH are critical components in the system that regulates this form of cue-induced feeding in sated rats. However, it is unknown if this effect is mediated by a direct connection between BLA and LH or a potential indirect connection from BLA to NAc to LH. The BLA is known to send major projections to the NAc that in turn send projections to the LH [57]. Together these findings suggest that projections from NAc and/or amygdala may stimulate orexin neurons in response to food-conditioned stimuli, which may play a role in conditioned overeating and obesity.
Other studies have investigated brain sites involved in context-induced reinstatement of sucrose-seeking using Fos immunohistochemistry [58,59]. During context-induced reinstatement, re-exposure to the contextual environment in which self-administration occurred drives reward-seeking following extinction training. In the ABA design of context-induced reinstatement, animals are trained to self-administer reward in the presence of one context (Context A), extinguished in a different environment (Context B), and then re-exposed to the original context (Context A) to elicit reinstatement [60,61]. Using this design, Hamlin et al. (2006) demonstrated that context-induced reinstatement of sucrose-seeking was associated with increased Fos expression in NAc shell, LH, BLA, and insular cortex [58]. Furthermore, they demonstrated that systemic injections of the dopamine 1 receptor antagonist SCH 23390 decreased context-induced reinstatement and Fos expression in the those brain areas. In subsequent studies, temporary inactivation of the LH with baclofen/muscimol blocked context-induced reinstatement of sucrose- and beer-seeking [59]. Using the retrograde tracer cholera toxin b subunit (CTb) in combination with Fos immunostaining, this study demonstrated that specific afferents from NAc shell projecting to LH were activated during reinstatement to beer-seeking. It is unknown whether these projections from NAc shell project directly or indirectly to orexin neurons within LH or to a separate distinct population of non-orexin neurons. Previous studies with sucrose-seeking showed increased Fos expression in LH, but specifically in non-orexin neurons [58]. Additional studies are needed to investigate these circuits as well as other potential pathways involved in context-induced reinstatement, but these studies demonstrate that the NAc to LH pathway is involved in context-induced reinstatement [59].
Orexin and cocaine-stimulus associations
As noted above, our lab has shown that LH orexin neurons exhibit conditioned responses to cocaine, morphine and food-associated contexts in proportion to behavioral preference [46]. In addition, systemic administration of the orexin 1 receptor antagonist SB-334867 (SB) attenuated expression of morphine preference [46]. Preliminary studies from our laboratory indicate that SB has similar effects on cocaine preference (G. Sartor & G. Aston-Jones, unpublished data). In this study, cocaine-conditioned Sprague Dawley rats received a systemic injection (i.p.) of SB (30mg/kg) or vehicle (within subjects) prior to the preference test. SB significantly blocked cocaine preference compared to vehicle injections (n=9; p<0.02). Therefore, orexin is necessary for the expression of cocaine preference in rats, as it is for morphine preference (above), although a recent study finds that it is not necessary for cocaine preference in C57BL/6J mice [62].
The orexin system also plays a role in the ability of conditioned stimuli to elicit cocaine-seeking behavior [43,63]. In these studies, rats self-administered intravenous cocaine (0.2 mg/infusion) during daily 2-hour sessions, followed by extinction training in the operant chamber or abstinence in the home cage. Following extinction, cocaine-seeking was reinstated via presentation of discrete cues previously paired with cocaine infusions or via re-exposure to the original self-administration context. Following abstinence (no explicit extinction training), cocaine-seeking was evaluated upon reintroduction to the self-administration environment.
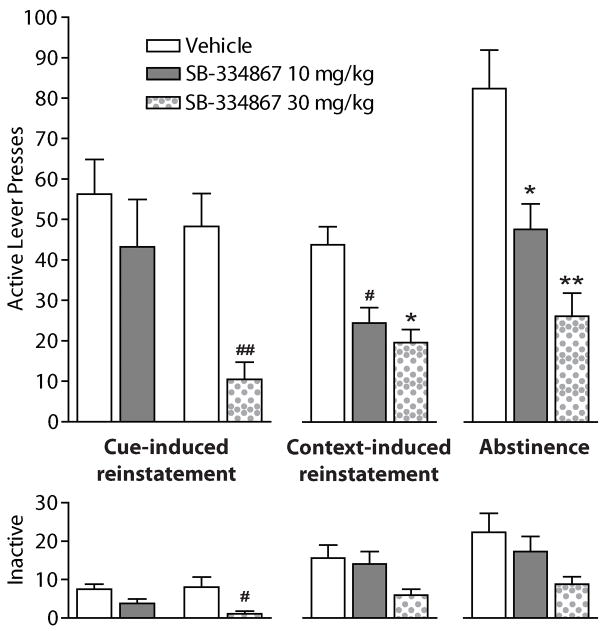
Cue-induced reinstatement of extinguished cocaine-seeking was significantly attenuated by systemic administration of 20 or 30 mg/kg, but not 10 mg/kg, SB (Fig. 6) [43]. Similarly, context-induced reinstatement of cocaine-seeking was reduced by 10, 20, or 30 mg/kg SB (Fig. 6) [63]. These results correspond with previous findings that SB reduced cue-induced reinstatement of extinguished ethanol-seeking [64]. Pretreatment with the high dose of SB also decreases inactive lever pressing following cue-induced reinstatement to cocaine, but not, following context-induced reinstatement or following abstinence. These data suggest that there may be a small contribution of decreased locomotor activity on lever pressing, however, it is unlikely that the large suppression of active lever pressing by SB during cocaine-seeking is due to a reduction in general locomotor activity [43]. Importantly, cue-induced reinstatement of cocaine-seeking was not affected by systemic administration of the OxR2 antagonist 4-pyridylmethyl (S)-tert-leucyl 6,7-dimethoxy-1,2,3,4-tetrahydrosisquinoline (4PT), indicating a unique role of orexin signaling specifically at OxR1 in cocaine-seeking [43]. This is consistent with a hypothesized role for OxR2 signaling primarily in arousal-related functions of the orexin system [8,65–68].
Figure 6.
Attenuation of cocaine-seeking behavior elicited by conditioned cues following pretreatment with the OxR1 antagonist SB-334867. Rats were given SB (10 or 30 mg/kg, i.p., shown here) or vehicle prior to: reinstatement of extinguished cocaine-seeking by discrete tone + light cues (left panels), reinstatement of extinguished cocaine-seeking by contextual cues (center panels), or cocaine-seeking following 2 weeks of abstinence in the home cage (right panels). Effects on active and inactive lever pressing are shown (#p < 0.05, *p < 0.01, ##p < 0.001, **p < 0.0005). For cue-induced reinstatement, SB was compared to vehicle treatment in the same animals using a within-subjects design. For context-induced reinstatement and abstinence, SB was compared to vehicle and other doses of SB in a between-subjects design. Modified from [43,63].
Recent animal models of relapse have also focused on drug-seeking following abstinence, which involves distinct neurocircuitry [61] and may represent more clinically relevant aspects of addiction. Our laboratory found that the orexin system is similarly involved in this type of contextual-driven cocaine-seeking observed in non-extinguished animals. Following chronic cocaine self-administration and 2 weeks of abstinence in the home cage, 10–30 mg/kg SB significantly attenuated cocaine-seeking upon re-exposure to the self-administration context (Fig. 6) [63]. These findings indicate that orexin signaling at OxR1 is involved in cocaine-seeking elicited by a variety of conditioned stimuli (discrete and contextual, and following either abstinence or extinction). Taken together with previous findings that SB reduced stress- or yohimbine-induced reinstatement of ethanol- or sucrose-seeking [41,69], these results indicate that orexin might play a general role in reward-seeking elicited by reward-conditioned stimuli.
In contrast to the above role of the orexin system in cue-induced cocaine-seeking, administration of 30 mg/kg SB had no effect on established self-administration of cocaine under a fixed ratio-1 schedule [43]. However, as discussed above, SB has been shown to significantly reduce self-administration of ethanol, nicotine, high-fat food, and sucrose [41,42,44,64,70], as well as ethanol intake in alcohol-preferring outbred rats [71] (discussed in further detail below). Interestingly, 10 mg/kg SB significantly reduced cocaine self-administration under a progressive ratio schedule, which is thought to measure motivation [45]. Together, these results demonstrate that orexin may be necessary for the reinforcing and motivating properties of some rewards, but not others.
Orexin and ethanol intake
Several studies have implicated the orexin system in ethanol consumption and abuse. The area of LH expressing orexin mRNA is increased after ethanol drinking in alcohol-preferring rats [64]. As noted above, SB decreased operant responding for ethanol and attenuated cue-and yohimbine-induced reinstatement of ethanol-seeking 41,64]. Furthermore, there was increased Fos expression in orexin neurons following context-induced reinstatement of ethanol-seeking (Dayas et al., 2008).
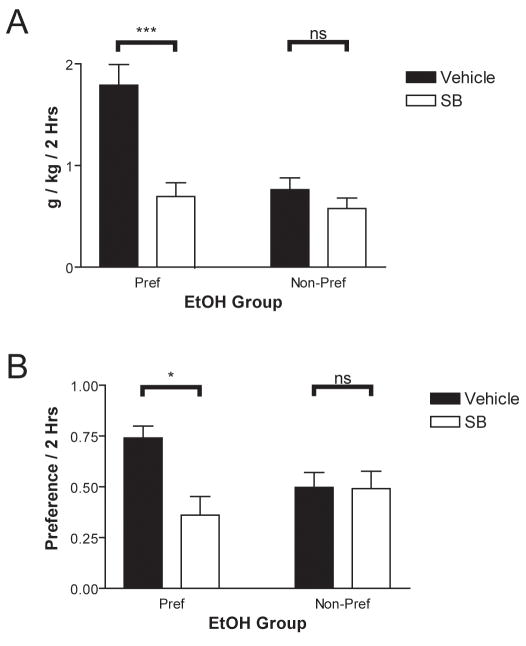
Recent studies from our laboratory have found that systemic administration of SB decreased the amount of ethanol consumed and preference for ethanol as measured by two-bottle tests, but only in individual rats that expressed a high preference for ethanol (Fig. 7) [47]. In these studies, preference was characterized in one of two ways: 1) alcohol acquisition method or 2) individual differences. In the case of alcohol acquisition method, we capitalized on the fact that the intermittent access paradigm, where rats receive either ethanol or water on alternating days, produces notably higher levels of drinking than does the sucrose fade paradigm, where rats are initially trained to drink sucrose which is gradually replaced with ethanol [72].
Figure 7.
Attenuation of ethanol intake and ethanol preference in ethanol-preferring rats following pretreatment with the Orx1 antagonist SB 33-4867. Effects on A) ethanol intake and B) ethanol preference are shown (*p < 0.05, ***p < 0.001). SB was compared to vehicle treatment in the same animals using a within-subjects design.
By training separate groups to drink using each of these techniques, we found that SB decreased ethanol drinking and preference more frequently in intermittent access-trained rats than in sucrose fade-trained rats. We extended these results by showing that, even within intermittent access-trained rats, SB administration primarily decreased ethanol preference and drinking in individuals with initially high preferences, bringing them down to the levels observed in those with initially low preferences. We concluded that orexin-1 receptor antagonism may play an important role in reducing compulsive drinking without abolishing the behavior entirely–finding that could equally be applied to compulsive eating scenarios. Importantly, SB had minimal effects on water intake, indicating that SB does not have a non-specific effect on fluid intake or arousal and suggesting that the orexin system might be a good target for treating compulsive reward-seeking behaviors. The fact that SB effectiveness tracks ethanol preference produced by intermittent access versus sucrose fade indicates that orexin may primarily be acting on conditioned rather than genetic factors in alcohol intake, a potentially clinically important attribute.
Orexin and altered hedonic processing during protracted abstinence
Chronic exposure to cocaine or morphine followed by protracted forced abstinence results in dramatically altered preferences for natural and drug rewards. Specifically, preference for environments associated with natural rewards such as food or novelty is decreased, whereas preference for drug (morphine or cocaine) is increased in a CPP paradigm following 2 or 5 weeks of protracted forced abstinence [73–77]. This increased interest in drug and decreased interest in natural rewards is similar to experiences reported by addicts [78] and could contribute to an addict’s inability to maintain drug abstinence.
To identify the neural substrates underlying such altered hedonic processing following protracted abstinence, Aston-Jones and Harris (2004) measured neuronal Fos activity following preference testing in animals subjected to protracted abstinence from chronic morphine [73]. This study identified three areas where Fos expression was lower than normal when abstinent animals were tested for food or novelty preference but higher than normal when abstinent animals were tested for drug preference (i.e., Fos expression mirrored behavioral changes): BLA, NAc shell, and LH [73]. Notably, the LH region that displayed altered Fos expression coincided with the area of the LH containing orexin cell bodies. Subsequent analysis of double-labeling for Fos and orexin immunohistochemistry revealed that, in fact, Fos activation in LH orexin neurons was reduced following food CPP, but enhanced following morphine CPP, in rats tested during protracted morphine abstinence as compared to naïve rats (Fig. 8) [73]. Taken together with results described above, these studies indicate that Fos activation in LH orexin neurons reflects normal preference for food-associated environments in naïve rats as well as altered food preference in rats following chronic morphine exposure and abstinence [46,73]. More recently, we also have found that orexin neurons exhibited increased Fos expression in proportion to enhanced preference for a cocaine context when CPP occurs during protracted withdrawal from cocaine [48].
Figure 8.

Percentage of LH orexin neurons expressing Fos following preference testing for food, morphine, or cocaine in a CPP paradigm. Some animals were implanted with morphine or placebo pellets for 2 weeks, remained in their home cages for 5 weeks, and then underwent CPP conditioning for morphine or food [73,76]. Naïve animals underwent CPP conditioning for cocaine, or had no conditioning at all. Note that a higher percentage of LH orexin neurons exhibited Fos in withdrawn animals than in placebo-pelleted animals following morphine CPP testing. Conversely, a lower percentage of LH orexin neurons exhibited Fos in withdrawn animals than in placebo-pelleted animals following food CPP testing. Cocaine, morphine, or food CPP testing increased Fos expression in LH orexin neurons, as compared to non-conditioned controls. Taken from [31].
Functional differences for LH versus DMH/PeF orexin neurons
As reviewed above, orexin neurons appear to be involved both in homeostatic processes that modulate arousal as well as feeding and reward-seeking; additional evidence indicates that orexin neurons are functionally dichotomous [79]. Thus, reward-seeking functions are associated primarily with orexin cells in LH, whereas arousal- and stress-related processes are linked with orexin neurons in the DMH and PeF [79]. Support for this hypothesis comes from several findings: PeF and DMH, but not LH, orexin neurons show increased Fos activation during waking compared to sleep [80]; neuroleptics that cause weight gain preferentially activate LH, rather than more medial, orexin neurons [81]; morphine withdrawal activates Fos in DMH/PeF but not LH orexin neurons [82]; chronic ethanol consumption increased the area of orexin mRNA expression in LH but not DMH/PeF [64]; and footshock stimulation induced Fos in DMH/PeF, but not LH, orexin neurons [46].
The dichotomy of orexin function implies that orexin neurons differ in their input-output connections according to reward-seeking versus arousal. There is some evidence to support this possibility. Fadel and colleagues found that orexin cells that project to VTA or medial prefrontal cortex (mPFC) originate preferentially from LH [81,83]. Yoshida et al. (2006) reported that PeF/DMH orexin neurons are innervated by other hypothalamic regions involved in homeostatic and arousal-related drive states, whereas LH orexin neurons are preferentially targeted by brainstem areas involved in autonomic and visceral processing and by reward-related areas such as VTA and NAc shell [84]. The specific circuit connections for LH vs. DMH/PeF are currently being further explored in our laboratory [85–89]. Additional studies are required to determine if a similar relationship between these projections to and from LH orexin neurons are involved in reward-seeking for palatable food.
Discussion
As described above, orexin is involved in reward-based feeding. Central orexin administration stimulated free-feeding in sated rats and antagonism of OxR1 blocked this effect. OxR1 antagonism in VTA attenuated high-fat feeding in sated rats induced by DAMGO in NAc. Additionally, self-administration studies found that central orexin administration increased fixed and progressive ratio responding for sweets, while recent studies from our lab have shown that OxR1 antagonism attenuates responding for established sucrose self-administration, suggesting that orexin influences the reinforcing properties of at least some types of food.
In addition to this role in reward-based feeding, the orexin system is involved in stimulus-induced reward-seeking for both food and drugs of abuse. Orexin neurons specifically within LH showed activation in proportion to preference for food, cocaine and morphine [46]. Following protracted abstinence from morphine, rats showed decreased preference for food reward and increased preference for drug reward, which was mirrored by alterations in Fos activation within LH orexin neurons [73,74,77]. Signaling at OxR1 has been implicated in both cue-and context-induced reinstatement of cocaine-seeking [43,63], as well as cue-induced reinstatement of alcohol-seeking [64]. Similarly, cues associated with reward-based, non-homeostatic feeding may also involve the orexin system. Preliminary findings suggest that blocking OxR1 signaling attenuates cue-induced reinstatement of sucrose-seeking, primarily in food-restricted rats [42].
Both food- and drug-related cues activate common neural pathways and are encoded via common biochemical mechanisms. Contextual cues associated with palatable food or drug administration produced similar patterns of Fos activation specifically within prefrontal and cingulate cortex [90–92]. Furthermore, contextual cues associated with highly palatable food or drugs upregulated homer 1A and arc gene expression within prefrontal and cingulate cortex, as well as sensorimotor cortex, striatum, and amygdala [93]. Homer 1A and arc have been implicated in learning, memory and plasticity. These findings support the hypothesis that drugs of abuse may produce neuroadaptations in brain circuitry evolved to process sensory information associated with environmental cues for motivationally salient natural rewards. Importantly, the prefrontal cortex sends projections to NAc, LH and VTA, and could interact with the orexin system directly or indirectly within these brain regions.
It is still unclear at which brain sites SB acts to attenuate self-administration of some natural rewards and cue-elicited reward-seeking. Orexin signaling may be important in structures related to food-seeking including VTA, BLA and mPFC. Previous studies have implicated VTA as an important site for orexin action in learning morphine-stimulus associations and likewise this structure may be important for food-stimulus associations. Microinjection of SB into VTA during conditioning reduced acquisition of a morphine CPP, and bilateral neurotoxic lesions of LH neurons or unilateral neurotoxic lesion of LH neurons combined with injections of SB into the contralateral VTA, prevented learning a morphine CPP [77,94]. On the other hand, systemic administration of SB did not attenuate acquisition of cocaine-associated cues [43], which again illustrates specificity for orexin involvement in different rewards. However, VTA may be an important site of action for behaviors driven by stimuli associated with a variety of rewards. PFC projections to VTA may provide conditioned response information to this key reward system. Recent studies in our laboratory showed that activation of VTA dopamine (DA) neurons by mPFC is modulated by orexin, frequently resulting in augmented responses to mPFC inputs in DA neurons [95].
It was hypothesized that SB attenuated responding for high-fat diet by accelerating food satiety [44]. However, additional mechanisms could also be involved. The finding that SB was more effective at decreasing lever-pressing, and the number of sucrose pellets obtained, in food-restricted rats than ad libitum fed rats indicates that SB may not only interfere with satiety but may influence motivation for sucrose reinforcement. In support of this hypothesis, SB reduces breakpoint, a measure of motivation, for cocaine and high-fat chocolate pellets, but not regular chow pellets, suggesting that signaling at the OxR1 modulates motivation to work for highly salient rewards [45]. Similarly, SB pretreatment in mice elevates reward thresholds for cocaine during intracranial self-stimulation (ICSS), a reward that is not subject to satiety (J. Muschamp & W. Carlezon, personal communication). Together these findings support the hypothesis that the orexin system may influence motivation to seek rewards in addition to influencing satiety. Furthermore, in rats self-administering cocaine or high-fat chocolate, orexin A-mediated potentiation of NMDA receptor-evoked EPSCs in VTA was increased compared to rats self-administering regular chow or vehicle controls, indicating that orexin-mediated glutamatergic synaptic transmission in VTA is especially involved in processing highly salient positive reinforcers [45]. These findings lead us to hypothesize that SB may reduce orexin A-potentiated glutamatergic transmission in VTA, and thereby attenuate DA-dependent conditioned behaviors following reward-conditioned stimuli. This would be in keeping with observations in our lab that orexin potentiates responses of VTA neurons to mPFC stimulation [95] (described above).
Alternative interpretations of the sucrose data include the possibility that SB may decrease sucrose intake by causing a conditioned taste aversion. However, studies examining the behavioral satiety sequence reveal that SB decreases palatable mash consumption in a different manner than the aversive agent LiCl [11–14]. For example, LiCl increases the duration of time spent eating and suppresses locomotor behaviors particularly during the initial portion of the test (i.e. before transitioning to resting). In contrast, SB decreases the duration of time spent eating and minimally affected locomotor behaviors. Importantly, the suppression of locomotor activity for SB occurred primarily following the transition to resting [11].
Additionally, the effects of SB on sucrose self-administration and cue-induced reinstatement may be due to decreased arousal and locomotor activity. As briefly discussed in this review, orexin increases arousal and increased arousal coincides with food consumption. However, this hypothesis does not seem likely given the fact that SB did not significantly decrease cue-induced reinstatement of sucrose-seeking in ad libitum fed rats, and did not decrease pellet-primed or yohimbine-induced reinstatement of food-seeking [44].
In conclusion, orexin has been implicated in feeding since its discovery and is involved in both homeostatic and reward-based food intake. Orexin’s role in reward-based feeding is of particular interest given the growing obesity epidemic; findings summarized here indicate that dysfunction of the orexin system may be a contributing factor to overeating associated with obesity. Currently, 66% of adults in the United States are overweight or obese leading to increased health risks and health care costs (http://win.niddk.nih.gov/statistics/#preval). Further investigation into the mechanisms by which the orexin system is involved in feeding may yield new therapeutic options for this obesity epidemic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, Sakurai T, Goto K. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
- 4.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 6.Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- 7.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 8.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 10.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Peptides. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 11.Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Rodgers RJ. Differential effects of the selective orexin-1 receptor anagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Rodgers RJ. Satiety enhancement by selective orexin-1 receptor anagonist SB-334867: influence of test context and profile comparison with CCK8-S. Behav Brain Res. 2005;160:11–24. doi: 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Arch JR, Blundell JE. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96:71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- 16.Broberger C, de Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent hypothalamus: relationship to neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 17.Horvath TL, Peyron C, Sabrina D, Ivanov A, Aston-Jones G, Kilduff T, van den Pol AN. Strong hypocretin (orexin) innervation of the locus coeruleus activates noradrenergic cells. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 18.Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai S, Matsuzaki I, Miwa Y, Goto K, Sakurai T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, Nomura M, Shibuya I, Matsukura S, Yamashita H. Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res. 1999;65:14–22. doi: 10.1016/s0169-328x(98)00320-9. [DOI] [PubMed] [Google Scholar]
- 20.White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8:373–399. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy with cataplexy. Sleep. 2009;32:993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Muhlethaler M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 27.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 30.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. doi: 10.1016/j.brainres.2009.09.106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players and addiction. Brain Res. doi: 10.1016/j.brainres.2009.10.068. in press. [DOI] [PubMed] [Google Scholar]
- 33.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. doi: 10.1016/j.brainres.2009.08.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 35.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;123:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7:607–612. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABA A receptr-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 40.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 41.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evan rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cason AM, Fallon R, Aston-Jones G. Effects of the orexin/hypocretin 1 receptor antagonist SB-334867 on sucrose-seeking in rats. 755.10 2009 Neuroscience Meeting Planner; Chicago, IL: Society fo Neuroscience; 2009. Online. [Google Scholar]
- 43.Smith R, See R, Aston-Jones G. Orexin/hypocretin signaling at the OX1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci S. Orexin A/Hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 47.Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Aston-Jones G, Liu S. Increased preference for cocaine during protracted forced abstinence: relationship with orexin/hypocretin neurons. Program No. 63. 18, 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. [Google Scholar]
- 49.Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (London) 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 50.Moore H, Sater M, Bruno JP. Bidirectional modulation of stimulated cortical acetylcholine release by benzodiazephine receptor ligands. Brain Res. 1993;627:267–274. doi: 10.1016/0006-8993(93)90330-p. [DOI] [PubMed] [Google Scholar]
- 51.Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during anticipation and consumption of a palatable meal. Neuroscience. 1994;62:1049–56. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 52.Fadel J, Moore H, Sarter M, Bruno JP. Trans-synaptic stimulation of cortical acetylcholine release after partial 192 IgG-saporin-induced loss of cortical cholinergic afferents. J Neurosci. 1996;16:6592–6600. doi: 10.1523/JNEUROSCI.16-20-06592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking. Neuroscience. 2007;146(4):1484–94. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirouac GJ, Ganguly PK. Topographical organization of the nucleus accumbens afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67(3):625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 58.Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143(1):25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 59.Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 62.Sharf R, Guarnieri DJ, Taylor JR, Dileone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res. doi: 10.1016/j.brainres.2009.12.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58(1):179–84. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akanmu MA, Honda K. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Res. 2005;1048:138–145. doi: 10.1016/j.brainres.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 66.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 67.Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 68.Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, Roche O, Rogers-Evans M, Wettstein JG, Moreau JL. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. Br J Phrarmacol. 156:1326–1341. doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;24:24. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Sprague Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induced high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(S1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- 75.Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 76.Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- 77.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Ralls TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. pp. 522–573. [Google Scholar]
- 79.Harris G, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richardson KA, Knackstedt PT, Aston-Jones G. Orexin neurons that project to the ventral tegmental area (VTA) are activated by morphine preference during protracted forced abstinence. Program No. 916.4. 2007 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2007. Online. [Google Scholar]
- 86.Richardson KA, Knackstedt PT, Aston-Jones G. Activation of VTA-projecting orexin/hypocretin neurons in the lateral, but not the dorsomedial and perifornical hypothalamus, correlates with morphine preference after prolonged forced abstinence. Program No. 63.16. 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. [Google Scholar]
- 87.Richardson KA, Aston-Jones G. Morphine conditioning activates orexin/hypocretin neurons that project to rostral VTA: Effects of protracted abstinence. Program No. 751.12. 2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- 88.Sartor GC, Aston-Jones G. Afferents that regulate lateral hypothalamic orexin/hypocretin neurons during drug seeking behaviors. Program No. 63.13. 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. [Google Scholar]
- 89.Sartor GC, Aston-Jones G. Afferents that regulate lateral hypothalamic orexin neurons during drug-seeking behaviors. Program No. 253.15. 2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- 90.Schroeder BE, Binzark JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–45. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 91.Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116:727–32. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- 92.Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- 93.Kelley AE, Schlitz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 94.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexigenic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moorman DE, Aston-Jones G. Modulation of ventral tegmental area neural responses to prefrontal stimulation by local orexin application in vivo. Program No. 916.2. 2007 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2007. Online. [Google Scholar]