Abstract
SIR2 (silent information regulator 2) proteins, now called “sirtuins,” are an evolutionarily conserved family of NAD-dependent protein deacetylases/ADP-ribosyltransferases. Sirtuins have recently attracted major attention in the field of aging research, and it has been demonstrated that SIR2 and its orthologs regulate aging and longevity in yeast, worms, and flies. In mammals, the SIR2 ortholog SIRT1 coordinates important metabolic responses to nutritional availability in multiple tissues. Most recently, it has been demonstrated that SIRT1 regulates the amplitude and the duration of circadian gene expression through the interaction and the deacetylation of key circadian clock regulators, such as BMAL1 and PER2. More strikingly, we and others have discovered a novel circadian clock feedback loop in which both the rate-limiting enzyme in mammalian NAD biosynthesis, nicotinamide phosphoribosyltransferase (NAMPT), and NAD levels display circadian oscillations and modulate CLOCK:BMAL1-mediated circadian transcriptional regulation through SIRT1, demonstrating a new function of NAD as a “metabolic oscillator.” These findings reveal a novel system dynamics of a recently proposed systemic regulatory network regulated by NAMPT-mediated NAD biosynthesis and SIRT1, namely, the NAD World. In the light of this concept, a new connection between physiological rhythmicity, metabolism, and aging will be discussed.
Introduction
In the history of aging research, there has been an idea that the life-long sequence of age-associated events might be controlled by a sort of “clock.” This idea of “the aging clock” was first proposed by Arthur Everitt in Australia in 1973 [1]. In his article, he speculated that such a clock or even multiple clocks might be located in the hypothalamus. He also tried to integrate this idea into the hypothalamic/neuroendocrine theory of aging proposed by Vladimir Dilman in Russia in 1971 [2]. Dilman recognized aging as “a process of disordered homeostatic stability of internal environment” and assumed that “permanent deviations from the law of constancy of the internal environment” could ultimately produce “the specific lesions of ageing” [2]. Both Dilman and Everitt suspected that some feedback mechanism that involves the hypothalamus might be running “the genetic programme of development and ageing” or ticking “the aging clock” [1, 2].
Intriguingly, almost at the same time when those ideas were proposed, studies began to reveal that a hypothalamic region called the suprachiasmatic nucleus (SCN) actually has such a “clock” function [3]. For example, it was reported in 1972 that bilateral lesions in the SCN eliminated circadian rhythms in rats [4, 5]. Since then, remarkable progress has been made in the biology of circadian rhythm, and major regulators in the core clock mechanism have been identified [6-8]. These findings have clearly shown that major clock regulators function not only in the SCN but also in many different peripheral tissues, such as the liver and white adipose tissue (WAT), suggesting that circadian rhythmicity of physiological events is regulated by a network of multiple oscillators throughout the body [8]. It has also been reported that both amplitude and phase of circadian rhythms are affected in the process of aging [9-11]. Although these age-associated changes in circadian rhythms are rather complex, aging might affect interactions among circadian oscillators and compromise “sustainability” of rhythmic tissues [11], which could cause the “permanent deviations” from stable rhythmicity that Dilman speculated in his idea [2].
Despite all these studies, there had been neither supportive evidence for the idea of “the aging clock” nor direct molecular connection between clocks and aging until recently. Most recently, however, there have been exciting developments in the juncture of the fields of aging and circadian rhythm researches which bring up important insights into a fascinating connection between physiological rhythmicity, metabolism, and aging. Therefore, in this review, I will first summarize these new findings and then revisit the idea of “the aging clock” in the light of a new concept of the NAD World, a systemic regulatory network for the regulation of metabolism and aging [12, 13].
SIRT1 and circadian clock regulators
For the past couple of decades, important regulators and signaling pathways have been identified in model organisms that, when modified, may slow down aging [14-17]. One such regulator has recently attracted major attention in the field of aging research. It is the SIR2 (silent information regulator 2) protein family, now called “sirtuins” [15, 18-21]. Sirtuins are an evolutionarily conserved family of NAD-dependent protein deacetylases/ADP-ribosyltransferases. In yeast, worms, and flies, SIR2 and its orthologs regulate aging and longevity [22-27] and in certain genetic backgrounds, also mediate the lifespan-extending effect of caloric restriction [25, 28-31], the most consistent dietary regimen that retards aging and extends longevity in many diverse species. There are seven mammalian sirtuins, SIRT1 through SIRT7, and the majority of mammalian sirtuin research has so far focused on the function of the SIR2 ortholog SIRT1 [15, 19, 21]. Although it has not yet been proven whether SIRT1 regulates aging and longevity in mammals, it has been firmly established that SIRT1 plays an important role in the regulation of metabolism in response to nutritional availability in multiple tissues. Details of reported SIRT1 functions have already been summarized and discussed in many review articles [19, 21, 32-34]. The most important theme through all of these SIRT1 studies is that SIRT1 coordinates physiological programs that allow animals to survive through nutritionally scarce conditions by mediating critical metabolic responses to nutritional cues, particularly in low nutritional conditions such as fasting and caloric restriction, [13, 32, 35]. This unique aspect of SIRT1 function and the absolute requirement of NAD for its activity place this protein at a central position as a key regulator that connects metabolism and aging.
In 2008, two groups further stretched the physiological importance of SIRT1 towards another fascinating direction. They reported that SIRT1 regulates the circadian clock oscillatory mechanisms and affects the expression of circadian clock genes (Fig. 1) [36, 37]. Nakahata et al. [37] found that SIRT1 shows the oscillation of its enzymatic activity and deacetylates BMAL1, one of the critical regulators in the core clock mechanism, and histone H3 in a circadian manner. SIRT1 physically interacts with CLOCK, another key clock regulator that heterodimerizes with and acetylates BMAL1, and is recruited to the CLOCK:BMAL1 complex at the promoters of circadian clock genes (Fig. 1). They also found that a SIRT1 deficiency causes changes in the expression of circadian clock genes, such as Period2 (Per2), in mouse embryonic fibroblasts (MEFs) in vitro and in the liver in vivo, including an augmentation of the expression levels and a broadening of the oscillatory cycles. On the other hand, Asher et al. [36] reported that SIRT1 protein levels oscillate in MEFs and in the liver and that SIRT1 activity is required for the robust oscillatory expression patterns of circadian clock genes. They likewise found that SIRT1 interacts with the CLOCK:BMAL1 complex in a circadian manner. They also showed that SIRT1 interacts with and deacetylates PER2, resulting in its degradation (Fig. 1). Although both groups indicate the importance of SIRT1 in the regulation of circadian clock gene expression, there are some discrepancies between results from these groups. One example is whether SIRT1 protein levels or activity levels oscillate. Another example is whether SIRT1 negatively or positively regulates the amplitude of the circadian gene expression. Even though there are no reconcilable explanations for these discrepancies at this moment, it is now clear that SIRT1 regulates the amplitude and the duration of circadian gene expression through the deacetylation of key circadian clock regulators, such as BMAL1 and PER2.
Fig. 1.
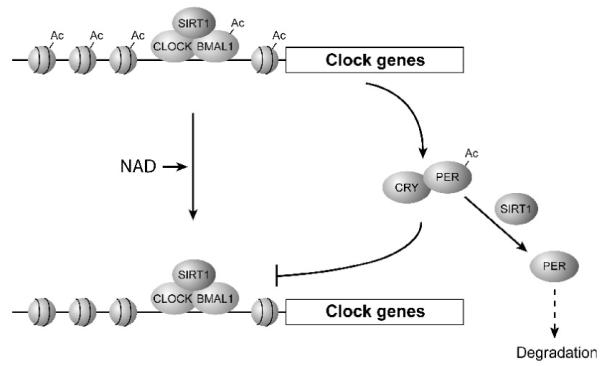
A schematic diagram of the SIRT1-mediated regulation of the CLOCK:BMAL1-dependent circadian transcription. SIRT1 physically interacts with CLOCK, a key clock regulator that heterodimerizes with BMAL1, and deacetylates BMAL1 and histone H3 in a circadian manner at the promoters of circadian clock genes. SIRT1 also interacts with and deacetylates PER2, which heterodimerizes with CRY proteins and inhibits the CLOCK:BMAL1 function, and promotes its degradation. See text for details.
These studies have demonstrated the first connection between key regulators for aging and circadian rhythm. Intriguingly, Bmal1-deficient mice and Per1/2-deficient mice develop some pathological features at earlier ages that resemble those in aged mice, providing indirect support for the connection between aging and circadian rhythm [38, 39]. Furthermore, the core molecular clock machinery has also been demonstrated to be one of the most powerful modifiers of metabolism [40, 41]. For example, homozygous Clock mutant mice exhibit metabolic dysregulation, including hyperlipidemia, hyperglycemia, hyperleptinemia, hypoinsulinemia, and hepatic steatosis [42]. Liver-specific deletion of Bmal1 causes loss of rhythmic expression of clock-regulated metabolic genes in the liver and hypoglycemia in the fasting phase of the daily feeding cycle [43]. Hepatic Bmal1 expression is also regulated by PGC-1α, another SIRT1 target transcription factor that regulates glucose production in the liver, and liver-specific PGC-1α knockdown in mice proves that this factor is required for hepatic clock function [44]. These findings, as well as new studies described above, have implicated a critical connection between physiological rhythmicity, metabolism, and aging, and SIRT1 might function at a central interface connecting these fundamental biological events. However, how exactly SIRT1 functions at such a fundamental junction was a big question back then, and indeed, this particular question awaited a much more surprising answer.
NAD as a metabolic oscillator in a novel circadian clock feedback cycle
The key to solve this question was the fact that sirtuins require NAD for its enzymatic activity [45-47]. NAD is an essential coenyzme that is synthesized from three major precursors - tryptophan, nicotinic acid, and nicotinamide [48, 49]. The predominant NAD biosynthetic pathway in mammals involves the conversion of nicotinamide (a form of vitamin B3) and 5′-phosphoribosyl-1-pyrophosphate (5′-PRPP) to nicotinamide mononucleotide (NMN) by the rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT) (Fig. 2). Whereas the enzymatic activity of NAMPT was originally reported in 1957 [50], it was in 2001 that the gene encoding NAMPT was first identified in Haemophilus ducreyi [51]. Since then, the enzymological features and the crystal structures of NAMPT have been studied extensively [52-56]. Interestingly, NAMPT has intra- and extracellular forms (iNAMPT and eNAMPT, respectively), and eNAMPT (a.k.a. PBEF/visfatin), which is positively secreted through a non-classical secretory pathway by fully differentiated mouse and human adipocytes, also exhibits robust NAD biosynthetic activity compared to iNAMPT, likely contributing to the extracellular biosynthesis of the key NAD intermediate NMN in mammals [57, 58]. Indeed, NMN and other NAD intermediates, such as nicotinamide riboside (NR), can be detected in both mouse and human plasma [58, unpublished observation]. NAMPT has recently been shown to play important roles in a variety of biological events and has drawn much attention in several different fields, including NAD biology, metabolism, and immune response [57, 59]. Most importantly, accumulating lines of evidence have demonstrated that NAMPT-mediated NAD biosynthesis plays a critical role in the regulation of SIRT1 activity in many different cell types [54, 60-67]. Increased dosage of iNAMPT enhances total cellular NAD levels and thereby SIRT1 activity in mouse fibroblasts [54]. In human vascular smooth muscle cells, iNAMPT promotes their maturation [66] and cellular life span [61, 68] through enhanced SIRT1 activity. In cardiac myocytes, increased iNAMPT levels protect them from cell death through SIRT1 [69]. In pancreatic β cells, both SIRT1 and NAMPT-mediated NAD biosynthesis play important roles in the regulation of glucose-stimulated insulin secretion [58, 70, 71]. In skeletal myoblasts, glucose restriction inhibits their differentiation through the AMP-activated protein kinase (AMPK)-dependent induction of Nampt expression and the resultant activation of SIRT1 [60]. The NAMPT/SIRT1 pathway also mediates granulocyte colony-stimulating factor (G-CSF)-triggered granulocyte differentiation in vitro and in vivo [65]. iNAMPT also plays an important role in NAD biosynthesis and sirtuin activation in mitochondria [72]. These findings led us to hypothesize that NAMPT-mediated NAD biosynthesis may coordinate physiological rhythmicity, metabolism, and aging through the regulation of SIRT1 in metabolic tissues.
Fig. 2.
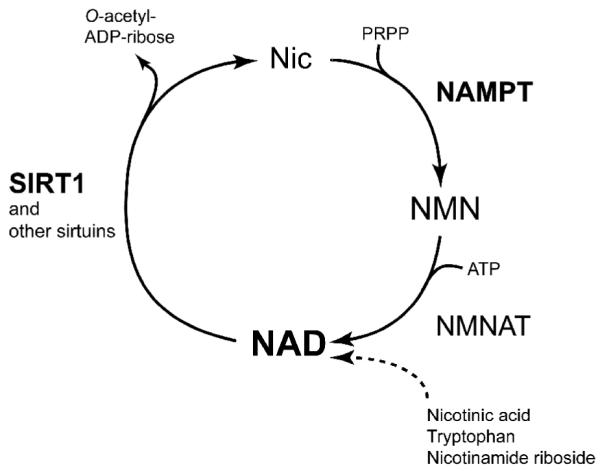
NAD biosynthesis from nicotinamide in mammals. Nicotinamide (Nic) is the main precursor for mammalian NAD biosynthesis, and nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step in this pathway, producing nicotinamide mononucleotide (NMN) from nicotinamide and 5′-phosphoribosyl-1-pyrophosphate (PRPP). Then, nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) completes the conversion of NMN and ATP to NAD. Other pathways from nicotinic acid, tryptophan, and nicotinamide riboside are not shown. SIRT1 and other sirtuins use NAD for their functions and produce nicotinamide and O-acetyl-ADP-ribose. Other NAD-consuming enzymes are not shown in this figure.
Pursuing this hypothesis with Joseph Bass’s group in Northwestern University, we have made a striking discovery that levels of NAMPT and NAD display circadian oscillations that are regulated by the core clock machinery in mice (Fig. 3) [64]. Almost at the same time, Paolo Sassone-Corsi’s group reached the same conclusion using MEFs [63]. The first important clue came from the observation that expression levels of Nampt RNA display a robust diurnal oscillation in the liver and WAT, with a peak around the beginning of the dark period (zeitgeber time (ZT) 14) [64]. A similar oscillatory pattern of Nampt expression was observed in serum-entrained wild-type MEFs [63]. Interestingly, expression levels of NAMPT protein also show a diurnal oscillation in the liver. This oscillation of NAMPT protein is bimodal, with a reduction in NAMPT protein levels prior to the onset of the dark period, which is consistent with the time when mice usually start eating. This would imply some post-translational regulation on NAMPT protein in response to a nutritional surge or ingestive behavior. The oscillation of Nampt RNA is still robust in the liver even when mice are maintained in constant darkness, demonstrating that Nampt RNA oscillation is circadian in nature. These robust diurnal and circadian oscillation patterns of Nampt RNA and protein are completely abolished in tissues from the circadian rhythm-deficient ClockΔ19 mutant mice, indicating that the core clock machinery is required for the circadian control of Nampt expression. Most importantly, NAD levels show a very similar bimodal circadian oscillation pattern to that of NAMPT in the liver, and NAD levels are significantly reduced in ClockD19 mutant liver during both the light (ZT2) and dark (ZT14) periods. This NAD oscillation is recapitulated in serum-entrained MEFs, although there is no bimodality in their oscillatory pattern [63]. Additionally, consistent with our in vivo results, NAD levels are significantly reduced in serum-stimulated Clock mutant cells [63]. Mice deficient in BMAL1, the heterodimeric binding partner of CLOCK, also exhibit a significant reduction in both Nampt RNA and NAD levels in the liver. Therefore, these findings demonstrate that the rhythmic oscillation in RNA and protein levels of NAMPT, which is regulated by the CLOCK:BMAL1-mediated core clock mechanism, leads to a circadian oscillation of NAD levels in vivo (Fig. 3).
Fig. 3.
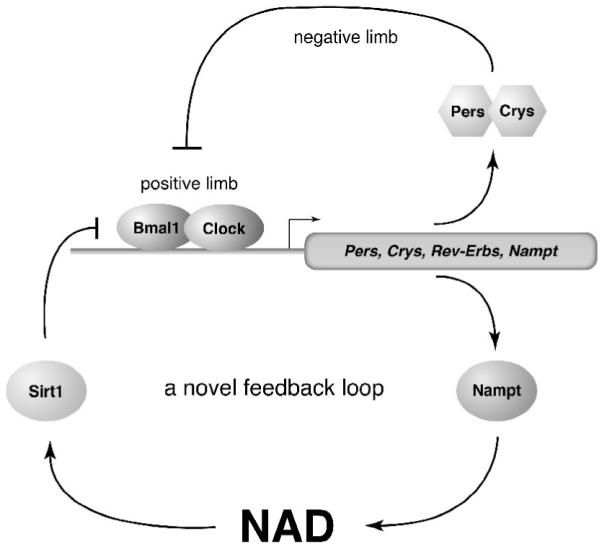
A novel NAMPT/NAD-driven feedback loop through SIRT1 and CLOCK:BMAL1. Whereas CLOCK:BMAL1-mediated circadian transcription comprises a positive limb of the transcription-translation circadian feedback loop, the complex of PER and CRY proteins inhibits the function of CLOCK:BMAL1, forming the negative limb of this feedback loop. NAMPT/NAD/SIRT1 comprises a novel circadian feedback cycle that mediates rhythmic regulation of many physiological events. In this NAMPT/NAD-driven feedback loop, NAD functions as a “metabolic oscillator.” See text for details.
Given that SIRT1 regulates CLOCK:BMAL1-mediated circadian transcription [36, 37], it is conceivable that NAMPT-mediated NAD biosynthesis might also control CLOCK:BMAL1-mediated transcription through SIRT1. Indeed, inhibition of NAMPT by FK866, a potent chemical inhibitor of NAMPT [73], abrogates the SIRT1-dependent suppression of CLOCK:BMAL1-mediated transcription and promotes a more robust oscillation of clock target gene expression in primary hepatocytes [64] or clock gene oscillations with earlier onsets and higher amplitudes in serum-entrained MEFs [63], suggesting that the NAMPT/NAD-driven pathway modulates circadian transcriptional regulation in mammals. The final important piece of evidence is that the CLOCK:BMAL1 complex binds to canonical and noncanonical E-box motifs in the promoter and the first intron of the Nampt gene and up-regulates Nampt transcription [63, 64], thus completing a novel circadian clock feedback loop involving NAMPT/NAD and SIRT1/CLOCK:BMAL1 (Fig. 3).
In the NAMPT/NAD-driven feedback loop, NAD functions as a “metabolic oscillator” and regulates the core clock machinery through SIRT1 (Fig. 3) [64]. Through this tight coupling of a classical transcriptional circadian loop to a novel enzymatic feedback loop, two major biological system, metabolism and circadian clock, are interlocked [63]. Given that NAD has long been considered a stable pool of energy in cells and tissues, the circadian oscillation of NAD levels is striking, and this discovery reveals a novel system dynamics of the regulatory network comprised of NAMPT-mediated NAD biosynthesis and SIRT1. Through the regulation of SIRT1 activity, the circadian oscillatory production of NAD might convey a cascade of effects on a variety of downstream pathways, including epigenetic chromatin regulation, stress response, metabolism, and possibly aging. Therefore, in the next section, I would like to discuss a possibility that this novel circadian oscillatory mechanism might function as “the aging clock” in a recently proposed systemic regulatory network regulated by NAMPT-mediated NAD biosynthesis and SIRT1, namely, the NAD World.
The aging clock(s) in the NAD World
The NAD World is a comprehensive concept that has been proposed to explain a systemic regulatory network connecting metabolism and aging. This concept also conveys the ideas of functional hierarchy and frailty for the induction of aging in mammals [12, 13]. NAMPT-mediated NAD biosynthesis and SIRT1 are two critical components that comprise the NAD World. Conceptually, NAMPT-mediated NAD biosynthesis functions as a driver or a pacemaker that keeps up the pace of metabolism throughout the body. iNAMPT and eNAMPT together play a critical role in the systemic regulation of NAD biosynthesis through the intra- and extracellular biosynthesis of NMN, promoting total NAD biosynthesis and thereby contributing to the fine-tuning of SIRT1 activity [57]. SIRT1 functions as a key downstream mediator that controls physiological responses to alterations in NAMPT-mediated NAD biosynthesis in a tissue-dependent manner. The intimate connection between these two critical components of the NAD World has been demonstrated in a number of different cell types, such as pancreatic β cells [74], vascular smooth muscle cells [61, 66], skeletal myoblasts [60], cardiac myocytes [62, 75], and others [63-65, 67]. Thus, through this interplay between NAMPT-mediated systemic NAD biosynthesis and SIRT1, the NAD World orchestrates physiological responses to a variety of nutritional and environmental inputs and maintains the robustness of the physiological system in mammals (Fig. 4) [12, 13].
Fig. 4.
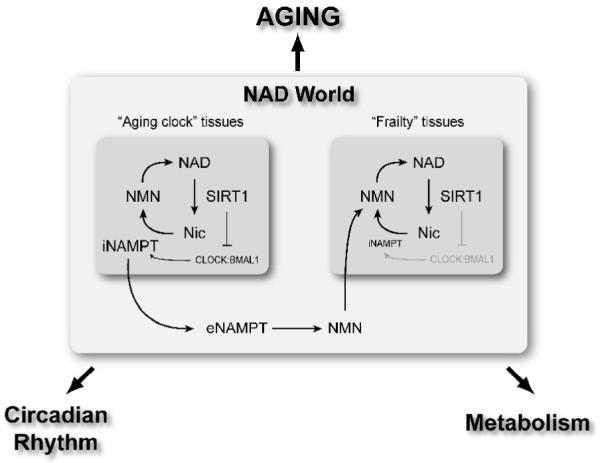
A schematic model of the interplay between tissues that provide “the aging clock” function and tissues that are frailty points in the NAD World. This interplay plays a critical role in the maintenance of systemic robustness through the entire body. The NAD World functions to orchestrate physiological responses to nutritional and environmental inputs and connects physiological rhythmicity, metabolism, and aging.
As described in the previous section, we have now come to know that NAMPT/NAD drives the circadian clock feedback cycle through SIRT1 and CLOCK:BMAL1. Whereas this “clock” likely ticks in major metabolic tissues, such as liver and WAT, there are tissues/organs that do not have adequate amounts of iNAMPT and therefore rely on circulating NMN and other NAD intermediates to maintain sufficient NAD biosynthesis for their functions. Pancreatic β cells and brain (neurons), both of which have very low levels of iNAMPT [58], are likely such tissues/organs. In those tissues/organs, iNAMPT-dependent oscillatory production of NAD may not be able to influence SIRT1 activity effectively. Then, an important question is whether plasma eNAMPT and NMN levels also display circadian rhythm. Further investigation will be required to have the answer to this particular question. However, if this is the case, one could speculate that eNAMPT and/or NMN might function as a key factor that synchronizes the circadian oscillation of NAD production and thereby SIRT1 activity through the whole body. This idea also implies that NAD-dependent “clocks” in tissues/organs like pancreatic β cells and neurons might be driven or influenced by other tissues that are capable of propagating their own rhythm through the control of eNAMPT secretion (Fig. 4). In this regard, it will be of great interest to examine whether the adipose tissue has any role in the synchronization of NAD-dependent physiological rhythmicity at a systemic level by knocking out the Nampt gene specifically in adipose tissues.
Another critical prediction is that those tissues/organs that do not have adequate amounts of iNAMPT are likely important frailty points in the NAD World [12, 13]. If systemic NAD biosynthesis starts declining, these frailty points would be the first that responds to this change and starts having functional problems due to insufficient NAD biosynthesis and thereby reduced SIRT1 activity. Indeed, we have previously demonstrated that a decrease in NAMPT-mediated systemic NAD biosynthesis, which appears to be happening during the course of aging, causes significant reductions in SIRT1 activity and glucose-stimulated insulin secretion in pancreatic β cells in aged mice [74]. Strikingly, administration of NMN restores higher levels of glucose-stimulated insulin secretion in aged mice, providing support for the notion that NAMPT-mediated systemic NAD biosynthesis declines over age [74]. Therefore, pancreatic β cells are definitely an important frailty point in the NAD World that is susceptible to changes in NAMPT-mediated systemic NAD biosynthesis. In the case of brain or neurons, it has long been known that one of the triad symptoms in pellagra, the vitamin B3 deficiency, is dementia [76]. Although the mechanistic connection between vitamin B3 deficiency and dementia is unknown, it is conceivable that the age-associated decline in systemic NAD biosynthesis causes functional deficits in neurons and results in neurological problems, including dementia. To address this possibility, the connection between NAMPT-mediated NAD biosynthesis and neural function is currently under investigation.
Once pancreatic β cells and brain start having functional problems due to insufficient NAD biosynthesis, other peripheral tissues/organs would also be affected through insulin secretion and central metabolic regulation, resulting in the gradual deterioration of the physiological robustness through the entire body. Therefore, in the concept of the NAD World, aging is considered as the process in which organismal robustness gradually breaks down according to a functional hierarchy determined by the susceptibility to systemic NAD biosynthesis. Additionally, tissues that control the NAD-dependent branch of circadian clock feedback regulations throughout the body could be considered as “the aging clock”, and any imbalance in this fine-tuning system, which could be imposed by long-term nutritional and environmental perturbations, might initiate the process of organismal robustness breakdown (Fig. 4). Although further investigation will be necessary, tweaking “the aging clock”, possibly by supplementing NAD intermediates to systemic NAD biosynthesis, might be an effective way to make all of our clocks tick robustly in this otherwise inevitable process of aging.
Conclusion
This review article focuses on a novel aspect of the connection between physiological rhythmicity, metabolism, and possibly aging. Particularly, the recent finding that NAD functions as a “metabolic oscillator” opens new possibilities to further dissect the complex interlocked feedback system controlling circadian rhythm and metabolism and to better understand the pathophysiology of metabolic complications caused by dysregulation or imbalance in this feedback system. Such dysregulation/imbalance might also be caused in the process of aging, and the concept of the NAD World provides some ideas and predictions on a potential role of “aging clock” tissues/organs in the systemic regulation of aging. Of course, further investigation will be necessary to address this novel concept. For example, evidence connecting altered sirtuin function and NAD levels to aging and longevity is still not strong in mammals. The primacy of NAD for the regulation of brain and β cell functions also needs to be studied extensively. Nonetheless, in the next decade, this new NAD World will be further explored, and we might eventually find a way to manipulate the process of aging based on our knowledge on the detailed system structure and dynamics of this complex regulatory network.
Acknowledgments
I apologize to those whose work is not cited due to the focus of this review and space limitations. I thank all members of the Imai lab for their helpful discussions and comments. This work was supported by grants from the National Institute on Aging (AG024150), Ellison Medical Foundation, and Longer Life Foundation to S. I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Everitt AV. The hypothalamic-pituitary control of ageing and age-related pathology. Exp. Gerontol. 1973;8:265–277. doi: 10.1016/0531-5565(73)90039-9. [DOI] [PubMed] [Google Scholar]
- [2].Dilman VM. Age-associated elevation of hypothalamic threshold to feedback control, and its role in development, ageing, and disease. Lancet. 1971;1:1211–1219. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- [3].van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res. Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- [4].Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- [5].Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- [8].Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- [9].Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res. Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [10].Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res. Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [11].Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Imai S. From heterochromatin islands to the NAD World: A hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbagen.2009.03.005. Epub on Mar 13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Imai S. The NAD World: a new systemic regulatory network for metabolism and aging - Sirt1, systemic NAD biosynthesis, and their importance. Cell. Biochem. Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- [15].Imai S, Guarente L. Sirtuins: A universal link between NAD, metabolism, and aging. In: Guarente L, Partridge L, Wallace D, editors. The Molecular Biology of Aging. Cold Spring Habor Laboratory Press; New York: 2007. pp. 39–72. [Google Scholar]
- [16].Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim. Biophys. Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- [18].Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- [19].Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann. Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- [20].Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [22].Astrom SU, Cline TW, Rine J. The Drosophila melanogaster sir2+ gene Is nonessential and has only minor effects on position-effect variegation. Genetics. 2003;163:931–937. doi: 10.1093/genetics/163.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- [24].Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- [27].Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- [28].Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin S-J, Defossez P-A, Guarente L. Life span extension by calorie restriction in S. cerevisiae requires NAD and SIR2. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- [30].Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- [31].Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [32].Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell. Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- [33].Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front. Biosci. 2009;14:2983–2995. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr. Opin. Chem. Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- [35].Imai S. SIRT1 and caloric restriction: an insight into possible trade-offs between robustness and frailty. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:350–356. doi: 10.1097/MCO.0b013e32832c932d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- [37].Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee CC. The circadian clock and tumor suppression by mammalian period genes. Methods Enzymol. 2005;393:852–861. doi: 10.1016/S0076-6879(05)93045-0. [DOI] [PubMed] [Google Scholar]
- [40].Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu. Rev. Nutr. 2007;27:219–240. doi: 10.1146/annurev.nutr.27.061406.093546. [DOI] [PubMed] [Google Scholar]
- [42].Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- [45].Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- [46].Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- [50].Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J. Biol. Chem. 1957;225:759–770. [PubMed] [Google Scholar]
- [51].Martin P, Shea R, Mulks M. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J. Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- [53].Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, Lee YS, Kim JB, Kim KK, Suh SW, Eom SH. Crystal Structure of Visfatin/Pre-B Cell Colony-enhancing Factor 1/Nicotinamide Phosphoribosyltransferase, Free and in Complex with the Anti-cancer Agent FK-866. J. Mol. Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- [54].Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- [55].Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [56].Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD(+) biosynthetic enzyme. Nat. Struct. Mol. Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- [57].Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 2009;15:20–28. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ho C, van der Veer E, Akiwa O, Pickering JG. SIRT1 markedly extends replicative lifespan if NAD(+) salvage is enhanced. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.08.031. Epub on Aug 28. [DOI] [PubMed] [Google Scholar]
- [62].Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ. Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat. Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- [66].van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ. Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- [67].Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J. Biol. Chem. 2009;284:20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- [69].Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J. Biol. Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- [70].Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [72].Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD(+) levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- [74].Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ. Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- [76].Williams AC, Ramsden DB. Pellagra: A clue as to why energy failure causes diseases? Med. Hypotheses. 2007;69:618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]


