Abstract
Background
The pathophysiological and neurochemical changes following spinal injury are not yet elucidated. This study was designed to evaluate the morphological changes of the dorsal horn of the spinal cord and profiles of pain behaviors following intraspinal injection of NMDA in rats.
Methods
Rats were randomized into three groups: a sham-operated control group and groups where the rats received 10 mM or 100 mM N-methyl-D-aspatate (NMDA) injected into their spinal dorsal horn. Following injection, hypersensitivity to cold and mechanical stimuli and excessive grooming behaviors were assessed serially for four weeks. Morphological changes of the spinal cord were evaluated four weeks after intraspinal injection.
Results
Few animals in the NMDA groups developed hypersensitivity to cold and mechanical stimuli. The number of groomers and the severity of excessive grooming were significantly higher in the 100 mM NMDA group than those values of the control and 10 mM NMDA groups. The size of the neck region (lamina III-IV) was significantly smaller in the 100 mM NMDA group than in the control and 10 mM NMDA groups.
Conclusions
In conclusion, intraspinal injection of NMDA in rats leads to the pathological sequela in the spinal cord and to excessive grooming behavior. These results support the use of NMDA and excessive grooming behavior after excitotoxic SCI as a model to study chronic pain after SCI.
Keywords: central pain, NMDA, rat, spinal cord injury
INTRODUCTION
Pain following spinal cord injury (SCI) is a major challenge for patients coping with the physical and life-threatening consequences of SCI [1]. For the development of more effective treatments for pain of spinal origin, the pathophysiological and neurochemical changes following spinal injury should be clearly illustrated.
An important consideration in the study of injury-induced spinal pain using an animal model is the pathological and/or behavioral responses associated with human SCI. Numerous experimental models, including photochemical [2], hemisection [3], contusion [4] and clip compression [5] models, have been developed to explicate the complex pathophysiological mechanisms of spinal cord injury. Although these models share many pathological characteristics with the human condition, the specific neural substrates responsible for the injury-induced abnormal sensations are not yet elucidated. Based on previous studies documenting the elevated excitatory amino acid levels commonly associated with spinal injury [6,7], Yezierski et al. [8] injected the AMPA-metabotropic receptor agonist quisqualic acid into the grey matter of the spinal cord in an effort to simulate injury-induced elevations of excitatory amino acids. They reported that intraspinal injections of the AMPA-metabotropic receptor agonist quisqualic acid induce the progressive pathological sequela resembling the cascade of events described following ischemic and traumatic SCI, and that the morphological changes of the spinal cord following intraspinal injection correlate with the spontaneous and evoked pain behaviors seen in the central neuropathic pain patients. Moreover, it has also been demonstrated that the injection of N-methyl-D-aspartate (NMDA) into the ventral horn of the spinal cord induces intense neuronal degeneration and acute inflammation in both the grey and white matter [9].
The present study was designed to evaluate the morphological changes of the dorsal horn of the spinal cord and profiles of pain behaviors following intraspinal injection of NMDA in rats.
MATERIALS AND METHODS
This study was performed with the approval of the Institutional Animal Care and Use Committee, and in accordance with NIH guidelines for the care and use of laboratory animals. Adult male Sprague-Dawley rats weighing 180-200 g were used. The rats were housed at a constant humidity and temperature, with a 12-hour light/dark cycle and free access to food and water.
1. Intraspinal injections
Rats were randomized into three groups (eight rats per group): a sham-operated control group, and groups receiving 10 mM or 100 mM of N-methyl-D-aspatate. Anesthetized rats with zoletil 12.5 mg and xylazine 3 mg intraperitoneally were immobilized with a hip bar. Spontaneous respiration was maintained and the rats were shaved, scrubbed with betadine, and wiped with 70% alcohol. Core body temperature was monitored using a rectal probe and was maintained at 37.0 ± 0.5℃ through employment of a heating pad (Homeothermic Blanket System, Harvard Apparatus Inc., USA).
After a midline incision, the spinous process and vertebral laminae of L1 were removed. For intraspinal injections, we used a 34 g beveled NanoFil needle (WPI, Sarasota, USA) attached to a Hamilton syringe (volume, 5 µl) mounted with a micromanipulator. Intraspinal injections were made into the left side of the spinal dorsal horn between the dorsal vein and dorsal root entry zone at a depth of 1,000 µm below the spinal cord surface. Stock solutions of 100 mM NMDA (Sigma, St. Louis, MO) were made using sterile saline. Animals of the 10 mM and 100 mM NMDA groups were injected with 1.2 µl of 10 mM or 100 mM NMDA, respectively, over a twenty-second interval (three tracks of 0.4 µl separated by 0.3 mm parallel to the long axis of the cord). The animals of the control group received the same operation without injection. After injections, muscles were sutured, the skin was closed, and the animals were returned to their home cages.
2. Behavioral testing
All animals were evaluated three days prior to intraspinal injections in order to establish baseline responses to mechanical and cold stimuli. Post-injection testing started seven days after intraspinal injection and continued for four weeks. All testing was done by one examiner 'blind' to the injection strategies. Due to the nature of responses required for behavioral evaluations, animals experiencing signs of post-injection motor dysfunction, e.g. hindlimb paresis and/or paralysis, were excluded from behavioral evaluations.
For the assessment of cold allodynia, the rat was placed under a transparent plastic dome on a metal mesh floor and acetone was applied to the plantar surface of the hind paw. An acetone bubble that formed at the end of a piece of small polyethylene tubing connected to a syringe was in contact with the heel. Acetone was applied five times to each paw at intervals of five minutes. A prompt foot withdrawal response to the acetone application was interpreted as a sign of cold allodynia. The frequency of paw withdrawal was expressed as a percentage (the number of paw withdrawals divided by the total number of trials, times 100).
Response to mechanical stimuli was tested with calibrated von Frey filaments. Stimulus intensities ranging from 0.5 to 50 g were applied six times to the glabrous skin of each hind paw while the animals were standing on an elevated screen. Filaments were applied to the point of bending, at which point evidence of responsiveness or non-responsiveness was determined. During each test session, the filament producing a threshold response, i.e. 50%, in each animal was determined for the left hind paws. Positive responses included withdrawal, licking and/or vocalizations.
After intraspinal injection, the animals were inspected weekly for signs of excessive grooming (removal of hair, superficial skin damage) for four weeks. Excessive grooming behavior was a progressive condition and the severity of grooming was categorized into four classes (I-IV) as described previously [10]: (a) Class I: hair removal over contiguous portions of a dermatome; (b) Class II: extensive hair removal combined with signs of damage to the superficial layers of skin; (c) Class III: hair removal and damage to dermal layers of skin; and (d) Class IV: subcutaneous tissue damage.
3. Tissue processing and analysis of histological damage
At the end of the four-week survival periods, animals were anesthetized with zoletil 12.5 mg and xylazine 3 mg intraperitoneally and perfused transcardially with 4% buffered paraformaldehyde. Spinal segments containing microinjection sites were sectioned at 10 um, after paraffin imbedding. Sections were stained with 0.1% cresyl violet solution and mounted with permanent mounting medium. Sections were examined with light microscopy and reconstructions of damaged areas were made with the aid of an overhead projector and camera lucida. We measured the size of spinal gray matter in two regions, superficial (lamina I and II) and neck (lamina III to V) by examining three serial transverse sections through the epicenter of injection sites using an Image Analysis System (Image J software, Universal Imaging Corp. USA). This analysis was conducted by an examiner blind to the behavioral results and injection protocols. Tissue blocks with mechanical damage due to histological processing were excluded from this analysis.
4. Data analysis
Response to mechanical and cold stimuli and the size of spinal grey matter were expressed as mean ± SE and mean ± SD, respectively. Significant differences were evaluated with analysis of variance followed by the Turkey-Kramer multiple comparisons. The number of animals that developed cold, mechanical allodynia and excessive grooming behaviors were compared with the χ2 test and Fisher's exact test among the three treatment groups. For analysis of the severity of grooming, the non-parametric Kruskal-Wallis test was used for multiple comparisons, followed by the Mann-Whitney U-test to compare individual groups. A P value less than 0.05 was considered significant.
RESULTS
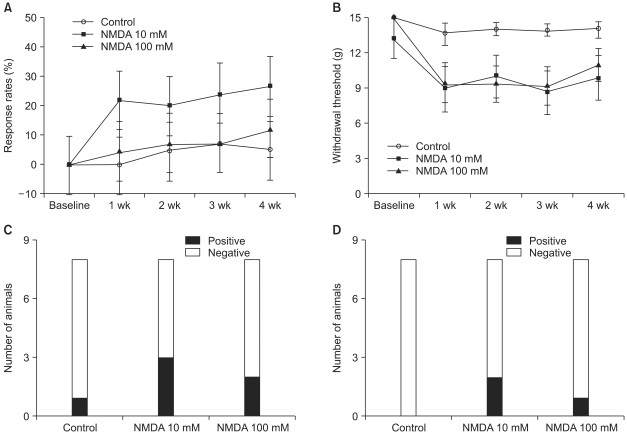
The percentage of the response rate to cold stimuli was not influenced by intraspinal injections of NMDA during the four-week observation period (Fig. 1A). The paw withdrawal threshold was lower in the NMDA groups after intraspinal injection when compared with the sham-operated control group, but there was no statistical significance (0.05 < P < 0.07, Fig. 1B). The number of animals that developed cold allodynia and mechanical allodynia (withdrawal threshold less than 5 g) was not significantly different (Fig. 1C, D).
Fig. 1.
The effects of intraspinal N-methyl-D-aspartic acid (NMDA) injection and sham surgery (control) on the responses to cold and mechanical stimuli delivered to the hind paws. Baseline responses were measured 3 days prior to the surgery done for intraspinal injection. Cold allodynia assessed with acetone was represented as percent response rates (A), and animals showing sustained sign of cold allodynia during the observation periods were interpreted as positive (C). Mechanical allodynia tested with calibrated von Frey filaments was expressed as withdrawal threshold (B), and animals that whose measured withdrawal threshold was less than 4 g at every testing were interpreted as positive (D). Responses to cold and mechanical stimuli were not influenced by intraspinaly injected NMDA. Data are expressed as mean ± SE or number of animals.
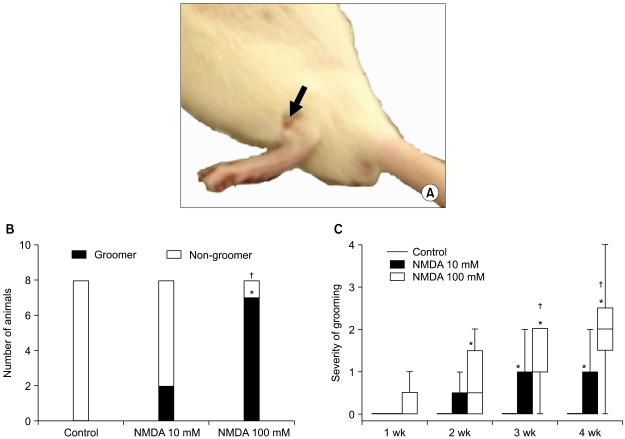
Excessive grooming targeted caudally or at the site of the injected dermatome started with excessive biting and scratching of the skin. In the early stages, it was accompanied with hair removal (Class I) and progressed over time to damage to the superficial (Class II) and dermal (Class III) layers of skin, and, on occasion, to damage to subcutaneous tissue (Class IV) (Fig. 2A). This behavior was observed in two out of eight rats in the 10 mM NMDA group, in seven out of eight rats in the 100 mM NMDA group but in none within the control group. There was a statistically significant difference between the two NMDA groups (P < 0.01, Fig. 2B). The severity of grooming behavior was significantly higher in the two NMDA groups than in the sham-operated control group (P < 0.01). Another finding in the severity of grooming behavior was that it was significantly higher in the 100 mM NMDA group than in the 10 mM NMDA group (P < 0.05; Kruskal-Wallis test with Mann-Whitney post hoc test) (Fig. 2C).
Fig. 2.
Excessive grooming behaviors. (A) An example of phase II grooming targeted dermatome at the injection site in a 100 mM NMDA injected rat is demonstrated (black arrow). (B) The number of animals that developed excessive grooming behavior was significantly higher in the 100 mM NMDA group than those values in the 10 mM NMDA or the sham operated control groups. (C) The severity of excessive grooming in the animals of the 100 mM NMDA group was higher than those values of control and 10 mM NMDA group. In (C) boxes show interquartile ranges and the bars are the 10th and 90th percentiles. *P < 0.05 compared with control group. †P < 0.05 compared with 10 mM NMDA group.
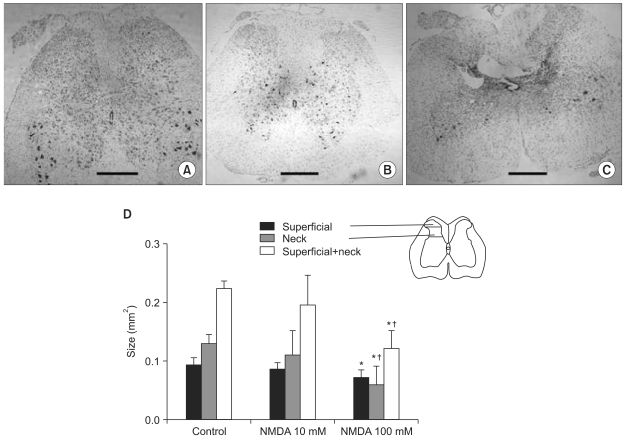
On the spinal cord sections of the 100 mM NMDA group prepared after the four-week survival periods, dilation of central canal and ipsilateral neuronal loss in the lamina III-V (neck of the dorsal horn) and intraspinal cavities were observed (Fig. 3A-C). The size of the neck in the 100 mM NMDA group, but not superficial laminae, was significantly smaller compared with those values of the control and 10 mM NMDA groups (Fig. 3D, P < 0.05)
Fig. 3.
Morphological changes of the spinal cord. Examples of the spinal cord sections of the sham-operated control (A) and morphological spinal cord damage following unilateral intraspinal injections of NMDA (B, C) are demonstrated. Dilation of central canal, ipsilateral neuronal loss of lamina III-V (neck) and intraspinal cavities were developed when NMDA was injected at a concentration of 100 mM (C). The estimated size of the dorsal horn of the spinal grey matter was significantly smaller in the 100 mM NMDA group compared with the control and 10 mM NMDA groups (D). Scale bars in (A-C), 500 um. Inset in (D) is a schematic representation on a standard cross-sectional drawing of the spinal cord on how the size of superficial and neck regions is measured. Values are mean ± SD. *P < 0.05 compared with the control and 10 mM NMDA groups. †P < 0.05 compared with 10 mM NMDA group.
DISCUSSION
The purpose of this study was to evaluate the morphological changes of the dorsal horn of the spinal cord and profiles of pain behaviors following intraspinal injection of NMDA in rats. A major finding of the present study is that intrarspinal injection of NMDA induced excessive grooming behaviors and intense neuronal degeneration in the ipsilateral dorsal horn of the spinal cord. The histopathological findings were similar to those previously reported quisqualic acid-induced excitotoxic damage to the spinal cord [2,11]. Although the incidence of mechanical and cold allodynia following intraspinal injection was low, excessive grooming behavior commonly seen in animals suffering from experimental central neuropathic pain was evident, especially in the 100 mM NMDA group. On the spinal cord sections of the 100 mM NMDA group prepared after four-week survival periods, dilation of the central canal and formation of intraspinal cavities were observed. Intraspinal NMDA-induced neuronal loss occurred mainly at the lamina III-V. The present data suggest the existence of a causal relationship between the neuronal loss of the dorsal horn and the evoked/spontaneous pain-like behavior.
Concentrated excitatory amino acids following trauma or ischemia can lead to a prolonged period of depolarization and the initiation of a cellular cascade that ultimately results in neuronal death. Both NMDA and non-NMDA receptors contribute to the excitotoxic effects of elevated glutamate levels in the spinal cord [12]. Several classes of excitatory amino acid receptors are thought to be involved in glutamate excitotoxicity, including NMDA, AMPA, kainate and metabotropic receptors [13]. Ionotropic receptors regulate the opening of voltage-gated ion channels, and metabotropic receptors are linked via G-proteins to phospholipase C. Glutamate-induced cell death that results from NMDA ionotropic receptor activation is well documented as largely due to the excessive influx of sodium and calcium ions [14]. The role of non-NMDA receptors in the injury of spinal neurons has been studied with a simulated excitotoxic spinal cord injury model using intraspinal quisqualic acid injection. The results suggest that the specific non-NMDA receptor agonist AMPA produces histological changes similar to those observed following quisqualic acid injections [8].
NMDA receptors are present on the endothelial cells of grey matter vasculature and damage to blood vessels in the grey matter can induce damage to the adjacent white matter [15]. NMDA molecules can bind to receptors in the endothelial cells, inducing blood-brain barrier breakdown, acute inflammation and tissue damage. The expansion of the primary necrotic area is a secondary pathological phenomenon involving a multitude of mechanisms, including pathological release of excitatory amino acids, free radical formation, nitric oxide synthesis, and inflammation [9]. Neuronal degeneration and activated macrophages/microglia in the grey matter could also release cytokines and other diffusible factors, such as nitric oxide, which can induce damage to white matter or potentiate deleterious tissue damage by pathological activation of non-NMDA receptors [16].
In the present study, excessive grooming behavior correlated with a lesion sparing the superficial laminae (lamina I-II, superficial in Fig. 3) and neuronal damage in the neck of the dorsal horn (lamina III-IV, neck in Fig. 3). These results are consistent with those of the previous study, documenting that destruction of lamina I cells can prevent or eliminate excessive grooming behavior [17]. Pain associated with spinal cord injury can be divided into nociceptive (including musculoskeletal and visceral) and neuropathic (including above-, at- and below-level) [1]. The primary characteristic of at-level pain is a hypersensitive band of skin in dermatomes associated with spinal segments adjacent to the site of injury. Injury-induced loss of intrinsic inhibitory control and changes in the functional state of neurons adjacent to the site of injury represent the current view of a mechanism responsible for this condition [17]. Consistent with the clinical profile of at-level pain, hypersensitivity to thermal and mechanical stimuli are characteristics of the area targeted for excessive grooming behavior. Pain can be present in NMDA-injected animals without excessive grooming behavior, as evidenced by the finding that a few of the injected animals developed cold and mechanical allodynia below the level of injury without any signs of excessive grooming. In this study, efforts to correlate the pattern of neuronal loss with the development of hypersensitivity to cold and mechanical stimuli proved unsuccessful. It can be postulated that cold and mechanical allodynia were not developed due to somatosensory dysfunction associated with NMDA-induced injury to the white matter and death of the entire primary afferent neuron as a consequence of the central nervous system lesion [18].
In conclusion, intraspinal injection of NMDA leads to the pathological sequela in the spinal cord and to excessive grooming behavior. The morphological changes of the spinal cord resemble the cascade of events described following ischemic and traumatic SCI. Excessive grooming behavior was associated with neuronal damage in the neck of the spinal dorsal horn and with sparing the superficial laminae. These results support the use of NMDA and excessive grooming behavior to study central neuropathic pain following SCI.
References
- 1.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- 3.Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- 5.Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Exp Neurol. 2002;178:33–48. doi: 10.1006/exnr.2002.8026. [DOI] [PubMed] [Google Scholar]
- 6.Simpson RK, Jr, Robertson CS, Goodman JC. Spinal cord ischemia-induced elevation of amino acids: extracellular measurement with microdialysis. Neurochem Res. 1990;15:635–639. doi: 10.1007/BF00973755. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- 8.Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 9.Gomes-Leal W, Corkill DJ, Freire MA, Picanço-Diniz CW, Perry VH. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Exp Neurol. 2004;190:456–467. doi: 10.1016/j.expneurol.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Gorman AL, Yu CG, Ruenes GR, Daniels L, Yezierski RP. Conditions affecting the onset, severity, and progression of a spontaneous pain-like behavior after excitotoxic spinal cord injury. J Pain. 2001;2:229–240. doi: 10.1054/jpai.2001.22788. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Ruenes GL, Yezierski RP. NMDA and non-NMDA receptor antagonists protect against excitotoxic injury in the rat spinal cord. Brain Res. 1997;756:160–167. doi: 10.1016/s0006-8993(97)00137-6. [DOI] [PubMed] [Google Scholar]
- 12.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 14.Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 16.Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 17.Yezierski RP, Yu CG, Mantyh PW, Vierck CJ, Lappi DA. Spinal neurons involved in the generation of at-level pain following spinal injury in the rat. Neurosci Lett. 2004;361:232–236. doi: 10.1016/j.neulet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Brewer KL, Lee JW, Downs H, Oaklander AL, Yezierski RP. Dermatomal scratching after intramedullary quisqualate injection: correlation with cutaneous denervation. J Pain. 2008;9:999–1005. doi: 10.1016/j.jpain.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]