Abstract
Aim
Liver fibrosis develops when chronic liver injury stimulates cells in the liver to produce mediators that activate hepatic stellate cells and stimulate them to secrete collagen. Recent studies suggest that the hypoxia-regulated transcription factor, hypoxia-inducible factor-1α, is essential for upregulation of profibrotic mediators, such as platelet-derived growth factor, in the liver during the development of liver fibrosis. What remains unknown, however, is the cell type-specific regulation of profibrotic mediators by hypoxia-inducible factors. Accordingly, in the present study the hypothesis was tested that hypoxia-inducible factors regulate production of profibrotic mediators by hypoxic Kupffer cells.
Methods
Kupffer cells were isolated from Control mice and hypoxia-inducible factor-1β-Deficient mice and exposed to room air or 1% oxygen (i.e., hypoxia). Levels of profibrotic mediators were quantified by real-time PCR.
Results
Exposure of Kupffer cells isolated from Control mice to 1% oxygen activated hypoxia-inducible factor-1α, and increased mRNA levels of platelet-derived growth factor-B, vascular endothelial growth factor, Angiopoietin-1, and monocyte chemotactic protein-1. Upregulation of all of these mediators by hypoxia was prevented in Kupffer cells isolated from hypoxia-inducible factor-1β-Deficient mice.
Conclusion
Results from these studies suggest that hypoxia-inducible factors are critical regulators of profibrotic mediator production by hypoxic Kupffer cells.
Keywords: Angiogenesis, Fibrosis, Hypoxia, Hypoxia-inducible Factors, Kupffer cells, Liver
INTRODUCTION
Liver fibrosis is characterized by excessive deposition of extracellular matrix in the liver during chronic injury. This disease has many causes including those of known etiology, such as chronic alcohol consumption and hepatitis virus, and those of unknown etiology, such as primary biliary cirrhosis, sclerosing cholangitis, and others. Liver fibrosis is initiated when liver injury stimulates cells to produce soluble mediators (i.e., profibrotic mediators), such as platelet-derived growth factors and transforming growth factor-β that cause cells in the liver, such as hepatic stellate cells, portal fibroblasts, hepatocytes, and bile duct epithelial cells to differentiate into myofibroblasts 1. Mediators are also released that stimulate chemotaxis of myofibroblasts to regions of injury and stimulate them to produce collagen, the primary component of fibrosis.
Studies suggest that hepatic macrophages may be an important source of profibrotic mediators during the development of fibrosis. Immunohistochemical studies and in situ hybridization techniques have shown that macrophages express transforming growth factor-β and platelet-derived growth factors (PDGFs) in the liver during the genesis of fibrosis 2, 3. In support of a role for these cells in the development of fibrosis, recent studies have demonstrated that depletion of macrophages reduces liver fibrosis in mice treated chronically with carbon tetrachloride 4. These studies demonstrate that macrophages are important for the development of fibrosis and may promote fibrosis by releasing profibrotic mediators, such as PDGFs and TGF-β. The mechanism by which hepatic macrophages are stimulated to release these mediators during the development of fibrosis, however, is not known. One possibility is hepatocellular hypoxia.
Studies have demonstrated that regions of hepatocellular hypoxia develop in animal models of liver fibrosis 5, 6. Hypoxia may be an important stimulator of profibrotic mediator production by macrophages and other cells types by activating a group of transcription factors called hypoxia-inducible factors (HIFs). HIFs are composed of an alpha subunit, typically HIF-1α or HIF-2α, and a beta subunit, HIF-1β 7, 8. In normoxic cells, HIFα subunits are constitutively produced and immediately targeted for proteolytic degradation. When cells become hypoxic, however, the mechanisms that target HIFα subunits for degradation are inhibited allowing HIFα protein levels to increase 9. HIFα subunits then translocate to the nucleus where they heterodimerize with HIF-1β and regulate expression of genes involved in glycolysis, angiogenesis, iron metabolism, pH control, and others functions 8. Interestingly, many of the genes that HIFs regulate, including various growth factors, have been implicated in the pathogenesis of liver fibrosis. Furthermore, recent studies have demonstrated that mice deficient in HIF-1α develop less liver fibrosis after bile duct ligation 10. In this study, PDGFs where upregulated to a greater extent in control mice subjected to bile duct ligation when compared to bile duct-ligated HIF-1α-deficient mice, suggesting that HIF-1α is an important regulator of profibrotic mediator production in the liver during the development of fibrosis 10. The cell type specific function of HIFs in regulating production of profibrotic mediators by liver cell types, however, is not known. Since hepatic macrophages are important for the development of fibrosis and express profibrotic mediators, such as PDGFs, the hypothesis was tested that hypoxia stimulates Kupffer cells to produce profibrotic mediators in a HIF-dependent manner.
METHODS
Mice
Deletion of HIF-1β in mice causes embryonic lethality 11. To selectively reduce HIF-1β levels in adult mice, HIF-1βfl/fl mice, described in detail previously 12, were crossed with mice expressing Cre recombinase under control of the Mx interferon-inducible promoter (Mx-Cre+/− mice; Jackson Laboratories, Bar Harbor, ME) 13. Offspring of the HIF-1βfl/fl mouse breeding were HIF-1βfl/fl-Mx-Cre+ (i.e., HIF-1β-deletable) or HIF-1βfl/fl-Mx-Cre- (i.e., HIF-1β-nondeletable littermate controls). PCR of genomic DNA was used to detect the floxed HIF-1β gene and the Cre transgene as described previously 12. To activate the MxCre promoter HIF-1βfl/fl-Mx-Cre+, and HIF-1βfl/fl-Mx-Cre- mice were treated with 500 µg of polyinosinic–polycytidylic acid (pIpC, Sigma Chemical Company, St. Louis, MO) dissolved in sterile saline by intraperitoneal injection every three days for a total of three injections. In mice containing the MxCre transgene, this treatment causes a near complete deletion of loxP-containing genes in liver 12, 13. Kupffer cells were isolated from the livers of these mice one week after the final pIpC injection.
All mice were maintained on a 12-h light/dark cycle under controlled temperature (18–21°C) and humidity. Food (Rodent Chow; Harlan-Teklad, Madison, WI) and tap water were allowed ad libitum. All procedures on animals were carried out in accordance with the Guide for the Care and Use of Laboratory Animals promulgated by the National Institutes of Health.
Kupffer cell isolation
Kupffer cells were isolated from the livers of mice as described in detail previously 14. Briefly, the livers were digested by perfusion with Hank’s Balanced Salt Solution (HBSS; Sigma Chemical Company, St. Louis, MO) containing Pronase (Roche Diagnostics, Indianapolis, IN) followed by perfusion with HBSS containing Collagenase B (Sigma Chemical Company). Hepatocytes were removed from the digested liver by centrifugation at 50 g for 2 minutes. Kupffer cells were isolated from the remaining nonparenchymal cell fraction by gradient centrifugation with Percoll (Sigma Chemical Company). The nonparenchymal cells were isolated from the supernatant by centrifugation at 250 g for 10 minutes. The resulting pellet was resuspended in 10.5 ml of 1× phosphate-buffered saline (PBS) containing Percoll at a final concentration of 52%. The cells were overlaid with 20 ml of 50% Percoll in 1XPBS. This layer was overlaid with 20 ml of 35% Percoll in 1XPBS followed by a layer containing 5 ml of 1XPBS. The gradient was centrifuged at 900 g for 30 minutes at 4 degrees C. After centrifugation, Kupffer cells, contained in the 50% Percoll fraction were removed, diluted with an equal volume of 1XPBS, and centrifuged at 900 g for 10 minutes at 4 degrees C. The Kupffer cell pellet was resuspended in RPMI-1640 (Sigma Chemical Company) containing 10% FBS and penicillin/streptomycin. They were plated in 100 mm dishes at a density of 2.5 × 106 cells/dish. After 24 hours, the medium was removed, the cells washed twice with 1XPBS, and the medium replaced with serum-free RPMI-1640. The cells were cultured for 24 hours and then placed in an environment containing room air or 1% oxygen in a NAPCO CO2 7000 cell culture incubator (NAPCO Precision, Winchester, VA) for an additional 16 hours. These environments also contained 5% CO2 and were balanced with nitrogen.
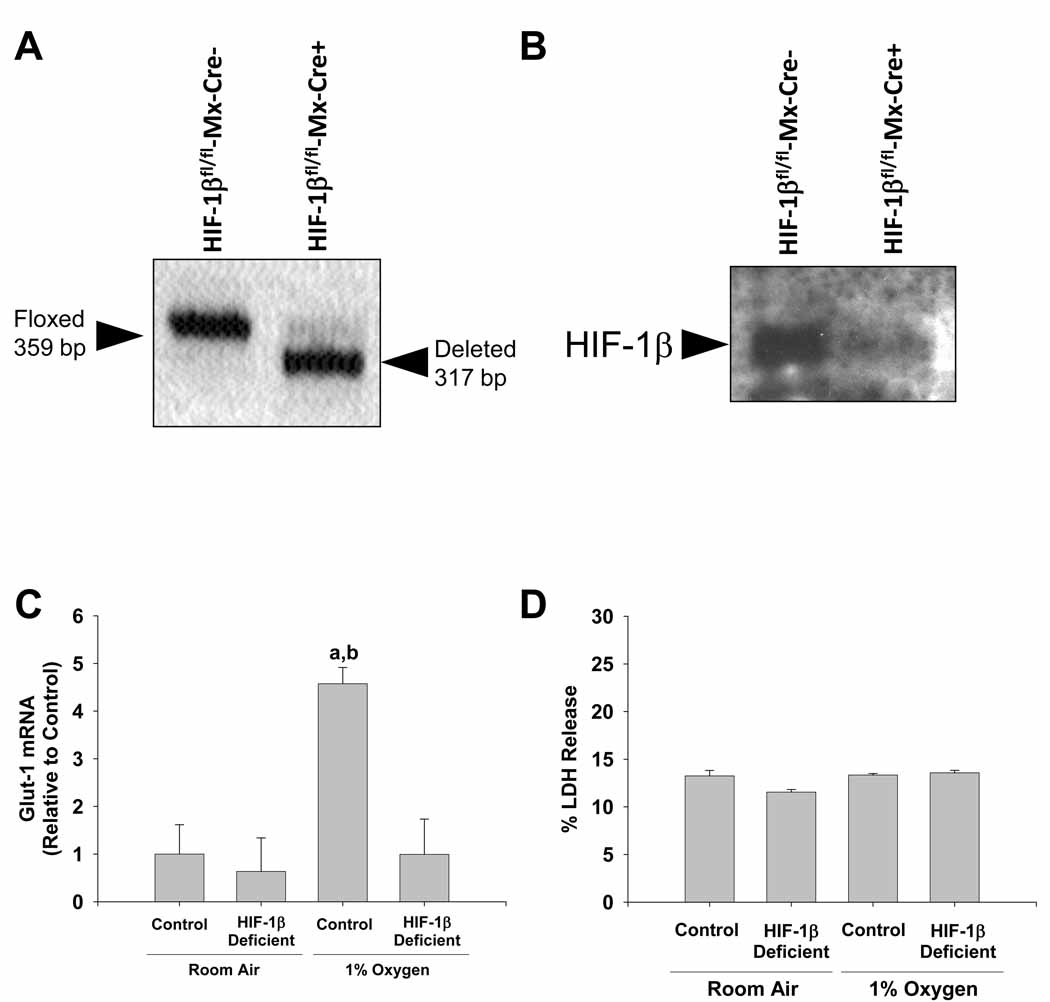
The Kupffer cell cultures were only used if greater than 98% pure as determined by F4/80 immunostaining. Representative F4/80 immunostaining of Kupffer cell cultures is shown in Figure 2.
Figure 2.
Deletion of HIF-1β in Kupffer cells. Kupffer cells isolated from HIF-1βfl/fl-Mx-Cre+ mice and HIF-1βfl/fl-Mx-Cre- mice treated with pIpC. (A) PCR was used to estimate the extent of deletion of Exon 6 in the HIF-1β gene. The floxed, undeleted Exon 6 of the HIF-1β gene produces a 359 bp PCR fragment, whereas the floxed, deleted Exon 6 of the HIF-1β gene produces a 317 bp PCR fragment as described previously 31. (B) Western blot was used to detect HIF-1β protein. Kupffer cells were isolated from HIF-1β-Control and HIF-1β-Deficient mice and exposed to room air or 1% oxygen. Sixteen hours later, (C) Glut-1 mRNA levels were quantified by real-time PCR and (D) % LDH release was calculated. aSignificantly different from Kupffer cells exposed to room air (p<0.05). bSignificantly different from Kupffer cells isolated from HIF-1β-Deficient mice and subjected to 1% oxygen (p<0.05).Data are expressed as means ± SEM; n = 3.
Analysis of HIF-1β deletion in Kupffer cells
Genomic DNA was isolated from Kupffer cells, and PCR was used to determine the degree of HIF-1β deletion as described previously 12.
Lactate Dehydrogenase Release
Release of lactate dehydrogenase (LDH) from Kupffer cells was quantified as a measure of cell viability. The activity of LDH in the medium and cell lysates was measure using a commercially available kit (Sigma Chemical Company) as per manufacturers’ instructions. Percent LDH release was measured by dividing the activity of LDH in the medium by the total LDH activity (i.e., activity in the medium plus the activity in cell lysates).
Real-time PCR
Total liver RNA was isolated using TRI reagent (Sigma Chemical Company), and reverse transcribed into cDNA as described by us previously 15. Real-time PCR was used to quantify vascular endothelial growth factor (VEGF), 18S RNA, platelet-derived growth factor-B, connective tissue growth factor (CTGF), glucose transporter-1 (Glut1), Angiopoeitin 1 (Ang1), Angiopoeitin 2 (Ang2), monocyte chemotactic protein-1 (MCP-1), and transforming growth factor-β1 (TGF-β1) and performed on an Applied Biosystems Prism 7300 Real-time PCR Instrument (Applied Biosystems, Foster City, CA) using the SYBR green DNA PCR kit (Applied Biosystems) as described by us previously. The sequences of the primers were as follows: 18S Forward: 5’-TTGACGGAAGGGCACCACCAG-3’; 18S Reverse: 5’- GCACCACCACCCACGGAATCG-3’; CTGF Forward: 5’-CTGCCAGTGGAGTTCAAATGC-3’; CTGF Reverse: 5’-TCATTGTCCCCAGGACAGTTG-3’; PDGF-B Forward: 5’- CCCACAGTGGCTTTTCATTT-3’; PDGF-B Reverse: 5’-GTGGAGGAGCAGACTGAAGG-3’; Ang1 Forward 5’-TTTGCATTCTTCGCTGCCA-3’; Ang1 Reverse 5’-GGCACATTGCCCATGTTGA-3’; Ang2 Forward 5’-ACCTTCAGAGACTGTGCGGAAA-3’; Ang2 Reverse 5’-CGTCCATGTCACAGTAGGCCTT-3’; Glut1 Forward 5’- TCGGCCTCTTTGTTAATCGCT-3’; Glut1 Reverse 5’-GGACTTGCCCAGTTTGGAGAA-3’; MCP-1 Forward 5’-ACATTCGGCGGTTGCTCTAGA-3’; MCP-1 Reverse 5’-ACATCCTGTATCCACACGGCAG-3’; VEGF Forward 5’-TGTTCAGAGCGGAGAAAGCATT-3’; VEGF Reverse 5’-TAACTCAAGCTGCCTCGCCTT-3’; TGF-β Forward 5’-TGCTAATGGTGGACCGCAA-3’; TGF-β Reverse 5’-CACTGCTTCCCGAATGTCTGA-3’.
HIF-1α immunocytochemistry
For HIF-1α immunostaining, Kupffer cells were fixed in 4% formalin in phosphate-buffered saline (PBS) for 10 min at room temperature. Cells were incubated with rabbit polyclonal anti-HIF-1α (NB100–449, Novus BIologicals, Littleton, CO) diluted 1:50 in PBS containing 3% goat serum at room temperature for 3 hours. The sections were washed with PBS, and then incubated with secondary antibody conjugated to Alexa 488 (green staining; Invitrogen). The sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) to stain DNA. Kupffer cells were additionally incubated with rat anti-F4/80 antibody (Serotec, Inc., Raleigh, NC, 1:100) to confirm that the plated cells were Kupffer cells. They were then incubated with secondary antibody conjugated to Alexa 594 (red staining, Invitrogen, Carlsbad, CA).
Western blot analysis
Nuclear extracts were isolated from Kupffer cells. Briefly, the Kupffer cells were scraped into 10 mM Hepes, pH 7.9 containing 100 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 0.5% NP-40, and Halt Protease Inhibitor Cocktail (Pierce Biotechnology, Rockford, IL) and incubated on ice. After 15 minutes, the cells were vortexed and centrifuged for 10 seconds at 10,000 g. The pellet was resuspended in 10 mM Hepes, pH 7.9 containing 420 mM NaCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 5% glycerol, and Halt Protease Inhibitor Cocktail mixed for 1 hour at 4°C. The samples were centrifuged at 10,000 g for 10 minutes at 4°C and the concentration of protein determined in the supernatant. For Western blot analysis, aliquots (15 µg) of nuclear extracts were subjected to 10% SDS-polyacrylamide gel electrophoresis, and proteins were transferred to Immobilon polyvinylidene difluoride transfer membranes (Millipore Corporation, Bedford, MA). The membranes were then probed with rabbit polyclonal anti-HIF-1α antibody (NB100-449, Novus Biologicals, Littleton, CO) diluted 1:500 followed by incubation with goat anti-rabbit antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology). Immunoreactive bands were visualized using the Immun-Star HRP Substrate Kit (Bio-Rad Laboratories, Hercules CA).
For HIF-1β western blotting, membranes were probed with a rabbit polyclonal anti-HIF-1β antibody (Novus Biologicals, Littleton, CO) diluted 1:500 followed by incubation with goat anti-rabbit antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology).
Statistical analysis
Results are presented as the mean ± SEM. Data were analyzed by Analysis of Variance (ANOVA). ANOVAs were performed on log X-transformed data in instances in which variances were not homogenous. Comparisons among group means were made using the Student-Newman-Keuls test. The criterion for significance was p < 0.05 for all studies.
RESULTS
Activation of HIF-1α in hypoxic Kupffer cells
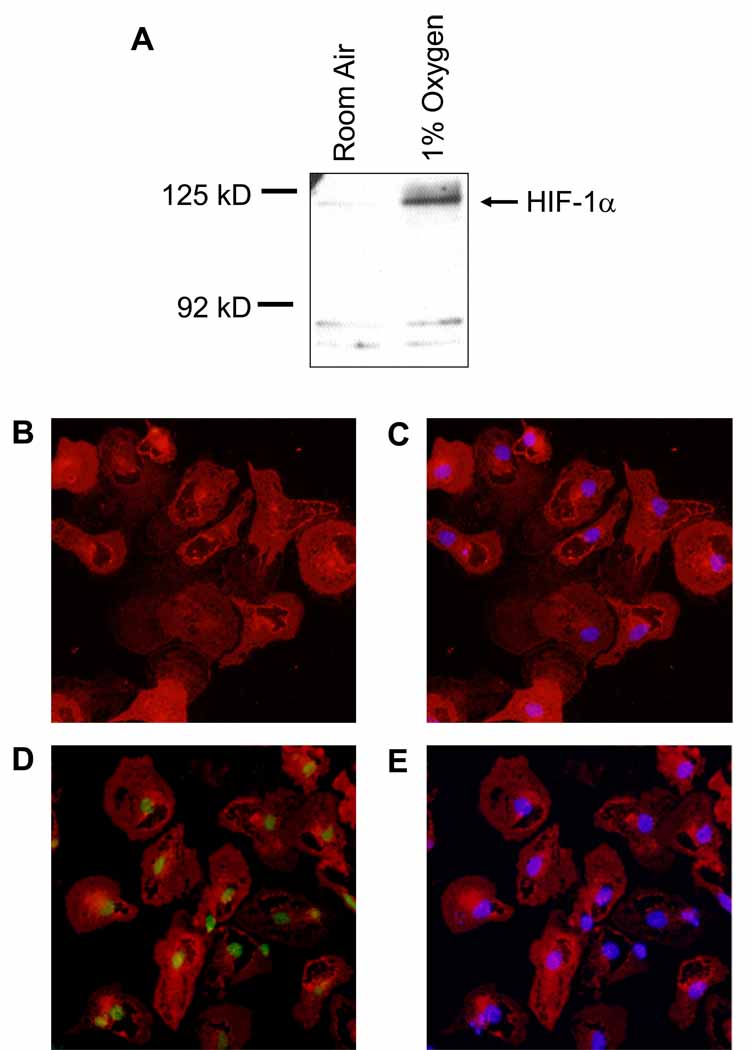
To determine whether HIF-1α is activated in hypoxic Kupffer cells, primary Kupffer cells were isolated from wild-type mice and exposed to room air or 1% oxygen for 1 hour. Western blot analysis of nuclear extracts from these cells showed increased levels of HIF-1α (120 kD) after exposure to 1% oxygen (Figure 1).
Figure 1.
Activation of HIF-1α in hypoxic kupffer cells. Kupffer cells were isolated from wild-type mice and exposed to room air or 1% oxygen for 1 hour. (A) HIF-1α was then detected in nuclear extracts by Western blot. Representative Western blot of an n=3. Immunohistochemistry was used to detect HIF-1α (green fluorescence in B and D) and Kupffer cells (red fluorescence in A–D). The same cells were counterstained with DAPI (C and E) to identify nuclei and show colocalization of HIF-1α immunostaining with nuclear staining. Representative of an n=3.
Immunohistochemistry was next used to confirm nuclear accumulation of HIF-1α in hypoxic Kupffer cells. HIF-1α (green staining) was not detected in the nuclei of Kupffer cells (red staining) exposed to room air (Figure 1B). Exposure of Kupffer cells to 1% oxygen, however, stimulated accumulation of HIF-1α in nuclei (Figure 1D). Nuclear accumulation of HIF-1α was confirmed by colocaliztion with 4’,6-diamidino-2-phenylindole (DAPI) which stains DNA (Figure 1E).
In contrast to HIF-1α, HIF-2α was not detected by either Western blot or immunohistochemistry in hypoxic Kupffer cells (data not shown).
Inhibition of HIF signaling in hypoxic Kupffer cells
To evaluate the role of HIFs in regulation of gene expression in hypoxic Kupffer cells, HIF-1βfl/fl mice, containing loxP sites flanking Exon 6 of the HIF-1β gene, were crossed with Mx-Cre+/− mice. Exon 6 of the HIF-1β gene encodes the conserved basic-helix-loop-helix domain. Deletion of this region completely abrogates all HIF signaling in cells 12. Treatment of HIF-1βfl/fl-Mx-Cre+ mice with pIpC upregulates Cre recombinase in most cell types in these mice and deletes Exon 6 in the HIF-1β gene. For the present studies, we first determined the efficiency of deletion of Exon 6 of the HIF-1β gene in Kupffer cells isolated from HIF-1βfl/fl-Mx-Cre+ mice and HIF-1βfl/fl-Mx-Cre- mice treated with pIpC. One week after the final pIpC injection, Kupffer cells were isolated from the mice and the extent of Exon 6 deletion was analyzed using PCR of genomic DNA. As shown in Figure 2A, there was a complete deletion of Exon 6 of the HIF-1β gene from Kupffer cells isolated from these mice. We next confirmed that this resulted in a reduction in HIF-1β protein. Whereas HIF-1β was detected in Kupffer cells isolated from HIF-1βfl/fl-Mx-Cre- mice, HIF-1β was not detected in lysates from HIF-1βfl/fl-Mx-Cre+ mice (Figure 2B). For the remainder of this manuscript, Kupffer cells isolated from HIF-1βfl/fl-Mx-Cre+ mice treated with pIpC will be referred to as HIF-1β-Deficient Kupffer cells, and Kupffer cells isolated from HIF-1βfl/fl-Mx-Cre- mice treated with pIpC will be referred to as HIF-1β-Control Kupffer cells.
Next to confirm that deletion of HIF-1β abrogated HIF signaling in hypoxic Kupffer cells, HIF-1β-Deficient and HIF-1β-Control Kupffer cells were exposed to room air or 1% oxygen for 16 hours, and glucose transporter-1 mRNA (Glut-1) levels were measured by real-time PCR. Glut-1 mRNA levels are increased in hypoxic cells in a HIF-dependent manner 16. Exposure of HIF-1β-Control Kupffer cells to 1% oxygen increased Glut-1 mRNA levels 4.5 fold (Figure 2C). Upregulation of Glut-1 was completely prevented in HIF-1β-Deficient Kupffer cells, confirming that deletion of Exon 6 of the HIF-1β gene completely abrogated HIF signaling in these cells.
Lastly, we confirmed that deletion of HIF-1β from these cells did not decrease their viability after hypoxia exposure. To investigate this, we measured release of lactate dehydrogenase (LDH) from the cells into the medium, a commonly used measure of cell death. Exposure of HIF-1β-Control and HIF-1β-Deficient Kupffer cells to 1% oxygen did not stimulate release of LDH when compared to cells cultured in room air (Figure 2D).
Role of HIFs in regulation of production of profibrotic growth factors by hypoxic Kupffer cells
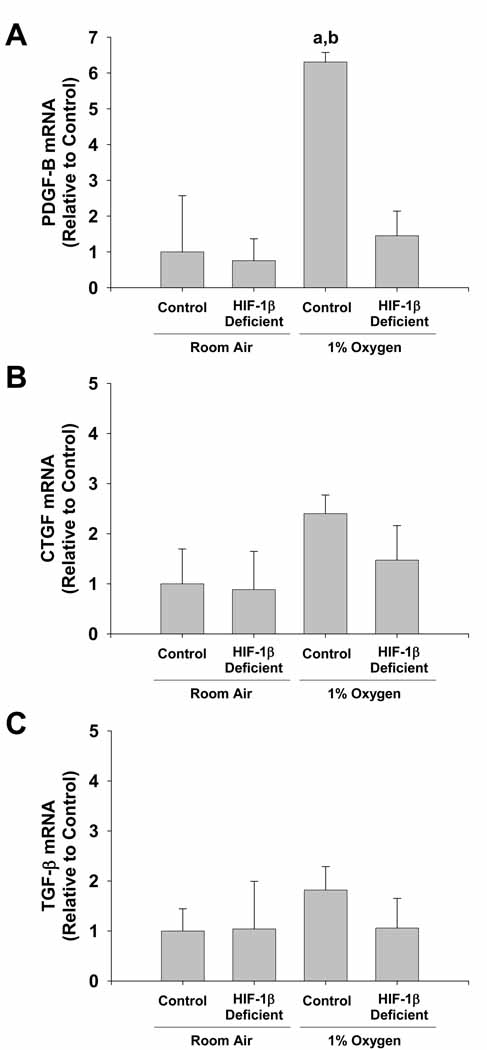
Next, HIF-1β-Deficient and HIF-1β-Control Kupffer cells were exposed to 1% oxygen to determine whether hypoxia upregulates profibrotic growth factors in Kupffer cells in a HIF-dependent manner. Exposure of HIF-1β-Control Kupffer cells to 1% oxygen increased PDGF-B mRNA levels by 6 fold (Figure 3A). Upregulation of PDGF-B by hypoxia was completely prevented in HIF-1β-Deficient Kupffer cells. In contrast, mRNA levels of CTGF and TGF-β were not affected by exposure of either HIF-1β-Control or HIF-1β-Deficient Kupffer cells to 1% oxygen (Figure 3B and 3C).
Figure 3.
HIF-dependent regulation of profibrotic growth factors in hypoxic Kupffer cells. Kupffer cells were isolated from HIF-1β-Control and HIF-1β-Deficient mice and exposed to room air or 1% oxygen. Sixteen hours later, PDGF-B, CTGF, and TGF-β mRNA levels were quantified by real-time PCR. aSignificantly different from Kupffer cells exposed to room air (p<0.05). bSignificantly different from Kupffer cells isolated from HIF-1β-Deficient mice and subjected to 1% oxygen (p<0.05). Data are expressed as means ± SEM; n = 3.
HIF-dependent production of proangiogenic mediators by hypoxic Kupffer cells
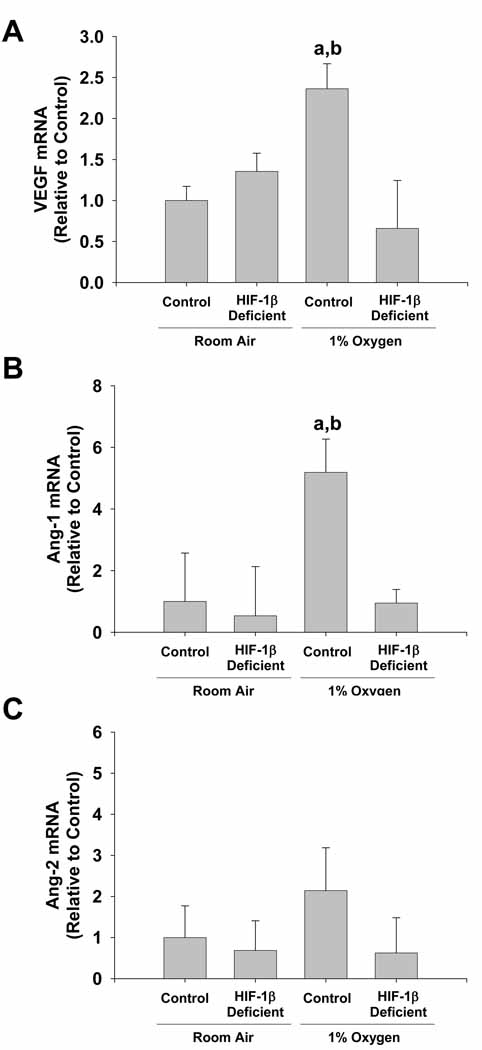
Studies suggest that angiogenesis is important for the development of fibrosis 5, 17, 18. In addition, studies have shown that HIFs are important regulators of proangiogenic mediators by hypoxic cells 19, 20. Therefore, we next determined whether hypoxia stimulates production of proangiogenic mediators by Kupffer cells in a HIF-dependent manner. Exposure of HIF-1β-Control Kupffer cells to 1% oxygen increased VEGF and Ang-1 mRNA levels (Figure 4A and 4B). Upregulation of both genes by hypoxia was completely prevented in HIF-1β-Deficient Kupffer cells. In contrast, mRNA levels of Ang-2 were not affected by exposure of either HIF-1β-Control or HIF-1β-Deficient Kupffer cells to 1% oxygen (Figure 4C).
Figure 4.
HIF-dependent regulation of proangiogenic mediators in hypoxic Kupffer cells. Kupffer cells were isolated from HIF-1β-Control and HIF-1β-Deficient mice and exposed to room air or 1% oxygen. Sixteen hours later, VEGF, Ang-1, and Ang-2 mRNA levels were quantified by real-time PCR. aSignificantly different from Kupffer cells exposed to room air (p<0.05). bSignificantly different from Kupffer cells isolated from HIF-1β-Deficient mice and subjected to 1% oxygen (p<0.05). Data are expressed as means ± SEM; n = 3.
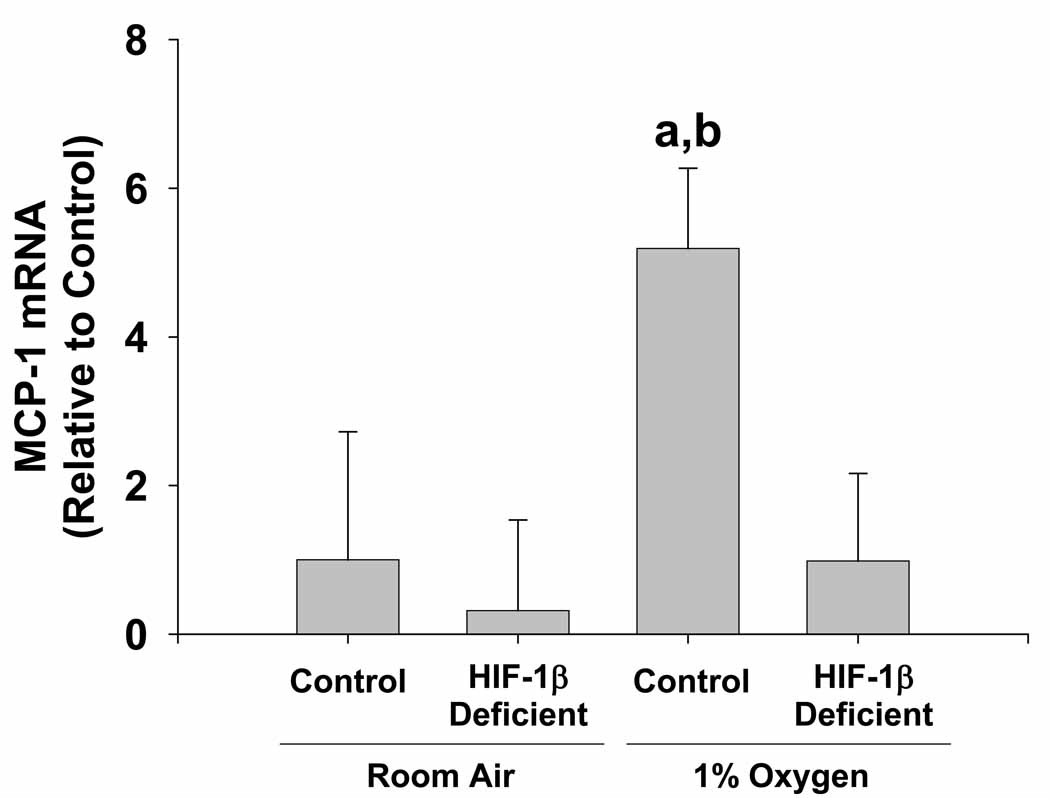
HIF-dependent production of MCP-1 by hypoxic Kupffer cells
MCP-1 is a chemokine that is well known for its ability to stimulate monocyte and hepatic stellate cell chemotaxis 21. Studies have shown that this chemokine is expressed by numerous cell types, including macrophages, in regions of fibrous liver 22. Furthermore, recent studies suggest that MCP-1 may be regulated by HIFs 23. Therefore, we next determine whether MCP-1 is upregulated in hypoxic Kupffer cells in a HIF-dependent manner. Exposure of HIF-1β-Control Kupffer cells to 1% oxygen increased MCP-1 mRNA levels by 5 fold (Figure 5). Upregulation of MCP-1 by hypoxia was completely prevented in HIF-1β-Deficient Kupffer cells.
Figure 5.
HIF-dependent regulation of MCP-1 in hypoxic Kupffer cells. Kupffer cells were isolated from HIF-1β-Control and HIF-1β-Deficient mice and exposed to room air or 1% oxygen. Sixteen hours later, MCP-1 mRNA levels were quantified by real-time PCR. aSignificantly different from Kupffer cells exposed to room air (p<0.05). bSignificantly different from Kupffer cells isolated from HIF-1β-Deficient mice and subjected to 1% oxygen (p<0.05). Data are expressed as means ± SEM; n = 3.
DISCUSSION
Studies have shown that macrophages are important for the development of liver fibrosis. These cells accumulate in injured regions of liver, and express mediators, such as PDGF-B and TGF-β, that activate hepatic stellate cells and stimulate them to produce collagen 24. What remains unclear, however, is the mechanism by which liver injury stimulates these cells to produce profibrotic mediators during the development of fibrosis. Our studies suggest that hypoxia, through activation of hypoxia-inducible factors, may be important for this process.
Our studies are the first to demonstrate that HIF-1α is activated in hypoxic Kupffer cells, and regulates expression of genes that promotes fibrosis. One gene that was upregulated in hypoxic Kupffer cells in a HIF-dependent manner was PDGF-B (Fig. 3), the most potent stimulator of hepatic stellate cell activation 25. In addition, PDGF-B is a key mediator of angiogenesis and inflammation, both of which are important for the development of liver fibrosis 26, 27. As mentioned, during the development of fibrosis, hepatic macrophages appear to be an important source of this mediator 24. Our studies demonstrate that hypoxia stimulates Kupffer cells to produce PDGF-B, and that this requires HIF signaling (Fig. 3). This suggests that during the development of fibrosis, Kupffer cells that accumulate in injured regions of liver, which are typically hypoxic 18, may become stimulated to produce PDGF-B in a HIF-dependent manner. In support of this, HIF-1α was activated in macrophages in livers of mice subjected to bile duct ligation, a model of liver fibrosis, and mice deficient in HIF-1α produced less PDGF-B in the liver after bile duct ligation 10. Interestingly, although some studies have indicated that TGF-β and CTGF are hypoxia-regulated genes 28, 29, this did not occur in Kupffer cells (Fig. 3). This suggests that regulation of growth factors by HIFs occurs in a cell type-specific manner. Overall, our results indicate that during chronic injury, hypoxia stimulates release of PDGF-B from Kupffer cells in a HIF-1α-depenedent manner, which stimulates activation of hepatic stellate cells, angiogenesis, and inflammation, all of which contribute to the development of fibrosis.
Our studies also demonstrated that hypoxia stimulates Kupffer cells to produce proangiogenic mediators in a HIF-dependent manner (Fig. 46). Studies have shown that levels of proangiogenic mediators are increased during fibrosis and that they may be important for the development of fibrosis by stimulating hepatic stellate cell activation and chemotaxis 5, 17, 18. Since many proangiogenic mediators are known HIF target genes 19, 20, we tested the hypothesis that they are upregulated in hypoxic Kupffer cells in a HIF-dependent manner. Our studies demonstrate that VEGF and Ang-1 are increased in a HIF-dependent manner (Fig. 4), suggesting that Kupffer cells may be an important source of these mediators in liver during the development of fibrosis. Both VEGF and Ang-1 are known HIF target genes and studies have shown that both of these mediators stimulate hepatic stellate cell chemotaxis 17, 19, 20. Interestingly, although Ang-2 is also a known HIF-target gene, this gene was not upregulated in hypoxic Kupffer cells.
Another gene stimulated in hypoxic Kupffer cells in a HIF-dependent manner was MCP-1. MCP-1 is a chemokine that is well known for its ability to stimulate monocyte chemotaxis by binding to CCR2 receptors on these cells 30. Studies have shown that this chemokine is expressed by numerous cell types, including macrophages, in regions of fibrous liver 22. Furthermore, it was shown that MCP-1 is chemotactic for activated hepatic stellate cells, suggesting that this chemokine may promote accumulation of activated hepatic stellate cells in regions of where fibrosis occurs 21. Recent studies have also shown that this gene is upregulated in hypoxic cells 23. Our studies show that MCP-1 is upregulated in hypoxic Kupffer cells in a HIF-dependent manner (Fig. 5). Therefore, during the development of fibrosis, hypoxic Kupffer cells may produce MCP-1 in a HIF-dependent manner that stimulates recruitment of hepatic stellate cells and additional monocytes to injured regions were fibrosis occurs.
Collectively, these studies demonstrate that hypoxia is an important stimulator of profibrotic mediator production by Kupffer cells, and that the mechanism by which hypoxia stimulates their production depends upon activation of HIFs. This suggests that during the development of fibrosis, the mechanism by which Kupffer cells become stimulated to release profibrotic mediators, such as PDGF-B, VEGF, Ang-2, and MCP-1 may occur through hypoxia-dependent activation of HIFs.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants DK073566 (B.L.C.) and COBRE (Center of Biomedical Research Excellence) P20 RR021940 as well as the Molecular Biology Core and the Histology Core supported by the COBRE grant. The authors thank Dr. Frank J. Gonzalez at the National Cancer Institute for providing the HIF-1βfl/fl-Mx-Cre+ and HIF-1βfl/fl-Mx-Cre- mice for these studies.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008 May;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faiz Kabir Uddin Ahmed A, Ohtani H, Nio M, et al. In situ expression of fibrogenic growth factors and their receptors in biliary atresia: comparison between early and late stages. The Journal of pathology. 2000 Sep;192:73–80. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH657>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85:1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005 Jan;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosmorduc O, Wendum D, Corpechot C, et al. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999 Oct;155:1065–1073. doi: 10.1016/S0002-9440(10)65209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copple BL, Moon J-O. Role of hypoxia-inducible factor-1α in the development of liver fibrosis. FASEB J. 2008;22:1190–1193. [Google Scholar]
- 7.Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. Essays Biochem. 2007;43:1–15. doi: 10.1042/BSE0430001. [DOI] [PubMed] [Google Scholar]
- 8.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005 Jul;64:971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med. 2007 Nov 1;43:1219–1225. doi: 10.1016/j.freeradbiomed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009 Mar;296:G582–G592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Developmental biology. 1997 Nov 15;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 12.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Molecular endocrinology. Vol. 14. Baltimore, Md: 2000. Oct, Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha; pp. 1674–1681. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995 Sep 8;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 14.Vrochides D, Papanikolaou V, Pertoft H, Antoniades AA, Heldin P. Biosynthesis and degradation of hyaluronan by nonparenchymal liver cells during liver regeneration. Hepatology. 1996 Jun;23:1650–1655. doi: 10.1002/hep.510230648. [DOI] [PubMed] [Google Scholar]
- 15.Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006 Apr;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 16.Wood SM, Wiesener MS, Yeates KM, et al. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of hif-1alpha-dependent and -independent hypoxia-inducible gene expression. J Biol Chem. 1998 Apr 3;273:8360–8368. doi: 10.1074/jbc.273.14.8360. [DOI] [PubMed] [Google Scholar]
- 17.Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007 Jun;170:1942–1953. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002 May;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 19.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996 Sep;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly BD, Hackett SF, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003 Nov 28;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 21.Marra F, Romanelli RG, Giannini C, et al. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999 Jan;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 22.Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998 Feb;152:423–430. [PMC free article] [PubMed] [Google Scholar]
- 23.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. Journal of neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malizia G, Brunt EM, Peters MG, Rizzo A, Broekelmann TJ, McDonald JA. Growth factor and procollagen type I gene expression in human liver disease. Gastroenterology. 1995 Jan;108:145–156. doi: 10.1016/0016-5085(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. The Journal of cell biology. 1994 May;125:917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyle GW, Li J, Finkelstein JB, et al. Emphysematous lesions, inflammation, and fibrosis in the lungs of transgenic mice overexpressing platelet-derived growth factor. Am J Pathol. 1999 Jun;154:1763–1775. doi: 10.1016/S0002-9440(10)65432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgin DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004 Dec;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 29.Falanga V, Qian SW, Danielpour D, Katz MH, Roberts AB, Sporn MB. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991 Oct;97:634–637. doi: 10.1111/1523-1747.ep12483126. [DOI] [PubMed] [Google Scholar]
- 30.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994 Mar 29;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Provost F, Riedlinger G, Hee Yim S, et al. The aryl hydrocarbon receptor (AhR) and its nuclear translocator (Arnt) are dispensable for normal mammary gland development but are required for fertility. Genesis. 2002 Mar;32:231–239. doi: 10.1002/gene.10037. [DOI] [PubMed] [Google Scholar]