Abstract
Methamphetamine (METH) is a psychostimulant that induces excessive release of dopamine (DA) in the striatum. In this study we have assessed the role of DA D1 and D2 receptors (D1R and D2R) on striatal METH-induced apoptosis and depletion of DA-terminal markers. Male mice were given one i.p. injection of METH (30 mg/kg). Apoptosis was assessed at 24 h, and DA-terminal marker depletion 3 days, after METH. A single toxic dose of METH induced apoptosis in approximately 10–13% of striatal neurons. This was completely prevented by pretreatment (30 min before METH) with either the D1R antagonist SCH-23390 (0.1 mg/kg) or the D2R antagonist raclopride (1 mg/kg). The same dose of METH induced depletion of DA transporter sites up to 61, 56, 71, and 69% in dorsal-medial, ventral-medial, dorsal-lateral, and ventral-lateral striatum, respectively, relative to vehicle-injected controls. Similarly, METH induced depletion of TH protein levels up to 80, 72, 87, and 90% in those respective quadrants. METH induced the expression of glial fibrillary acidic protein throughout the striatum. All these neurochemical changes were significantly attenuated by pretreatment with SCH-23390 (0.1 mg/kg) or raclopride (1 mg/kg). However, pretreatment with either raclopride or SCH-23390 did not prevent METH-induced hyperthermia in mice. These data demonstrate that the induction by METH of both striatal apoptosis and DA-terminal damage requires the activity of the postsynaptic DA receptors in the mouse brain. Moreover, since blockade of either receptor subtype protected from METH, the activity of both DA receptor subtypes is required for the induction of toxicity by METH in the striatum.
Keywords: DA receptors, methamphetamine, striatum, transporter, apoptosis, tyrosine hydroxylase
INTRODUCTION
Methamphetamine (METH) is an addictive agent that increases the concentration of catecholamines in the extrasynaptic space of neurons of the CNS (Liang and Rutledge, 1982; Raiteri et al., 1979; Schmidt et al., 1985; Sulzer et al., 1995). METH increases extrasynaptic dopamine (DA) levels in the striatum, a brain region associated with various neurological disorders affecting motor function such as Parkinson’s disease and Huntington’s chorea (Hickey and Chesselet, 2003, Lev et al., 2003). The high levels of extraneuronal DA in the striatum, and to a lesser extent in the nucleus accumbens, induces the development of neurotoxicity of the DA-terminals with long-term deficits of the DA transporter (dopamine transporter (DAT)), tissue DA content and its metabolites, and loss of tyrosine hydroxylase (TH) (Pu and Vorhees, 1995; Villemagne et al., 1998; Yu et al., 2002). Recent reports indicate that exposure to METH induces toxicity leading to cell death of striatal neurons postsynaptic to DA and cortical neurons (Choi et al., 2002; Deng et al., 1999; Deng et al., 2001; Eisch and Marshall, 1998; Pu et al., 1996; Zhu et al., 2002). The contribution of the DA receptors on the striatal apoptosis induced by METH has not been investigated. In this study we use selective D1R or D2R antagonists to evaluate the role of these receptors on METH-induced striatal apoptosis.
The induction of toxicity in the DA-terminals as well as the apoptosis of neurons postsynaptic to the DA-terminals by METH may be due primarily to the high extracellular levels of this neurotransmitter and its impact on DA receptors. Striatal neurons expressing a high density of D1 receptors project to the substantia nigra pars reticulata and also coexpress the neuropeptides dynorphin, neurokinin A, and substance P (Gerfen et al., 1990). Striatal neurons that project to the globus pallidum express a high density of D2 receptors as well as the neuropeptide enkephalin (Le Moine et al., 1990). However, recent evidence supports the conjoint expression of D1 and D2 receptors by all striatal projection neurons, as demonstrated by confocal microscopy (Aizman et al., 2000) and PCR to detect mRNAs for both DA receptor subtypes (Surmeier et al., 1996). D1 receptors increase the intracellular concentration of the second messenger cyclic AMP with concomitant activation of the downstream effector enzyme protein kinase A (Kebabian and Calne, 1979; Stoof and Kebabian, 1981). Alternatively, D2 receptors have been linked to the activation of calci-neurin (PP-2B), a protein phosphatase that dephosphorylates DARPP-32 (DA- and cyclic AMP-regulated phosphoprotein, having an apparent molecular mass of 32 kDa). The latter protein integrates signaling from both D1 and D2 receptors in striatal medium spiny neurons (Greengard et al., 1998; Svenningsson et al., 2003). D1 and D2 receptors exert opposing effects on the state of phosphorylation of striatal DARPP-32 (Nishi et al., 1997). Thus, excessive stimulation of these receptors by DA, such as when METH is present, can lead to the activation of downstream effectors activating pathways that are ultimately damaging to neurons postsynaptic to the DA-terminals. Alternatively, agents that block DA receptors should protect striatal neurons from METH.
The mechanism by which METH causes damage to neurons involves the activity of DA receptors. Antagonists of the D1 or D2 receptor prevented METH-induced loss of tissue DA content in the striatum (Sonsalla et al., 1986). The experiments presented here were undertaken to investigate if DA receptor activation is required for METH-induced striatal apoptosis. The DA-terminal markers TH and DAT were also assessed because METH causes long-term deficits in these markers. It is important to compare the time of appearance of presynaptic and postsynaptic damage induced by METH. Finally, we assessed the effect of DA receptor antagonists on the induction of glial fibrillary acidic protein (GFAP), a marker of reactive astrocytosis in the striatum of mice. DA receptor antagonists attenuate both pre- and postsynaptic neural damage but peak levels are reached at different times.
MATERIALS AND METHODS
Animals, drugs, and drug administration
Single i.p. (i.p.) injections of METH (30 mg/kg) (Sigma, St. Louis, MO) were given to 10-week-old ICR mice (a strain developed by the Institute for Cancer Research and were obtained from Taconic, Germantown, NY). All animals were housed singly with food and water available ad libitum on a 12-h light/dark cycle. Animals were habituated for ∼2 weeks before any drug treatment. In cases where the D1R antagonist or D2R antagonist applied, R-(+)-SCH-23390 hydrochloride, (Sigma/RBI) or S(−)-Raclopride (+)-tartrate salt (Sigma/RBI) was dissolved in distilled water and administered (i.p.) 30 min before METH treatment. After drug treatment, animals were sacrificed by decapitation at 24 h or 3 days after treatment. Brains were dissected out and immediately placed on dry ice. Tissue was stored at −80°C until use. All animal use procedures were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Hunter College of the City University of New York.
Body temperature
Rectal body temperature was determined by a BAT-12 thermometer coupled to a RET-3 mouse rectal probe (Physitemp Instruments, Clifton, NJ). Ambient room temperature was maintained at 20–22°C.
Terminal deoxyncleotidyl transferase-mediated dUTP nick end labeling (TUNEL) histochemistry
The method is adapted from Deng et al. (2001) with minor modifications. In brief, fresh frozen 20-µm coronal sections were taken between bregma 0.38 ± 0.1 mm and fixed in 4% paraformaldehyde for 30 min. After PBS wash, sections were immersed in 0.4% Triton-X 100 in PBS for 5–10 min at 70°C. Sections were washed and TUNEL reactions (Roche Applied Science, Indianapolis, IN) were applied directly onto sections and incubated for 1 h in a humidified chamber. After TUNEL staining, sections were counterstained with DAPI. Stained sections were washed in PBS and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Images were taken with a Nikon Eclipse E400 epifluorescent scope attached to a Hamamatsu digital camera C4742-95 using FITC filters.
Autoradiographic analysis of DAT
Fresh frozen 20-µm coronal sections were dried in a dessicator and then incubated in 0.073 nM [125I]RTI-121 (2,200 Ci/mmol, New England Nuclear, Boston, MA) buffered solution (137 mM NaCl, 2.7 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, 10 mM NaI) for 1 h at room temperature. Nonspecific binding was determined using 10 µM GBR-12909 (Sigma). After incubation, sections were washed twice with chilled buffer for 20 min and then quickly rinsed with chilled distilled water. Slides were allowed to air-dry overnight and exposed on Hyperfilm MP (Amersham Pharmacia, Piscataway, NJ) together with a [125I] microscale. Binding of [125I]RTI-121 was quantified by densitometry with use of a computer-based NIH image analysis system.
GFAP immunohistochemistry
Fresh frozen 20-µm coronal sections from bregma 0.38 ±0.1 mm were air-dried and fixed in absolute ethanol at −20°C for 10 min. Sections were allowed to air-dry again and washed in PBS. Nonspecific sites were blocked with MOM® Mouse Ig Blocking Reagent (Vector Laboratories) in 0.3% Triton-X 100/PBS for 1 h. After being washed with PBS, sections were incubated with MOM® diluent for 15 min and then with Cy3-conjugated mouse anti-GFAP (1:25, Sigma) for 3 h. After washing, slides were mounted with Vecta-shield (Vector Laboratories). All incubations were performed at room temperature with gentle rocking. Images were taken with a Nikon Eclipse TE200 inverted-epifluorescent scope attached to a CE 3.2.0 digital camera using rhodamine filters.
TH Western blot
Striata were dissected out after decapitation and separated into dorsal-medial (DM), dorsal-lateral (DL), ventral-medial (VM), and ventral-lateral (VL) compartments. Tissues were then homogenized with lysis buffer (50 mM Tris-HCL pH 7.4, 150 mM NaCl, 320 mM sucrose, 5 mM HEPES, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DTT, 1% inhibitor cocktail [1.04 mM AEBSF, 0.8 µM aprotinin, 0.02 mM leupeptin, 0.04 mM bestatin, 0.015 mM pepstatin A, 0.014 mM E-64 (Sigma)]). Homogenates were centri-fuged at 800g for 5 min at 4°C. Supernatants were further centrifuged at 3,000g, and supernatants were then used for Western blot analysis. After protein concentration was determined by the Bradford assay, samples were denatured in Laemmli sample buffer containing 20% β-mercaptoethanol for 10 min at 85°C. Samples were then subjected to 10% SDS-PAGE gel, and proteins were transferred onto PVDF membranes. Membranes were blocked with 5% nonfat dry milk and probed with mouse anti-TH (1:1,000, Chemi-con, Temecula, CA) overnight at 4°C with gentle rocking. Membranes were washed with TBS and incubated with HRP-conjugated goat antimouse (1:1,000, Santa Cruz Biotech, Santa Cruz, CA) for 1 h at room temperature. After a wash with TBS, proteins were detected with the use of the SuperSignal® West Pico Chemilumescent Substrate (Pierce, Rockford, IL) and exposed on Hyperfilm™ ECL film (Amersham Bio-sciences Corporation). For internal standards, membranes were stripped and reprobed with mouse anti-β-actin (1:10,000, Sigma). Densitometry was performed with a NIH image analysis system, and the relative density of each band was normalized against that of β-actin.
Cell counts and quantification
All coronal sections were taken from bregma 0.38 ± 0.1 mm. All cells of interest were quantified from 20-µm thick coronal sections in an area of 260 µm2 for each region of interest (ROI) in the striatum (DM, DL, VM, VL). Average neuronal cell counts done previously were then used to quantify percentage of TUNEL-positive neurons. TUNEL cell counts were averaged from five 20-µm serial sections per animal. The number of GFAP-positive cells was quantified from three 20-µm serial sections per animal.
Statistical analysis
Analysis was performed from mean ± SEM. Differences between groups were analyzed by ANOVA followed by post hoc comparison using Fisher’s protected least significance test. Significance criteria was set at P ≤ 0.05.
RESULTS
To quantify the apoptosis induced by a single bolus injection of METH (30 mg/kg of body weight), we counted the total number of neurons stained with the neuronal-specific marker NeuN and the total number of TUNEL-positive cells. The magnitude of cell death is expressed as the ratio of TUNEL/NeuN-labeled cells. To assess the impact of METH at the subregional level, we subdivided the striatum into four quadrants: DM, VM, DL, and VL. The analysis comprised an area of 260 µm2 within each quadrant of the striatum. We assessed the depletion of DA-termi-nal markers at day 3 and apoptotic cell death at 24 h post-METH because in another study we determined that these time points represent the peak of pre- and postsynaptic damage induced by METH.*
A bolus injection of METH induced TUNEL staining in the striatum 24 h after the treatment. TUNEL-positive cells (nuclear staining) are shown in Figure 1A as green immunohistofluorescence against a dark background. Approximately 10–13% of the striatal neurons are TUNEL-positive 24 h after a single bolus injection of METH (Figure 1B). An injection of the D1R antagonist SCH-23390, 30 min before METH significantly attenuated apoptosis at a dose of 0.05 mg/kg and completely prevented cell death at a dose of 0.1 mg/kg or higher (Figure 1A and 1B). Similarly, pharmacological blockade of the striatal D2R with 1 mg/kg of the selective antagonist raclopride completely prevented the induction of TUNEL staining in all quadrants of the striatum (Figure 2A and 2B). Both raclopride or SCH-23390 failed to induce striatal ap-optosis when given alone (Figure 1 and Figure 2).
Fig. 1.
D1R antagonist protects against METH-induced cell death. Pretreatment with D1R antagonist, SCH 23390 (SCH [0.05 mg/kg, 0.1 mg/kg, 0.25 mg/kg, 0.5 mg/kg, or 1 mg/kg of body weight]), 30 min before METH (30 mg/kg of body weight, i.p.) abrogated TUNEL-staining in the mouse striata in a dose-dependent fashion. Antagonist alone had no effect. (A) Epifluorescent images of TUNEL-stained mouse striata. Scale bar = 50 µm. (B) Figure shows mean ± SEM percentage of TUNEL-positive staining relative to total neuronal cell counts done previously (data not shown) with neuron-specific nuclear protein NeuN. *P < 0.01 compared with vehicle + saline, !P < 0.005 compared with vehicle + METH 30 mg/kg. No significance is found between regions of the same experimental group.
Fig. 2.
D2R antagonist protects against METH-induced cell death. Pretreatment with D2R antagonist, raclopride (RAC [0.025 mg/kg, 0.5 mg/kg, or 1 mg/kg of body weight]), 30 min before METH (30 mg/kg of body weight, i.p.) attenuated TUNEL-staining in the mouse striata in a dose-dependent fashion. Antagonist alone had no effect. (A) Epifluorescent images of TUNEL-stained mouse striata. Scale bar = 50 µm. (B) Figure shows mean ± SEM percentage of TUNEL-positive staining relative to total neuronal cell counts done previously (data not shown) with neuron-specific nuclear protein NeuN. *P < 0.01 compared with vehicle + saline, !P < 0.05 compared with vehicle + METH. No significance is found between regions of the same experimental group.
Exposure to METH induced depletion of DAT sites of 61, 56, 71, and 69% in DM, VM, DL, and VL quadrants of the striatum, respectively, relative to vehicle-injected controls. The striata of mice pretreated with the D1R antagonist (SCH-23390), 30 min before METH displayed less severe depletion of DAT sites: 31, 36, 26, and 22% in DM, VM, DL, and VL quadrants, respectively, relative to control (Figure 3A and 3B). Similarly, depletions of striatal DAT in mice pre-treated with 1 mg/kg of raclopride were attenuated to 30, 34, 24, and 25% in DM, VM, DL, and VL quadrants of the striatum, respectively (Figure 3B). Neither SCH-23390 nor raclopride significantly affected striatal DAT when administered alone (Figure 3B).
Fig. 3.
D1R and D2R antagonists protect against METH-induced DAT depletion. (A) DAT [125I]RTI-121 autoradiographs demonstrate that METH-induced DAT depletion is attenuated by SCH 23390 or raclopride pretreatment (B) in the DM, DL, VM, and VL regions of the striatum, respectively. Antagonists alone had no significant effects on binding sites compared with vehicle + saline. †P < 0.0001 compared with the DM region within each experimental group. !P < 0.0001 compared with the VM region within each experimental group. *P < 0.0001 compared with the corresponding region of vehicle + saline. aP < 0.005 compared with the corresponding region of vehicle + METH (30 mg/kg). n = 6 per group. Cx and CPu refer to the cortex and caudate-putamen (striatum), respectively.
To assess the impact of METH on activation of striatal astrocytes, we performed immunohistofluores-cence for the astrocytic marker GFAP in coronal sections of striatal tissue. METH induced intense staining of striatal astrocytes at day 3 after treatment. Pretreatment with either SCH-23390 or raclopride nearly abrogated the induction of GFAP in the astro-cytes (Figure 4A and 4B). SCH-23390 or raclopride had no effect on the levels of GFAP in striatal astro-cytes (Figures 4A and 4B).
Fig. 4.
D1R and D2R antagonists protect against METH-induced reactive astrocytosis. (A) Figure shows epifluorescent images of GFAP-stained mouse striata. Arrows point to astrocytes stained with anti-GFAP conjugated to Cy3. Scale bar = 10 µm. (B) Pretreatment with D1R (SCH 23390 [0.1 mg/kg of body weight] i.p.) or D2R antagonist, raclopride (RAC [1 mg/kg of body weight] i.p.), 30 min before METH (30 mg/kg of body weight, i.p.) exposure attenuated the induction of reactive astrocysts in the mouse striata. Antagonists alone had no effect. *P < 0.0001 compared with vehicle + saline, !P < 0.0001 compared with vehicle + METH 30 mg/kg. No significance is found between regions. n = 6 per group.
In addition to depletion of DAT sites and induction of GFAP by METH, we assessed another marker associated with neurotoxicity to this psychostimulant. Like DAT, it is located on the DA presynaptic terminals of the striatum. The rate-limiting enzyme of catecholamine biosynthesis, TH, was significantly depleted in striatum: 80, 72, 87, and 90% in DM, VM, DL, and VL, respectively, relative to vehicle-injected controls at day 3 after treatment, as determined by Western blot analysis (Figure 5A and 5B). Exposure to either SCH-23390 (0.1 mg/kg) or raclopride (1 mg/ kg) 30 min before METH attenuated depletion of TH. These agents had no effect on TH levels when administered alone (Figure 5A and 5B). It is worth noting that male rats receiving 40 mg/kg of METH displayed ∼50% depletion of TH by Western blot analysis of protein levels (Cappon et al., 2000). The discrepancy observed here may represent a species difference between mice and rats.
Fig. 5.
Western blot analysis showed that D1R and D2R antagonists protect against METH-induced TH depletion. (A) Immunoblots of TH and corresponding internal β-actin controls. (B) Analysis of TH immunoblots showing magnitude of the effect. Mice were injected with METH (30 mg/kg) and were sacrificed 3 days after METH. Separate groups of mice received either SCH-23390 or raclopride 30 min before METH at the doses indicated in the figure. The striatum was subdivided into DM, DL, VM, and VL quadrants. Note that pretreatment with either SCH-23390 or raclopride 30 min before METH resulted in loss of TH in all quadrants of the striatum. D1R or D2R antagonist alone had no significant effects. *P < 0.0001 compared to saline, †P < 0.001 compared to + METH 30 mg/kg, n = 6 in each group.
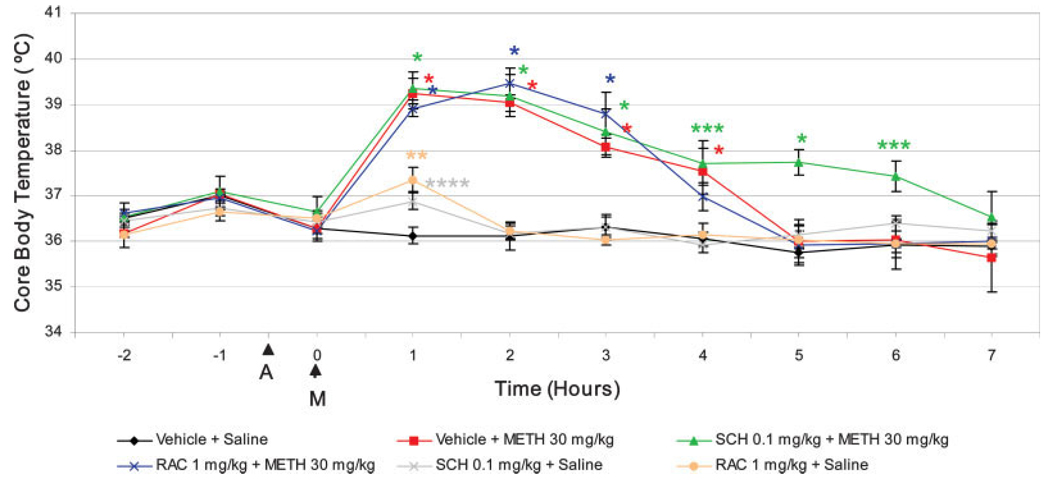
It has been demonstrated that environmental factors such as ambient temperature play an important role in METH-induced toxicity of the DA-terminals of the striatum (Bowyer et al., 1992; Ali et al., 1994; Bowyer et al., 1994). We assessed the effect of SCH-23390 and raclopride on METH-induced hyperthermia to determine if the neuroprotective effects of these agents on DA-terminal markers and striatal apoptosis are independent of the rise in body core temperature. We measured body core temperature hourly for 9 h. SCH-23390 (0.1 mg/kg) and raclopride (1 mg/kg) had no effect on METH-induced hyperthermia (Figure 6). SCH-23390 extended METH-induced hyperthermia up to 6 h after METH exposure (Figure 6).
Fig. 6.
D1R or D2R antagonists protects from METH-induced terminal toxicity and cell death without preventing hyperthermia. Rectal temperature was recorded every hour starting from 2 h prior to METH treatment. Ambient room temperature was 20–22°C. *P < 0.0001, **P < 0.001, ***P< 0.005, ****P < 0.05 compared with vehicle + saline. Results were from mean ± SEM of 6 animals per experimental group.
DISCUSSION
The experiments described here demonstrate that a single high dose of METH induces striatal injury extending to both afferents (DA-terminals) and the loss of striatal neurons postsynaptic to DA in the mouse brain. Deficits of DA-terminal markers measured in this study (DAT sites and TH levels) and loss of striatal neurons can be attenuated by pretreatment with selective DA receptor antagonists. For example, pretreatment with either SCH-23390 (D1R antagonist) or raclopride (D2R antagonist) protected DAT sites, TH levels, and striatal neurons from the neurotoxic effects of METH. Pharmacological blockade of these receptors also prevented the induction of GFAP in striatal astrocytes. These observations will be discussed in the context of potential neurochemical targets downstream of the neurotransmitter DA that may participate in the neurotoxic actions of this drug in the striatum.
Various laboratories have demonstrated unequivocally that manipulation of DA neurotransmission bears directly on the vulnerability to METH of the striatal DA-terminals. For example, inhibition of DA synthesis by systemic administration of α-methyl-ptyrosine depletes DA from presynaptic terminals and prevents METH-induced deficits in TH (Gibb and Kogan, 1979; Hotchkiss and Gibb, 1980). Pharmacological blockade of DA receptors with the neuroleptic drug haloperidol attenuates METH-induced changes in TH activity in the striatum (Buening and Gibb, 1974). Similarly, administration of the D1R antagonist SCH-23390 or the D2R antagonist sulpiride with METH prevents METH-induced deficits of striatal TH activity and depletion of tissue DA content in rats (Sonsalla et al., 1986). A study using in vivo microdialysis in rats demonstrated convincingly that either a D1R antagonist (SCH-23390) or a D2R antagonist (eticlopride) attenuates METH-induced DA efflux in the striatum, and the magnitude of inhibition of METH-induced DA efflux correlates with the extent of DA depletion observed 1 week after METH treatment (O’Dell et al., 1993). These studies support the present observation that blockade of DA receptors prevented METH-induced depletion of DAT sites and TH protein levels in the striatum. Our study extends these observations by demonstrating for the first time that pharmacological blockade of DA receptors with selective antagonists prevented the loss of striatal neurons induced by a high dose of METH.
Histologic (Aizman et al., 2000) and PCR (Surmeier et al., 1996) studies have unequivocally demonstrated the presence of both DA D1 and D2 class of receptors and their respective mRNAs in striatal projection neurons. The conjoint expression of D1 and D2 receptors within the same neuron provides an anatomical site for the interaction of these receptors and their intracellular effectors. It also supports numerous behavioral and electrophysiological studies of DA and dopamimetic drugs that effect a response only when the two receptor subtypes are activated concurrently (Clark and White, 1987). For example, D2 receptor stimulation results in the inhibition of some striatal neurons but only when the D1 receptor is activated concurrently (Hu et al., 1990). The immediate early gene c-fos is regulated by DA and by agents that increase dopaminergic activity in the striatum (Graybiel et al., 1990, Nguyen et al., 1992). Stimulation of either D1 or D2 receptor alone induces marginal induction of c-fos in rat striatal neurons; however, concurrent stimulation by the D1 agonist SKF 38393 and the D2 agonist quinpirole results in robust induction of c-fos immunoreactivity in striatal patches (LaHoste et al., 1993). A separate study in rats also found that concurrent stimulation of D1 and D2 receptors is required to induce striatal c-fos expression as well as stereotypy (Capper-Loup et al., 2002). A pharmacological study also demonstrated that striatal neuropeptide synthesis is under the control of D1 and D2 receptors (Angulo, 1992). The present study demonstrates that blockade of either the D1 receptor with SCH-23390 or the D2 receptor with raclopride suffices to significantly attenuate METH-induced depletion of DAT and TH from the DA-terminals as well as the induction of apoptotic cell death in the striatum of mice. These data suggest that METH-induced striatal injury at both pre- and postsynaptic sites requires the concurrent activation of the D1 and D2 class of DA receptors. It would be interesting to assess in a future study the augmentation of METH-induced striatal damage by selective D1 and D2 agonists.
The majority of studies on METH-induced toxicity in the CNS have focused on monoaminergic pathways. However, recent findings suggest that other systems in addition to monoamines are involved in the development of toxicity and neural degeneration induced by high doses of METH. For example, the neuropeptide substance P mediates METH-induced toxicity of the DA-terminals of the striatum as assessed by the use of selective antagonists of the neurokinin-1 receptor (the substance P receptor) at both neurochemical (Yu et al., 2002) and histologic levels of analysis (Yu et al., 2004). Moreover, it has been demonstrated that METH induces apoptotic cell death in various brain regions including the striatum (Deng et al., 2001, Loonam et al., 2003). We have found that pharmacological blockade of the neurokinin-1 receptor abrogates METH-induced apoptosis in the striatum (manuscript in preparation). Several laboratories have demonstrated that the synthesis of substance P in the striatum is under positive control by DA acting through the D1R (for review see Angulo and McEwen, 1994). We have observed that in the striatum of the mouse brain METH induces endocytosis of the neurokinin-1 receptor into endosomes in interneurons, a process that can be prevented by preexposure to a selective D1R antagonist (SCH-23390) 30 min before METH (manuscript in preparation). This observation suggests that the effects of METH on substance P release and signaling through the striatal neurokinin-1 receptor are downstream of the DA receptors. Experiments in progress in this laboratory are attempting to elucidate this important issue.
The overflow of striatal DA induced by a high dose of METH can affect glutamate transmission by activating striatal glutamate receptors and glutamate overflow from glutamatergic afferents of the striatum. The activation of these intra- and extrastriatal mechanisms may be a consequence of excessive signaling through DA receptors of the striatum. For example, D1Rs of the nucleus accumbens regulate the state of phosphorylation of the NR1 subunit of the NMDA receptor via a protein kinase A mechanism and DARPP-32/protein phosphatase-1 (Snyder et al., 1998). Similarly, D1Rs regulate the state of phosphorylation of the GluR1 subunit of the AMPA receptor by a protein kinase A pathway in postnatal cultures of nucleus accumbens neurons. D1R agonists induce phosphorylation at the protein kinase A site (Ser845) of GluR1 (Chao et al., 2002a). That study also observed that activation of the D2R attenuates D1Rstimulated phosphorylation of GluR1 at Ser845, thus suggesting that the D2R exerts inhibition of GluR1 phosphorylation (Chao et al., 2002a). In another study, the same group discovered that phosphorylation of GluR1 precedes increased surface expression of this subunit of the AMPA receptor in nucleus accumbens cultures (Chao et al., 2002b). Thus METHinduced DA overflow may lead to the activation of intrastriatal mechanisms that enhance the sensitivity of this structure to glutamate transmission.
Extrastriatal mechanisms may also contribute to augment glutamate transmission and exacerbate neurotoxicity in the striatum in the presence of DA overflow induced by METH. For example, pharmacological agents that attenuate glutamate transmission also protect the striatum from the neurotoxic effects of METH (O’Dell et al., 1992; Pu and Vorhees, 1995; Sonsalla et al., 1991; Yamamoto et al. 1998). METH induces a delayed release of glutamate in the striatum, as assessed by in vivo microdialysis (Nash and Yamamoto, 1992). Blockade of DA receptors with haloperidol augments DA overflow but attenuates the METH-induced overflow of glutamate and toxicity of the DA-terminals (Stephans and Yamamoto, 1994). METH induces damage to glutamate neurons of layer III of the parietal cortex (Eisch and Marshall, 1998), suggesting that increased glutamate overflow in the striatum may be due to increased activity in these neurons that project to the striatum and have been shown to be activated by the psychostimulant cocaine (Jimenez-Rivera and Waterhouse, 1991). These studies lend strong support in favor of the glutamate hypothesis of METH-induced toxicity and neural damage in the brain and suggest a causal relationship between METH-induced DA overflow and glutamatergic transmission.
In summary, our data demonstrate that blockade of DA receptors with selective antagonists attenuates the depletion of the DA-terminal markers TH and DAT from a single high dose injection of METH. The same DA receptor antagonists prevent METH-induced cell death in the striatum as assessed by TUNEL staining. The DA receptor blockers SCH-23390 (D1) and raclopride (D2) protect the DA-terminals and the striatal neurons from METH without preventing hyperthermia, thus, suggesting dissociation between METH-induced hyperthermia and stria-tal toxicity. Our results show that both DA D1 and D2 receptor subtypes are needed for the induction of striatal toxicity by METH, since blockade of either receptor subtype was protective. One possible mechanism accounting for these observations involves the neuropeptide substance P acting at a step between the DA receptors and the excessive activity of gluta-mate receptors. Experiments in progress are assessing the mechanism by which the DA receptors affect METH-induced toxicity of the DA-terminals and the death of striatal neurons.
ACKNOWLEDGMENTS
We thank Gertrude Rivera for her help in the preparation of the manuscript. Special thanks are due to Dr. Patricia Stephens for editorial assistance.
Contract grant sponsor: National Institute for Neurological Disorders and Stroke (Specialized Neuroscience Research Program); Contract grant number: NS41073; Contract grant sponsor: National Institute on Drug Abuse; Contract grant number: DA12136 (to J.A.A.); Contract grant sponsor: National Center for Research Resources of the National Institutes of Health (NCRR/NIH, Research Centers in Minority Institutions’ award).
Footnotes
Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res (submitted).
REFERENCES
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Angulo JA. Involvement of dopamine D1 and D2 receptors in the regulation of proenkephalin mRNA abundance in the striatum and accumbens of the rat brain. J Neurochem. 1992;58:1104–1109. doi: 10.1111/j.1471-4159.1992.tb09368.x. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Buening MK, Gibb JW. Influence of methamphetamine and neuroleptic drugs on tyrosine hydroxylase activity. Eur J Pharmacol. 1974;26:30–34. doi: 10.1016/0014-2999(74)90070-3. [DOI] [PubMed] [Google Scholar]
- Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM. Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci. 2002;22:6218–6227. doi: 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002a;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002b;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Yoo TM, Chung SY, Yang JS, Kim JI, Ha ES, Hwang O. Methamphetamine-induced apoptosis in a CNS-derived cat-echolaminergic cell line. Mol Cells. 2002;13:221–227. [PubMed] [Google Scholar]
- Clark D, White FJ. D1 dopamine receptor-the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxic-ity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Kogan FJ. Influence of dopamine synthesis on methamphetamine-induced changes in striatal and adrenal tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:185–187. doi: 10.1007/BF00500283. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in strio-some-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Nairn AC, Girault JA, Ouimet CC, Snyder GL, Fisone G, Allen PB, Fienberg A, Nishi A. The DARPP-32/protein phosphatase-1 cascade: a model for signal integration. Brain Res Brain Res Rev. 1998;26:274–284. doi: 10.1016/s0165-0173(97)00057-x. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Chesselet MF. Apoptosis in Huntington’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:255–265. doi: 10.1016/S0278-5846(03)00021-6. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Hu XT, Wachtel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci. 1990;10:2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Waterhouse BD. Effects of systemically and locally applied cocaine on cerebrocortical neuron responsiveness to afferent synaptic inputs and glutamate. Brain Res. 1991;546:287–296. doi: 10.1016/0006-8993(91)91493-k. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensi-tivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci USA. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev N, Melamed E, Offen D. Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:245–250. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- Liang NY, Rutledge CO. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem Pharmacol. 1982;31:2479–2484. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- Loonam TM, Noailles PA, Yu J, Zhu JP, Angulo JA. Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci. 2003;73:727–739. doi: 10.1016/s0024-3205(03)00393-x. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxyme-thamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. MK-801 prevents methamphetamine-induced striatal dopamine damage and reduces extracellular dopamine overflow. Ann N Y Acad Sci. 1992;648:317–319. doi: 10.1111/j.1749-6632.1992.tb24567.x. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neuro-chem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Pu C, Vorhees CV. Protective effects of MK-801 on methamphetamine-induced depletion of dopaminergic and serotonergic terminals and striatal astrocytic response: an immunohistochemical study. Synapse. 1995;19:97–104. doi: 10.1002/syn.890190205. [DOI] [PubMed] [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979;208:195–202. [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233:539–544. [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J Pharmacol Exp Ther. 1986;238:932–937. [PubMed] [Google Scholar]
- Sonsalla PK, Riordan DE, Heikkila RE. Competitive and noncompetitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J Pharmacol Exp Ther. 1991;256:506–512. [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of do-pamine receptors in neostriatal medium spiny neurons. J Neuro-sci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Gudelsky GA, Stephans SE. Amphetamine neurotoxicity: roles for dopamine, glutamate and oxidative stress. In: Qureshi GA, editor. Neurochemical Markers of Degeneratives Nervous Diseases and Drug Addiction. Netherlands: VSP Press; 1998. pp. 223–244. [Google Scholar]
- Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J Neurochem. 2002;83:613–622. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Cadet JL, Angulo JA. Blockade of neurokinin-1 receptors attenuate methamphetamine-induced TUNEL staining. Society for Neuroscience Abstract. 2002;28:701.9. [Google Scholar]