SIRT1 is the closest mammalian homologue of enzymes that extend life in lower organisms. Its role in mammals is incompletely understood, but includes modulation of at least 34 distinct targets through its nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase activity. Recent experiments using small molecule activators and genetically engineered mice have provided new insight into the role of this enzyme in mammalian biology and helped to highlight some of the potentially relevant targets. The most widely employed activator is resveratrol, a small polyphenol that improves insulin sensitivity and vascular function, boosts endurance, inhibits tumor formation, and ameliorates the early mortality associated with obesity in mice. Many of these effects are consistent with modulation of SIRT1 targets, such as PGC1α and NFκB, however, resveratrol can also activate AMPK, inhibit cyclooxygenases, and influence a variety of other enzymes. A novel activator, SRT1720, as well as various methods to manipulate NAD+ metabolism, are emerging as alternative methods to increase SIRT1 activity, and in many cases recapitulate effects of resveratrol. At present, further studies are needed to more directly test the role of SIRT1 in mediating beneficial effects of resveratrol, to evaluate other strategies for SIRT1 activation, and to confirm the specific targets of SIRT1 that are relevant in vivo. These efforts are especially important in light of the fact that SIRT1 activators are entering clinical trials in humans, and “nutraceutical” formulations containing resveratrol are already widely available.
Sirtuins
SIRT1 came to the attention of the pharmaceutical industry via an unlikely route. A screen for particularly stress-resistant strains of budding yeast turned up a mutation in a gene called SIR4 (Silent Information Regulator 4) [1] that, as the name implies, had previously been shown to mediate transcriptional silencing at specific loci [2]. Further experimentation revealed that in addition to being stress-resistant, yeast carrying the mutant sir4-42 allele are able to produce more buds before entering a terminal senescent state – the yeast equivalent of an extension of lifespan. The authors then went on to show that two additional SIR genes, SIR2 and SIR3 are required to mediate the effect, and subsequently, that increasing the gene dosage of wild type SIR2 in the absence of other modifications is sufficient to extend yeast lifespan [3]. Intriguingly, SIR2 is conserved all the way up to the level of mammals, where seven homologues have been identified (SIRT1-7), with SIRT1 being the closest based on sequence homology [4]. In fact, closer inspection has revealed that almost all organisms, including yeast, contain multiple SIR2 homologues (termed “sirtuins”), but it is SIR2 itself, and the closest corresponding gene in each organism that have thus far received the most attention, and been most firmly linked to longevity. Extra copies of these genes extend lifespan in worms [5] and flies [6], and the relevance of the mammalian homologue, SIRT1, to human health has been a subject of much discussion in recent years.
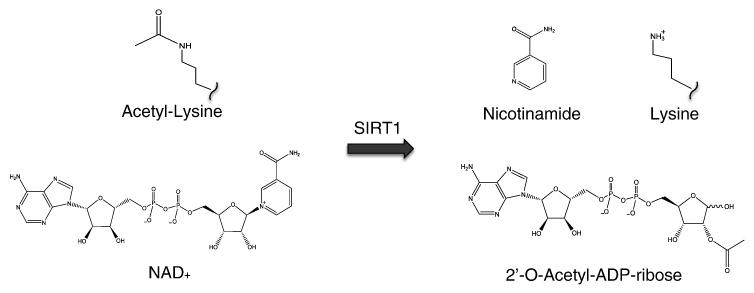
The first enzymatic activity described for Sir2 was a weak ability to transfer the ADP-ribose moiety of nicotinamide adenine dinucleotide (NAD+) to other proteins and itself [7]. However the major physiologically relevant activity of this enzyme, and its mammalian counterpart, SIRT1, is the NAD+-dependent deacetylation of acetylated lysine residues on histone and non-histone substrates [8, 9], which proceeds through a novel mechanism involving an ADP-ribosylated intermediate [10]. In fact, the description of ADP-ribosyltransferase activity was prescient, since other sirtuins, such as SIRT4 in mammals, appear to function exclusively as ADP-ribosyltransferases [11]. The use of NAD+ as a cofactor for deacetylation is unique to sirtuins and may provide a way to couple their activity to its metabolic state of the cell and/or allow their activity to be sensed by other enzymes. Whereas class I and II histone deacetylases simply release acetate, sirtuins (also called class III deacetylases) release nicotinamide and a novel metabolite, O-acetyl-ADP-ribose (AAR), that has been speculated to act as a second messenger (Figure 1). Although its lack of commercial availability and inherent instability have been a barrier to probing its function, experiments in yeast have shown that AAR can contribute to changes in the stoichiometry and structure of the Sir2/3/4 complex, and may influence heterochromatin spreading [12]. AAR is readily hydrolyzed to generate ADP-ribose in mammalian cells [13], and both bind to an inactive Nudix hydrolase domain in transient receptor melastatin-related ion channel 2 (TRPM2), potentiating its ability to induce cell death in response to oxidative and other insults [14, 15]. The normal function of TRMP2 is poorly understood, however cell death is accompanied by an influx of Na+ and Ca2+ ions, and it has been speculated that milder stimulation may play a role in Ca2+ signaling [14]. The quantitative contribution of SIRT1 to the cellular AAR pool has not been assessed, although there is evidence that disruption of other sirtuins is sufficient to decrease the concentration in yeast [16] and mammalian cells [14]. Moreover, some effects of AAR, such as its contribution to heterochromatin spreading in yeast [12], may be dependent on local production. Recently, evidence has been presented that both AAR and ADP-ribose can reduce free radical production in yeast and divert glucose into the pentose phosphate pathway, thereby increasing cellular NADPH levels [17]. The former occurs via inhibition of electron transport at complex I, and the latter through inhibition of glycolysis at the glyceraldehyde-3-phosphate dehydrogenase step. These observations highlight the potential importance of AAR as a product of SIRT1 activity, even though to date much more progress has been made on the characterization of its deacetylation substrates.
Figure 1.
Substrates and products of the reaction catalyzed by SIRT1. Note that only the side chain is shown for lysine, since SIRT1 does not have a conventional consensus sequence [139], although preference for specific peptides can be demonstrated [140].
Histones are a major class of substrates for sirtuins. In yeast, Sir2 deacetylates histone H4 at lysine 16 to maintain heterochromatin at the mating type loci, telomeres, and the rDNA [8]. The inherent instability of tandem repeats at the rDNA leads to formation of extrachromosomal rDNA circles (ERCs) that are thought to limit yeast lifespan [18]. Loss of Sir2 dramatically accelerates ERC formation and shortens lifespan, while increasing Sir2 activity reduces ERCs and extends yeast lifespan [3]. Interestingly, Sir2 appears to also promote longevity in an ERC-independent manner through its effects on telomeric heterochromatin [19]. In human cells, SIRT1 can deacetylate histones H4 at lysine 16, H3 at lysine 9, and H1 at lysine 26 [20], and appears to act at discrete sites, to which it is directed by binding partners such as Peroxisome proliferator activated receptor gamma (PPARγ) [21] and Clock [22]. Through these interactions, SIRT1 is able to transcriptionally repress genes involved in adipogenesis, and to contribute to circadian oscillations, respectively. There is no evidence for ERC formation in mammalian cells, although the rDNA appears to be one of the regions in which SIRT1 mediates transcriptional silencing [23]. At telomeres, a conserved role in chromatin maintenance may have been picked up by another Sir2 homologue, SIRT6, which deacetylates histone H3 lysine 9 in that region [24]. Even in the absence of ERCs, there is some evidence for a conserved role of SIRT1 in maintaining global genomic stability in mammals [25], however, non-histone substrates have thus far been linked to most of its reported effects.
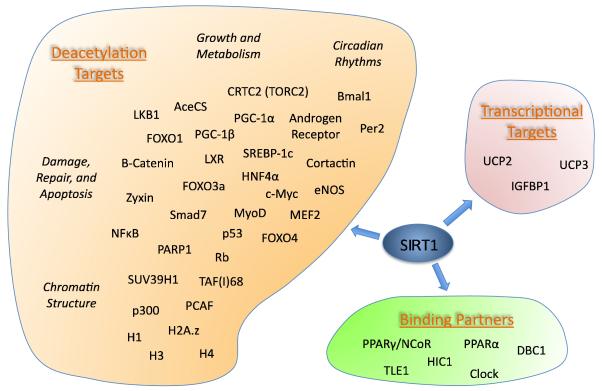
The first non-histone substrate identified for SIRT1 was p53 [26, 27]. Deacetylation of human p53 at lysine 382 suppresses its transcriptional activity and renders cells resistant to DNA damage and oxidative stress-induced apoptosis. Surprisingly, p53-dependent gene transcription and apoptosis were found to be roughly equivalent in mice lacking SIRT1 [28]. Although evidence continues to accumulate that the interplay between SIRT1 and p53 is important in cancer development, the mouse results clearly suggest that other targets of SIRT1 mediate many of its effects in vivo. Consistent with this, and with the view that sirtuins might use their deacetylase activity to fine-tune cellular metabolism based on NAD+ availability, a wide range of SIRT1 targets have since been identified (at least 34 distinct proteins), with roles in processes ranging from differentiation to circadian rhythms (Figure 2). Some of the best-characterized effects of SIRT1 include increasing stress-resistance and influencing metabolism through class O Forkhead box (FOXO) transcription factors [29], suppressing Nuclear factor kappa B (NFκB)-dependent inflammatory responses [30], and promotion of gluconeogenesis, fatty acid oxidation, and mitochondrial biogenesis through Peroxisome proliferator activated receptor gamma coactivator 1α (PGC1α) [31, 32]. However, nearly every target that has been identified is a potential mediator of crucial effects in vivo, and there is no doubt that more remain to be discovered. Resolving the relative importance, or net effect, of SIRT1’s deacetylation targets will be an enormous task, but may prove well worth the effort.
Figure 2.
Substrates, transcriptional targets, and binding partners of SIRT1. At least 34 direct deacetylation targets are known, with activities that impact almost every aspect of cellular physiology. For simplicity, only a few of the major themes are indicated. Several transcriptional targets of SIRT1 have been indicated because they are likely to be physiologically important and are not obvious results of the deacetylation targets and binding partners listed (although silencing of IGFBP1 is probably related to deacetylation of FOXOs). Note that the interaction of SIRT1 with binding partners can have varying results. SIRT1 overexpression silences PPARγ targets [21], but enhances expression of PPARα targets [64]. DBC1 directly inhibits SIRT1 activity [141, 142], while HIC1 directs it to its own promoter, silencing SIRT1 expression [143]. TLE1 mediates deacetylation of NFκB [144], and Clock directs SIRT1 to the promoters of genes involved in circadian rhythms [22, 145].
Resveratrol
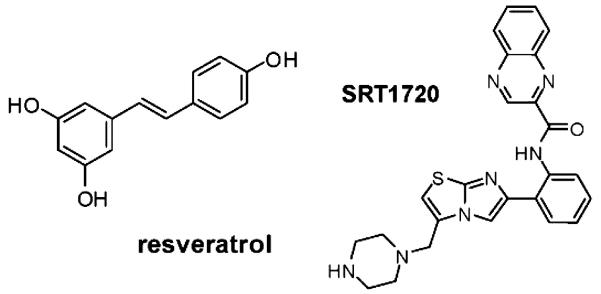
A major advance in the effort to understand the role of SIRT1 in vivo was the discovery that resveratrol (3,5,4′-trihydroxystilbene), a small polyphenol found at low doses in wine (Figure 3), can activate the enzyme in an in vitro assay and extend yeast lifespan [33]. Resveratrol has since been reported to extend lifespan in worms and flies, and in all three organisms, the effect is dependent on their respective SIRT1 homologues [34]. These invertebrate results have become somewhat controversial, since they have been alternately disputed [35, 36] and reproduced [37-41] in all three organisms. (Fly results were reproduced by the original lab using a different assay.) More recently, resveratrol has been shown to extend lifespan in a short-lived species of fish, although it has not been possible to test the involvement of sirtuins in that organism. Beneficial effects of resveratrol have also been reported in mammalian cells, and, as discussed in more detail below, in mice. A subset of these effects have been shown to depend on SIRT1 [42-44] including the prevention of skin cancer in vivo [45]. However, resveratrol influences many mammalian enzymes, and possesses intrinsic antioxidant capacity, greatly complicating the interpretation of its effects. Moreover, it has come to light that the in vitro activation of SIRT1 by resveratrol is substrate-dependent. Specifically, resveratrol activates the enzyme against an acetylated peptide containing a covalently attached fluorophore, but fails to affect deacetylation of the same peptide when the fluorophore is removed [36, 46]. This result raises questions about the validity of the original screen, and suggests that it will be important to test the effect of resveratrol using full-length endogenous substrates of SIRT1. Despite this controversy, many of resveratrol’s effects in mice are consistent with activation of SIRT1 and modulation of its targets. The following section highlights some of the salient effects of resveratrol treatment in rodents, along with SIRT1-dependent and independent pathways that may contribute to the observations.
Figure 3.
Structures of SIRT1 activators. Resveratrol is a naturally occurring polyphenol, and SRT1720 was synthesized by Sirtris Pharmaceuticals.
Cancer
Resveratrol potently inhibits carcinogenesis at multiple stages in rodent models [44, 47]. Direct inhibition of cyclooxygenases [47], as well as the aryl hydrocarbon receptor [48, 49] and cytochrome P450 enzymes [50, 51], likely account for much of this protection. However, SIRT1-dependent mechanisms may also play a role, since SIRT1 overexpression is sufficient to blunt intestinal tumorigenesis [52], and mice lacking SIRT1 exhibit a markedly reduced protective effect when given resveratrol as a preventive agent for skin cancer [45]. Moreover, SIRT1-dependent inhibition of NFκB could contribute to a decrease in cyclooxygenase activity through transcriptional silencing of the Cox2 isoform, and a decrease in Cox2 message has been reported following resveratrol treatment [53]. In vitro mechanistic studies have implicated a large number of downstream pathways in resveratrol’s antiproliferative, pro-apoptotic, and other tumor-suppressive effects, and a complete discussion is beyond the scope of this review. For a more comprehensive analysis, the reader is directed to recent reviews dedicated exclusively to this topic [54, 55].
Cardiovascular Disease
Resveratrol has at least three distinct properties that confer protection against cardiovascular disease. It induces a “preconditioning” effect that limits the damage during acute ischemia/reperfusion injuries [56], it improves vascular function [57], and it blocks platelet aggregation [58].
The protective effect of resveratrol against ischemic injuries is incompletely understood, but can be blocked by antagonists of nitric oxide synthase or adenosine in isolated hearts [59], and is absent in hearts from inducible nitric oxide synthase (iNOS)-null mice [60]. The protective effect of resveratrol against ischemic injury in brain is lost in PPARα-null mice [61], and again appears to require nitric oxide, although an endothelial nitric oxide synthase (eNOS)-specific inhibitor is sufficient to completely block the effect in this tissue [62]. At the time when most of these observations were made, no relationship was known between SIRT1 and PPARα or adenosine, and nitric oxide had been shown to function upstream of SIRT1, by increasing expression [63]. However, SIRT1 has since been shown to positively regulate PPARα [64], and to create a potential positive feedback loop by deacetylating and activating eNOS [65], raising the possibility that direct activation of SIRT1 could have a role in preconditioning. Moreover, inhibition of sirtuins by sirtinol or nicotinamide blocks the protective effect of resveratrol against ischemia in brain [66], and in cultured cardiomyocytes [67], respectively.
Resveratrol induces vasorelaxation in vitro [68] and lowers blood pressure in obese Zucker rats [69], as well as several experimentally-induced models of hypertension [44]. In aortas from obese or aged animals, resveratrol restores acetylcholine-dependent relaxation [70], which is dependent on nitric oxide signaling. Resveratrol’s protective effect appears to be mediated by suppression of age- and obesity-induced increases in NADPH oxidase expression. This enzyme is a major contributor to the production of superoxide radicals, which cause local oxidative stress and impair nitric oxide signaling by reacting to form peroxynitrate. Resveratrol also modestly induces eNOS expression in vascular tissue, which may help overcome the inactivation of nitric oxide by oxidative stress. The mechanism by which resveratrol suppresses NADPH oxidase in vasculature has not been firmly established, however SIRT1 can inhibit production of an upstream signal, tumor necrosis factor α (tnfα), by macrophages, most likely through inhibition of NFκB [71]. In addition, overexpression the SIRT1 target PGC-1α in vascular endothelial cells is sufficient to suppress NADPH oxidase expression [72], providing a second potentially SIRT1-dependent mechanism. Resveratrol may further stimulate PGC-1α through activation of AMP-activated protein kinase (AMPK). AMPK is a particularly attractive hypothesis to explain resveratrol’s effects, since there is evidence that the PPARγ ligand rosiglitazone reduces oxidative stress through an AMPK-dependent mechanism involving suppression of NADPH oxidase [73]. AMPK activation by resveratrol can be independent of SIRT1 [74], but can also be mediated by SIRT1-dependent deacetylation of the upstream kinase LKB1 [75]. Whether any or all of these mechanisms are relevant to the suppression of NADPH oxidase by resveratrol in vivo remains to be seen.
Resveratrol is able to inhibit platelet aggregation in vitro [76] and in vivo in hypercholesterolemic rabbits [58] and normal mice [77]. Its activity may be partially related to disruption of Mitogen-activated protein kinase (MAPK) signaling [78] and phosphoinositide metabolism [79], as well as enhanced nitric oxide signaling to guanylate cyclase [77]. It is also tempting to speculate that a major contribution to the in vivo effects might be mediated by direct and irreversible inhibition of cyclooxygenase I activity [80], resulting in decreased production of thromboxane A2, a potent inducer of clotting and vasoconstriction. One attractive aspect of this model, given the short half-life of resveratrol in vivo, is that it does not require a sustained dose. Inhibition of cyclooxygenase I is the same mechanism proposed to account for the cardioprotective effects of low-dose aspirin [81], and intriguingly, resveratrol remains effective even in platelets from aspirin-resistant individuals [82].
Insulin Sensitivity
Resveratrol has consistently been found to ameliorate insulin resistance in obese animals [32, 83]. This effect does not appear to be directly related to overall body weight, but is accompanied by a dramatic reduction of ectopic fat deposits in non-adipose tissues, particularly the liver [83]. In human studies, such ectopic fat deposits have been shown to precede clinical disease in subjects at risk for type II diabetes, and to associate with reduced respiratory capacity and tissue mitochondrial content [84]. In rodents, the protective effects of resveratrol are accompanied by an increase in mitochondrial biogenesis and respiratory capacity that offset effects of obesity [32, 83], and could thereby explain the restoration of insulin sensitivity. PGC-1α, the “master regulator” of mitochondrial biogenesis is activated upon deacetylation by SIRT1 [31], and this has been shown to occur in tissues of resveratrol-treated animals, ostensibly accounting for the increase in mitochondrial content [32, 83]. In addition, a SIRT1-dependent decrease in the expression of protein tyrosine phosphatase 1B (PTP1B) has been described in resveratrol-treated animals [42]. Since PTP1B inactivates the insulin receptor, this provides a second mechanism by which resveratrol might act through SIRT1 to improve insulin sensitivity. Furthermore, overexpression of SIRT1 is sufficient to eliminate ectopic fat deposits in liver and to improve insulin sensitivity in obese mice [85]. Although the mechanism is far from completely elucidated, these results provide significant support for the idea that resveratrol might protect against the metabolic syndrome and type II diabetes by activating SIRT1.
Energy Expenditure
Resveratrol has a biphasic effect on energy expenditure. Without any significant effect on food intake, resveratrol causes a modest, but significant increase in the body weight of mice at low doses [70], and a loss of body weight and reduction of adiposity at high doses [32]. Notably, the elimination of ectopic fat deposits described above occurs even at doses too low to decrease total body weight. Although the decrease in body weight at high doses of resveratrol is accompanied by an increase in oxygen consumption, cold tolerance, and endurance, resting body temperature is unchanged and spontaneous locomotion is decreased, raising interesting questions about where the excess energy actually goes [32]. Overexpression of SIRT1 from the β-actin locus, which results in high expression in adipose and brain, decreases body weight and adiposity, similar to the effect of a high dose of resveratrol [86]. However overexpression under the endogenous promoter from a bacterial artifical chromosome leads to a different expression profile, and can actually decrease energy expenditure in some cases, and possibly increase body weight on a leptin-deficient background [85, 87]. Therefore it is conceivable that the disparate effects of resveratrol at high and low doses reflect effects on SIRT1 in different tissues. Indeed, there is evidence that SIRT1 is differentially regulated across tissues by diet, suggesting that whole-body activation may be too simplistic an approach [88]. Of course, many other targets of resveratrol could contribute to its effects on body weight. One such target is the cannabinoid receptor CB1 [89], which can regulate body weight through food intake and appetite-independent mechanisms.
Learning and Memory
Resveratrol improves cognitive function in models of neurodegenerative diseases, and following neuronal injury, but has not been clearly demonstrated to improve cognition in normal, healthy rodents [90, 91]. Normal age-related cognitive impairment is ameliorated by resveratrol in a short-lived species of fish, in parallel with an increase in lifespan [92], but similar data have not been reported for any mammalian species. In mice, resveratrol improves rotarod performance [32, 83], which can have a cognitive component, but may also be explained by changes in endurance. Further research in this area is needed to clarify the involvement of SIRT1 in neuroprotective effects of resveratrol, and whether activation of SIRT1 could be detrimental, rather than protective, in neurons under certain conditions [93, 94]. Intriguingly, a SIRT1 allele has been linked to cognition in a human association study, although is not yet possible to know what consequence the allele has on SIRT1 function [95].
Survival
Placing mice on a high fat diet (60% by energy content) at one year of age results in an approximately 25% decrease in remaining lifespan, and this effect is completely blocked by resveratrol administration, independent of any effect on body weight [83]. This effect appears to represent amelioration of the detrimental effects of obesity, rather than slowing the rate of aging, since no significant change in longevity has been detected in mice fed a standard diet plus resveratrol at similar or higher doses [70]. These results are in accordance with the well-known correlation between insulin sensitivity and longevity, since resveratrol dramatically improves glucose tolerance only in the context of obesity. Similarly, whole-body overexpression of SIRT1 improves glucose tolerance only in obese mice [85, 87], although overexpression in a more limited subset of tissues from the β-actin promoter is effective even in lean animals [86]. To date, lifespans have not been published for SIRT1 overexpressing or heterozygous animals. SIRT1 null animals are short-lived, and do not respond favorably when placed on a calorie-restricted diet [94], however these mice manifest a number of developmental defects and altered metabolism [96, 97], that make it difficult to interpret their phenotypes with respect to aging. Two critical questions remaining to be answered are whether SIRT1 overexpression can recapitulate the effect of resveratrol on survival in obese mice, and whether it will influence longevity in lean animals.
“Off-Target” Effects of Resveratrol
Perhaps the biggest liability of resveratrol as a tool to probe the function of SIRT1 is its lack of specificity. Resveratrol has other direct targets in mammalian cells, some of which were identified prior to SIRT1, and many of which have their own complex and potentially beneficial consequences. For example, some of the cardioprotective and anti-inflammatory effects or resveratrol may be due to direct inhibition of cyclooxygenases [47], and an alternate explanation for many of the effects attributed to direct SIRT1 activation, such as increases in mitochondrial biogenesis and fatty acid oxidation, could be provided by indirect activation of AMPK [83, 98]. In fact, the relationship between SIRT1 and AMPK is more complex, since each has been reported to influence the other [75, 99], however, activation of AMPK by resveratrol is at least partially independent of SIRT1 [74, 100]. Additional direct effects of resveratrol that could have important consequences in vivo include inhibition of kinases [101, 102], modulation of the estrogen receptor [103], the aryl hydrocarbon receptor [49], and a cannabinoid receptor [89], and inhibition of quinone reductase 2 [104], to name a few.
Another important caveat is that the bioavailability of resveratrol in mammals is low enough that the doses required to activate SIRT1 in vitro and in cells are only briefly or never achieved in serum in vivo [105]. This has even led to the suggestion that metabolites of resveratrol might be the active forms, rendering most published work on the molecule irrelevant [106]. Notably, concentrations insufficient to activate SIRT1 would also be insufficient to affect most of the other targets, or stimulate AMPK, however a minority of higher affinity targets might still be affected. These include the aryl hydrocarbon receptor (AhR), quinone reductase 2 (NQO2, QR2), and the cannabinoid receptor CB1, each of which is affected by nanomolar concentrations of resveratrol. The CB1 receptor in particular is intriguing, since it is already considered a promising drug target for the treatment of obesity and related comorbidities. In fact, the antiobesity drug rimonabant, like resveratrol, is an antagonist of this receptor [107]. Although rimonabant was taken off the market due to a high incidence of psychiatric disorders, it has been shown to increase energy expenditure, improve insulin sensitivity, suppress inflammation, and prevent cancer, similar to resveratrol. One discrepancy is that rimonabant also suppresses food intake, which does not occur with resveratrol treatment, at least in mice. Suppression of AhR-dependent gene transcription by resveratrol could account for some of its anti-carcinogenic and anti-inflammatory effects, and it has been suggested that this mechanism could account for cardioprotective effects [108]. The function of NQO2, and consequences of its inhibition are less clear, but it has been suggested that blocking its activity could lead to induction of phase II antioxidant enzymes [104], which has been observed following resveratrol treatment [109]. The abundance of cellular targets and questions about bioavailability raise the possibility that SIRT1 activation might occur through an indirect mechanism, downstream of one of the higher affinity targets. This requires an enormous fluke, since resveratrol was selected through an in vitro assay, however it is almost impossible to distinguish experimentally from direct activation unless the upstream pathway is known. Ultimately, reconciling the pharmacokinetics of resveratrol with its biological consequences will require a better understanding of its distribution and accumulation within specific tissues or compartments, and a thorough consideration of many potentially relevant targets. These complications highlight the need to verify effects that have been attributed to SIRT1 using knockout animals and more specific methods of SIRT1 activation in vivo.
SRT1720
Besides resveratrol, a number of other naturally occurring polyphenols, such as quercetin, fisetin, and butein activate SIRT1 and extend lifespan in lower organisms [33, 34]. However, all are structurally related and share the same caveats. Recently, a more potent synthetic SIRT1 activator that is structurally unrelated to resveratrol was described and designated SRT1720 (Figure 3) [110]. Although its full spectrum of effects remains to be determined, SRT1720 would ostensibly not share “off-target” effects of resveratrol, and is therefore a useful tool for verifying putative SIRT1-dependent effects in vivo. Importantly, SRT1720 does not acutely activate AMPK [111]. Similar to both resveratrol treatment and SIRT1 overexpression, SRT1720 improves insulin sensitivity and glucose tolerance in obese mice. Moreover, a longer treatment course lowers body weight, induces mitochondrial biogenesis, eliminates ectopic fat deposits, and increases endurance [111]. These phenotypes are associated with deacetylation of the SIRT1 target PGC-1α, suggesting a direct mechanism, and providing further correlation with the effects of resveratrol. Deacetylation of the SIRT1 substrates FOXO1 and p53 were also observed following SRT1720 treatment, and modulation of their activities might contribute the observed phenotypes as well. One important caveat to the long-term studies with SRT1720 is that activation of AMPK eventually occurs. However, this is most likely a downstream consequence of the metabolic changes induced by SIRT1 activation, since unlike resveratrol, SRT1720 does not stimulate AMPK in cells. Furthermore, a microarray based study has shown a striking degree of overlap in the transcriptional effects of resveratrol and SRT1720 following a relatively short treatment, suggesting that activation of SIRT1 is responsible for many of the observed changes [112]. Experiments are currently underway to test whether SRT1720 recapitulates other salient effects of resveratrol, such as preconditioning, reduction of vascular oxidative stress, prevention of platelet aggregation, neuroprotection, cancer prevention, and delaying mortality in obese mice.
Isonicotinamide
Nicotinamide, which is produced by sirtuin enzymes, is a potent inhibitor of their activity. It has been estimated that physiological concentrations of nicotinamide are sufficient to reduce basal Sir2 activity in yeast by 2.5-6 fold, and SIRT1 activity in mouse cells by up to 20-fold [113]. The mechanism of inhibition involves re-entry of nicotinamide into the catalytic site of the enzyme after its release, where it can combine with a relatively stable reaction intermediate, resulting in regeneration of the original acetylated lysine and NAD+. Based on this observation, Sauve et al [113] were able to show that isonicotinamide can relieve inhibition of Sir2 activity by competing with nicotinamide for binding in the same pocket within the enzyme. Since isonicotinamide cannot participate in the reverse reaction, the intermediate is stabilized and more likely to complete the deacetylation step. Isonicotinamide is therefore expected to be an activator of sirtuins under normal physiological conditions (i.e when their activity is partly held in check by nicotinamide). Consistent with this, effects of isonicotinamide have been correlated with those of resveratrol in cell culture models, although in most cases the SIRT1-dependence was not thoroughly tested [114, 115]. Recently, it was shown that isonicotinamide represses expression of cytosolic phophoenolpyruvate carboxykinase (PEPCK) and that the effect depends on the presence of both SIRT1 and its substrate, hepatocyte nuclear factor 4α (HNF4α) [116]. Activation of SIRT1 with resveratrol or other polyphenols, or overexpression of the enzyme also repressed PEPCK transcription, suggesting that a reduction in gluconeogenesis might contribute to the long-term improvements in insulin sensitivity in mice treated with resveratrol or overexpressing SIRT1. The high concentrations of isonicotinamide required for efficacy likely limit any in vivo applications, although it appears to be well tolerated in mice, even at 1% in the drinking water over the entire lifespan [117].
NAD+ Metabolism
Another strategy for increasing sirtuin activity is by increasing the availability of the cofactor NAD+. There is an unresolved debate in the field about whether NAD+:NADH ratio or some combination of absolute NAD+ and nicotinamide concentrations best predicts the activity of sirtuins in vivo [118, 119]. However, it is widely agreed that increasing flux through the NAD+ salvage pathway, which generates NAD+ from nicotinamide, is sufficient to cause activation. In yeast, Pnc1 catalyzes the rate-limiting step in this pathway, and its overexpression is sufficient to extend lifespan in a Sir2-dependent manner [120]. In mammals, Nicotinamide phosphoribosyltransferase (Nampt) plays an analogous role and its overexpression is sufficient to increase the catalytic activity of SIRT1 [121], as well as promote cell survival in a SIRT3 and SIRT4-dependent manner [122]. In vivo, the product of its reaction, nicotinamide mononucleotide, appears to be limiting for SIRT1 activity in pancreatic beta cells [123], and circadian oscillations in Nampt expression cause SIRT1 activity to fluctuate in liver and white adipose tissue [22, 124]. Overexpression of Nampt in the heart is sufficient to promote survival of cardiac myocytes under a number of stresses, although the involvement of sirtuins in this process is not known [125]. These results underscore the fact that much additional work will be required to fully elucidate the consequences of increasing cellular NAD+, which serves as a cofactor in many crucial processes. Another potential route to increased NAD+ synthesis is through the administration of nicotinamide riboside, a precursor that is enriched in milk and may be involved in normal NAD+ metabolism [126]. Much like Pnc1 overexpression, supplementing yeast media with nicotinamide riboside is sufficient to confer Sir2-dependent lifespan extension, although the latter would presumably increase NAD+ without lowering nicotinamide levels.
It is important to keep in mind that resveratrol and SRT1720 are generally considered to target SIRT1 (albeit that resveratrol may also affect SIRT7 [127], and data on the specificity of SRT1720 are not widely available), while isonicotinamide and NAD+ availability are likely to affect all seven mammalian sirtuins. As has been discussed, each of these strategies to increase SIRT1 activity also comes with a large number of caveats and probable off-target effects. However, by combining mechanistically distinct approaches, and incorporating data from overexpression and loss of function studies, it will be possible to move forward and improve our understanding of the role of SIRT1 in vivo. Based on rodent studies, there is good reason to be optimistic that this could lead to the development of therapies that will benefit human health.
Human Trials
Nicotinamide and nicotinic acid are forms of vitamin B3 (niacin), and their biological functions, including the ability to act as NAD+ precursors, have been adequately reviewed elsewhere [128]. There is also significant interest in the potential use of nicotinamide riboside to drive NAD+ synthesis, particularly in nervous tissue, where it may be more effective than niacin [129]. However, it is resveratrol that has thus far received the most attention as a potential strategy to activate SIRT1 therapeutically. Humans have historically been exposed to low doses of resveratrol primarily through consumption of red wine, and traditional Chinese and Japanese medicines, and it is attractive to speculate that the health benefits associated with these substances might be at least partially attributed to their resveratrol content. Intriguingly, a recent study showed that light wine consumption is associated with a ~5 year increase in life expectancy [130], however alcohol alone has a significant protective effect (~2 years in the aforementioned study), and many other wine components are thought to improve health. Moreover, the resveratrol doses employed in animal studies significantly exceed those that could be obtained from wine. With the more recent availability of higher doses resveratrol in “nutraceutical” formulations, claims of benefits based on anecdotal evidence have become widespread. In many cases, studies are poorly controlled or uncontrolled, and in almost all cases, the formulations administered contain multiple compounds or complex extracts, making it even more difficult to assess the effect of resveratrol per se [131]. Based on peer-reviewed literature, it seems that resveratrol is well tolerated in humans over short periods of time, but further trials are needed to establish its ultimate effects on health and disease. The first measurements of resveratrol and its metabolites in human plasma and urine following an oral dose were reported in 2001 [132], and since then, and at least 9 additional human trials have been conducted to assess pharmacokinetic parameters [133, 134]. Safety and potential side effects have been investigated in two phase I clinical trials. The first employed a single dose of up to five grams, with a 14-day follow-up [135]. The second employed doses of up to 150 mg every four hours for two days, followed by an additional 24 hours of observation [136]. Notably, the doses from both studies greatly exceed the ~5-22 mg/kg that increased survival in obese mice when the preferred method of allometric scaling is employed [137], and the five gram dose remains well in excess even by direct extrapolation from body weight. In both studies, the effects reported were minor, and not clearly linked to the treatment, including symptoms such as headache, dizziness, and anomalous biochemical or hematological measurements that were not consistent across treatment groups. It has also been reported in company press releases that SRT501, a proprietary formulation of resveratrol developed by Sirtris Pharmaceuticals, has undergone phase I trials employing up to five grams daily for 28 days (www.sirtrispharma.com/news-press.html). This was reported to result in improved glucose tolerance, although neither these data, nor any other beneficial effect of a SIRT1 activator in humans, has yet been published in a peer-reviewed journal. This may soon change, since a number of more advanced clinical trials have been announced to test resveratrol against cancer, type II diabetes, Alzheimer’s disease, and viral infections. In addition, at least one controlled study of resveratrol has been initiated in monkeys [138], and Sirtris has announced its intention to proceed with phase I clinicals trials of another proprietary SIRT1 activator, designated SRT2104. The entry of SIRT1 activators into human trials is exciting, but also highlights the need to better define the pathways that mediate their effects in vivo.
Summary
As the homologue of enzymes that promote longevity in lower organisms, SIRT1 provides a tantalizing drug target. The ever-growing list of proteins whose activities are influenced by SIRT1-dependent deacetylation supports its potential importance in mammalian biology, but also add to the difficulty in understanding its function. Overexpression studies and treatment of rodents with small molecule activators have led to significant improvements in physiology, many of which are consistent with effects on specific SIRT1 targets. However, many caveats and uncertainties remain. Much of the strongest evidence for beneficial effects has been obtained with resveratrol, the strategy with perhaps the least certain mechanism of action. Moreover, disparate phenotypes have been reported even between independent lines of SIRT1-overexpressing mice, suggesting that the consequences SIRT1 activation will be complex and context-dependent. Data from three independent transgenic lines, as well as resveratrol and SRT1720 treatment, support a role for SIRT1 in promoting insulin sensitivity and glucose tolerance in obese animals, highlighting the therapeutic potential of modulating this pathway. Establishing a clear mechanistic explanation for these benefits should be a high priority, given the need for tools to combat obesity and the metabolic syndrome, and the number of humans already entering controlled or self-administered clinical trials with SIRT1 activators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kennedy BK, Austriaco NR, Jr., Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- [2].Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- [5].Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- [6].Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- [8].Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- [9].Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL. Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry. 2001;40:15456–15463. doi: 10.1021/bi011858j. [DOI] [PubMed] [Google Scholar]
- [11].Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- [12].Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell. 2005;121:515–527. doi: 10.1016/j.cell.2005.03.035. [DOI] [PubMed] [Google Scholar]
- [13].Rafty LA, Schmidt MT, Perraud AL, Scharenberg AM, Denu JM. Analysis of O-acetyl-ADP-ribose as a target for Nudix ADP-ribose hydrolases. J Biol Chem. 2002;277:47114–47122. doi: 10.1074/jbc.M208997200. [DOI] [PubMed] [Google Scholar]
- [14].Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL, Scharenberg AM, Denu JM. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem. 2006;281:14057–14065. doi: 10.1074/jbc.M513741200. [DOI] [PubMed] [Google Scholar]
- [15].Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- [16].Lee S, Tong L, Denu JM. Quantification of endogenous sirtuin metabolite O-acetyl-ADP-ribose. Anal Biochem. 2008;383:174–179. doi: 10.1016/j.ab.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tong L, Lee S, Denu JM. Hydrolase regulates NAD+ metabolites and modulates cellular redox. J Biol Chem. 2009;284:11256–11266. doi: 10.1074/jbc.M809790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- [19].Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- [21].Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, De Oliveira R. Machado, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Murayama A, Ohmori K, Fujimura A, Minami H, Yasuzawa-Tanaka K, Kuroda T, Oie S, Daitoku H, Okuwaki M, Nagata K, Fukamizu A, Kimura K, Shimizu T, Yanagisawa J. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133:627–639. doi: 10.1016/j.cell.2008.03.030. [DOI] [PubMed] [Google Scholar]
- [24].Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vaziri H, Dessain SK, Eaton E. Ng, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- [27].Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- [28].Kamel C, Abrol M, Jardine K, He X, McBurney MW. SirT1 fails to affect p53-mediated biological functions. Aging Cell. 2006;5:81–88. doi: 10.1111/j.1474-9726.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- [29].Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- [30].Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- [32].Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [33].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- [34].Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- [35].Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [36].Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell S, Napper A, Curtis R, Distefano PS, Fields S, Bedalov A, Kennedy BK. Substrate specific activation fo sirtuins by resveratrol. J Biol Chem. 2005 doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- [37].Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jarolim S, Millen J, Heeren G, Laun P, Goldfarb DS, Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- [40].Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- [41].Yang H, Baur JA, Chen A, Miller C, Adams JK, Kisielewski A, Howitz KT, Zipkin RE, Sinclair DA. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6:35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [43].Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- [45].Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009 doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- [46].Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- [47].Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- [48].Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- [49].Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- [50].Chan WK, Delucchi AB. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci. 2000;67:3103–3112. doi: 10.1016/s0024-3205(00)00888-2. [DOI] [PubMed] [Google Scholar]
- [51].Chang TK, Lee WB, Ko HH. Trans-resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen-activating human cytochrome P450 1B1. Can J Physiol Pharmacol. 2000;78:874–881. [PubMed] [Google Scholar]
- [52].Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- [54].Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- [56].Bradamante S, Piccinini F, Barenghi L, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Does resveratrol induce pharmacological preconditioning? Int J Tissue React. 2000;22:1–4. [PubMed] [Google Scholar]
- [57].Orallo F, Alvarez E, Camina M, Leiro JM, Gomez E, Fernandez P. The possible implication of trans-Resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol. 2002;61:294–302. doi: 10.1124/mol.61.2.294. [DOI] [PubMed] [Google Scholar]
- [58].Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- [59].Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465:115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- [60].Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2002;282:H1996–2003. doi: 10.1152/ajpheart.01013.2001. [DOI] [PubMed] [Google Scholar]
- [61].Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [62].Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- [63].Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- [64].Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- [68].Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- [69].Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- [70].Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- [73].Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- [74].Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, Bertelli A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- [77].Shen MY, Hsiao G, Liu CL, Fong TH, Lin KH, Chou DS, Sheu JR. Inhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol. 2007;139:475–485. doi: 10.1111/j.1365-2141.2007.06788.x. [DOI] [PubMed] [Google Scholar]
- [78].Kirk RI, Deitch JA, Wu JM, Lerea KM. Resveratrol decreases early signaling events in washed platelets but has little effect on platelet in whole blood. Blood Cells Mol Dis. 2000;26:144–150. doi: 10.1006/bcmd.2000.0289. [DOI] [PubMed] [Google Scholar]
- [79].Olas B, Wachowicz B, Holmsen H, Fukami MH. Resveratrol inhibits polyphosphoinositide metabolism in activated platelets. Biochim Biophys Acta. 2005;1714:125–133. doi: 10.1016/j.bbamem.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [80].Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- [81].Williams A, Hennekens CH. The role of aspirin in cardiovascular diseases--forgotten benefits? Expert Opin Pharmacother. 2004;5:109–115. doi: 10.1517/14656566.5.1.109. [DOI] [PubMed] [Google Scholar]
- [82].Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol. 2006;48:1–5. doi: 10.1097/01.fjc.0000238592.67191.ab. [DOI] [PubMed] [Google Scholar]
- [83].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- [87].Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.151654. [DOI] [PubMed] [Google Scholar]
- [90].Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- [92].Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- [93].Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kuningas M, Putters M, Westendorp RG, Slagboom PE, van Heemst D. SIRT1 gene, age-related diseases, and mortality: the Leiden 85-plus study. J Gerontol A Biol Sci Med Sci. 2007;62:960–965. doi: 10.1093/gerona/62.9.960. [DOI] [PubMed] [Google Scholar]
- [96].Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lemieux ME, Yang X, Jardine K, He X, Jacobsen KX, Staines WA, Harper ME, McBurney MW. The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech Ageing Dev. 2005;126:1097–1105. doi: 10.1016/j.mad.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [98].Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- [99].Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Baur JA, Sinclair DA. 2006. Unpublished results.
- [101].Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- [102].Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SN, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging. 2009;1:515–525. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- [106].Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- [107].Bifulco M, Santoro A, Laezza C, Malfitano AM. Cannabinoid receptor CB1 antagonists state of the art and challenges. Vitam Horm. 2009;81:159–189. doi: 10.1016/S0083-6729(09)81007-8. [DOI] [PubMed] [Google Scholar]
- [108].Savouret JF, Berdeaux A, Casper RF. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2003;13:104–113. doi: 10.1016/s0939-4753(03)80026-1. [DOI] [PubMed] [Google Scholar]
- [109].Hebbar V, Shen G, Hu R, Kim BR, Chen C, Korytko PJ, Crowell JA, Levine BS, Kong AN. Toxicogenomics of resveratrol in rat liver. Life Sci. 2005;76:2299–2314. doi: 10.1016/j.lfs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- [110].Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- [112].Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sauve AA, Moir RD, Schramm VL, Willis IM. Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell. 2005;17:595–601. doi: 10.1016/j.molcel.2004.12.032. [DOI] [PubMed] [Google Scholar]
- [114].Li Y, Backesjo CM, Haldosen LA, Lindgren U. Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur J Pharmacol. 2009;609:13–18. doi: 10.1016/j.ejphar.2009.03.004. [DOI] [PubMed] [Google Scholar]
- [115].Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- [116].Yang J, Kong X, Martins-Santos ME, Aleman G, Chaco E, Liu GE, Wu SY, Samols D, Hakimi P, Chiang CM, Hanson RW. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4alpha. J Biol Chem. 2009;284:27042–27053. doi: 10.1074/jbc.M109.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Toth B. Lack of carcinogenicity of nicotinamide and isonicotinamide following lifelong administration to mice. Oncology. 1983;40:72–75. doi: 10.1159/000225695. [DOI] [PubMed] [Google Scholar]
- [118].Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Schmidt MT, Smith BC, Jackson MD, Denu JM. Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem. 2004;279:40122–40129. doi: 10.1074/jbc.M407484200. [DOI] [PubMed] [Google Scholar]
- [120].Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- [122].Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cellspecific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–49. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- [127].Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- [128].Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324:883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- [129].Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- [130].Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term wine consumption is related tocardiovascular mortality and life expectancyindependently of moderate alcohol intake: theZutphen Study. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2008.082198. [DOI] [PubMed] [Google Scholar]
- [131].Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- [132].Soleas GJ, Yan J, Goldberg DM. Measurement of trans-resveratrol, (+)-catechin, and quercetin in rat and human blood and urine by gas chromatography with mass selective detection. Methods Enzymol. 2001;335:130–145. doi: 10.1016/s0076-6879(01)35238-2. [DOI] [PubMed] [Google Scholar]
- [133].Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila Pa) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- [134].Vaz-da-Silva M, Loureiro AI, Falcao A, Nunes T, Rocha JF, Fernandes-Lopes C, Soares E, Wright L, Almeida L, Soares-da-Silva P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–570. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- [135].Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- [136].Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- [137].Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- [138].Pearson KJ, de Cabo R. 2009.
- [139].Blander G, Olejnik J, Krzymanska-Olejnik E, McDonagh T, Haigis M, Yaffe MB, Guarente L. SIRT1 shows no substrate specificity in vitro. J Biol Chem. 2005;280:9780–9785. doi: 10.1074/jbc.M414080200. [DOI] [PubMed] [Google Scholar]
- [140].Garske AL, Denu JM. SIRT1 top 40 hits: use of one-bead, one-compound acetyl-peptide libraries and quantum dots to probe deacetylase specificity. Biochemistry. 2006;45:94–101. doi: 10.1021/bi052015l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- [143].Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- [144].Ghosh HS, Spencer JV, Ng B, McBurney MW, Robbins PD. Sirt1 interacts with transducin-like enhancer of split-1 to inhibit nuclear factor kappaB-mediated transcription. Biochem J. 2007;408:105–111. doi: 10.1042/BJ20070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]