Abstract
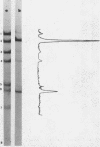
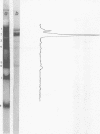
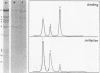
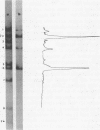
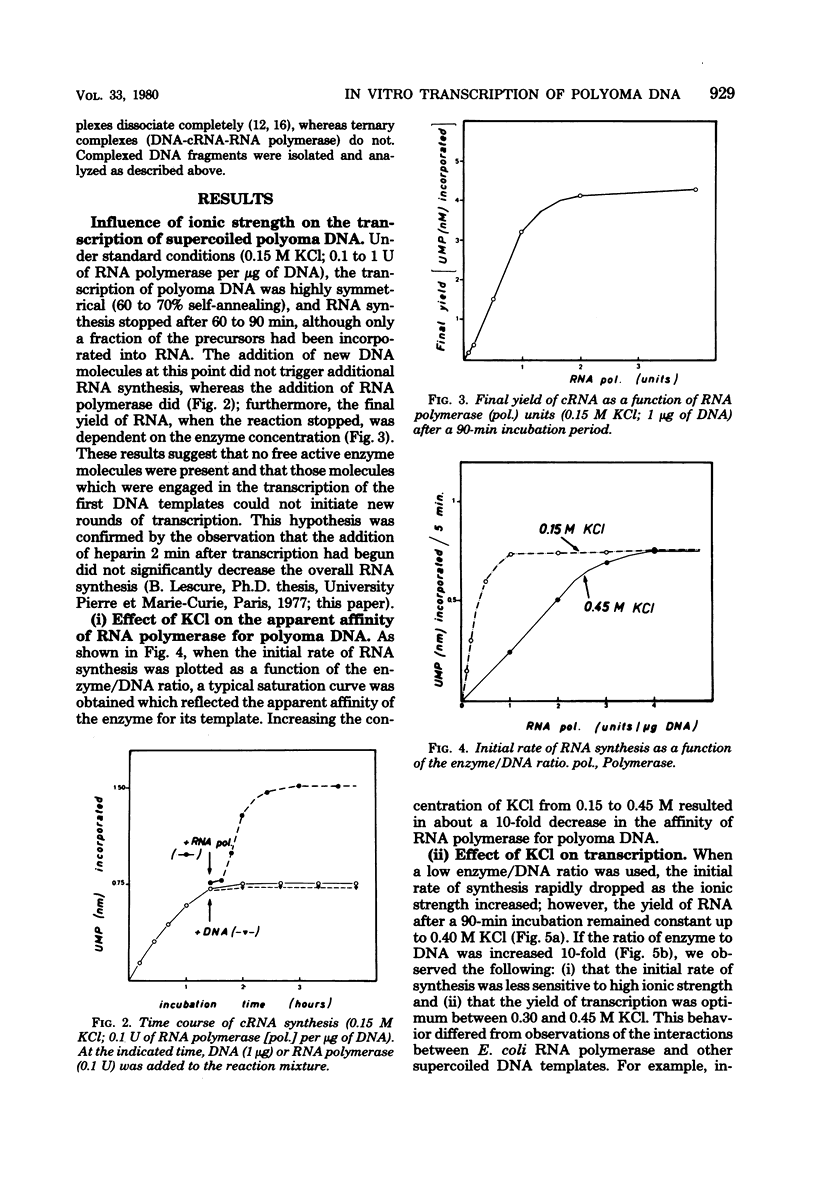
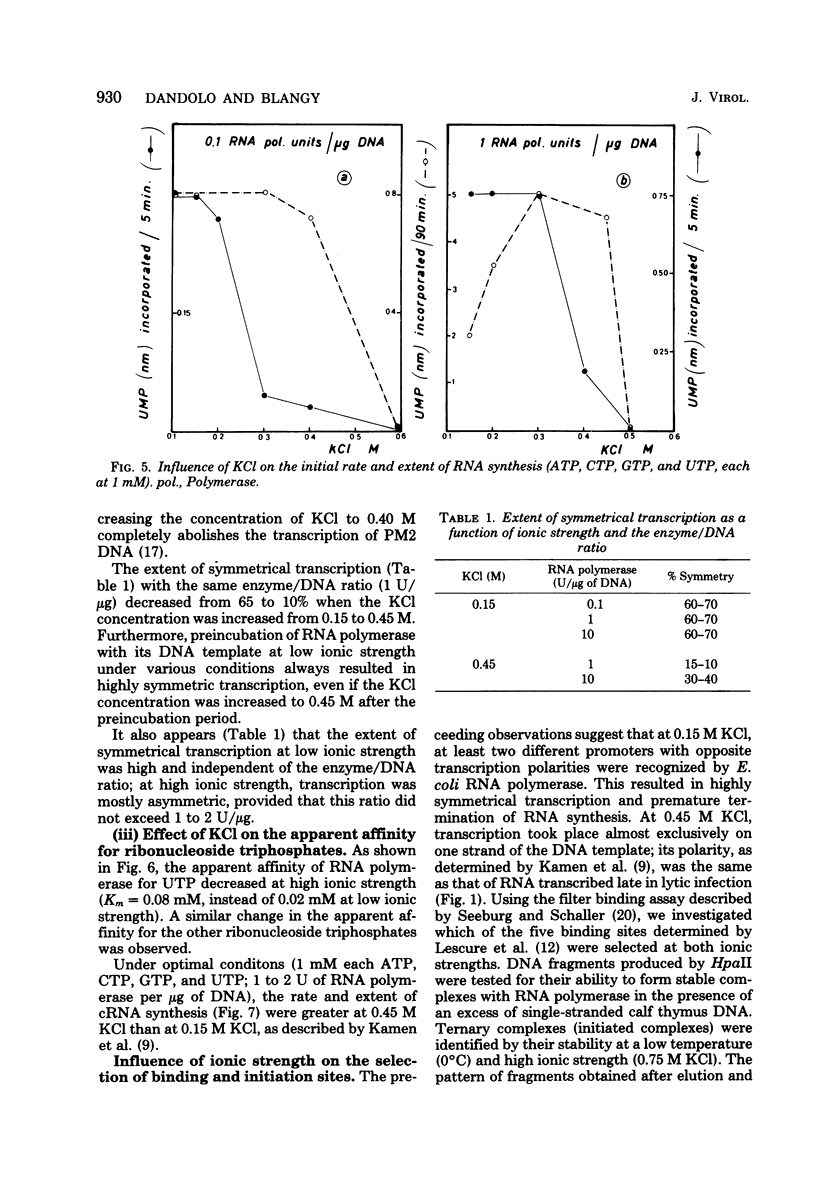
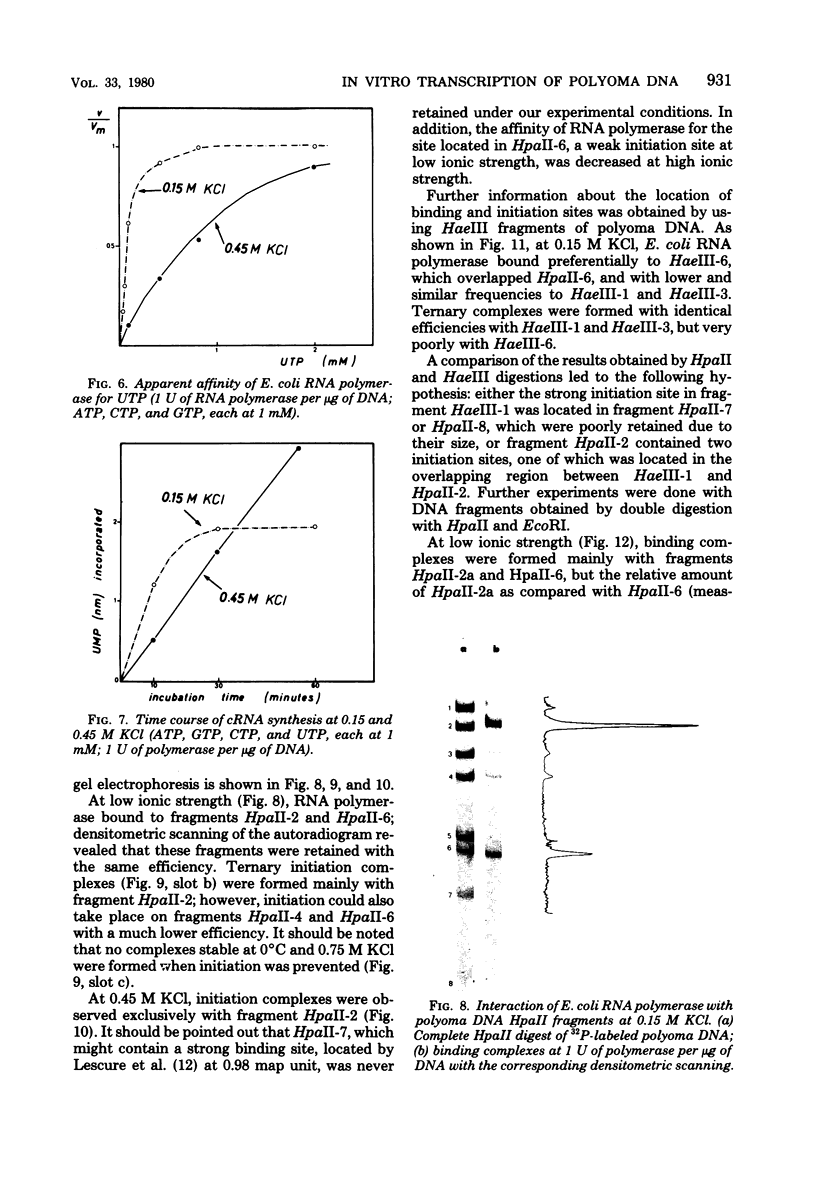
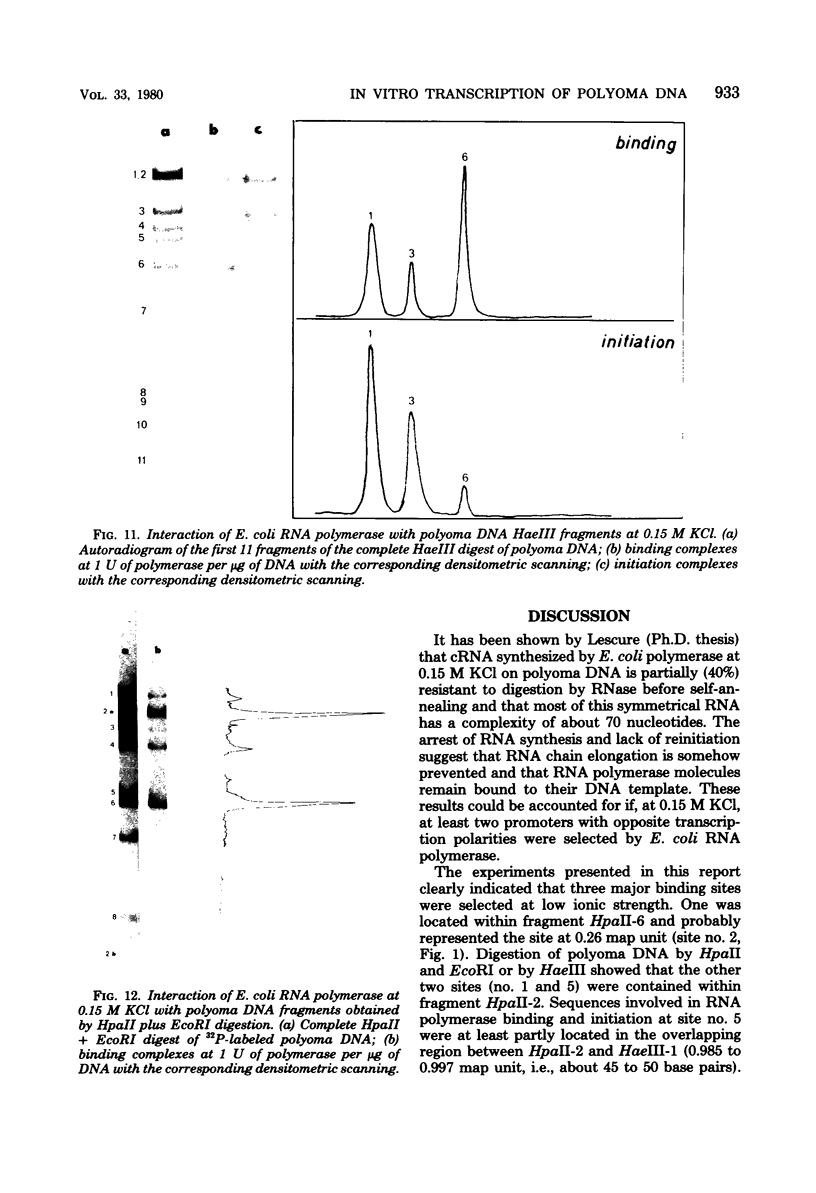
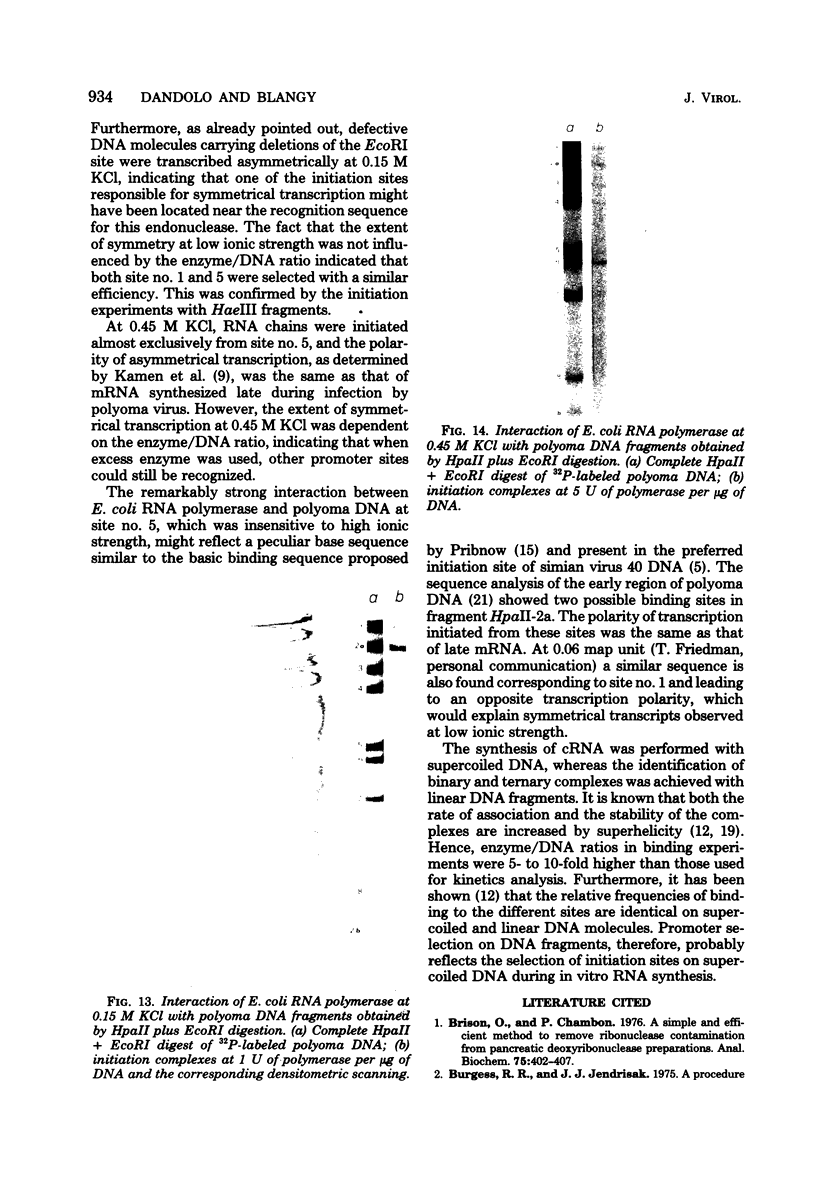
The influence of ionic strength on transcription of polyoma DNA by Escherichia coli RNA polymerase was investigated. At 0.15 M KCl, transcription was highly symmetrical and, due to the lack of reinitiation, a limited extent of RNA synthesis was observed. When the concentration of KCl was raised to 0.45 M, the affinity of the enzyme for its template, as well as its apparent affinity for ribonucleoside triphosphates, was reduced. Under optimal conditions, the rate and extent of RNA synthesis at 0.45 M KCl were greater than at 0.15 M KCl, and transcription was mostly asymmetric. Binding and initiation sites at both ionic strengths were identified; at 0.15 M KCl, transcription was initiated from two major sites, located at 0.99 and 0.06 map unit, whereas at 0.45 M KCl, a unique initiation site, at 0.99 map unit, was selected by RNA polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brison O., Cambon P. A simple and efficient method to remove ribonuclease contamination from pancreatic deoxyribonuclease preparations. Anal Biochem. 1976 Oct;75(2):402–409. doi: 10.1016/0003-2697(76)90094-4. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Delius H., Westphal H., Axelrod N. Length measurements of RNA synthesized in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1973 Mar 15;74(4):677–687. doi: 10.1016/0022-2836(73)90056-9. [DOI] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Zain S., Weissman S. M., Pan J., Subramanian K. Nucleotide sequences of RNA transcribed in infected cells and by Escherichia coli RNA polymerase from a segment of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):371–375. doi: 10.1073/pnas.71.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
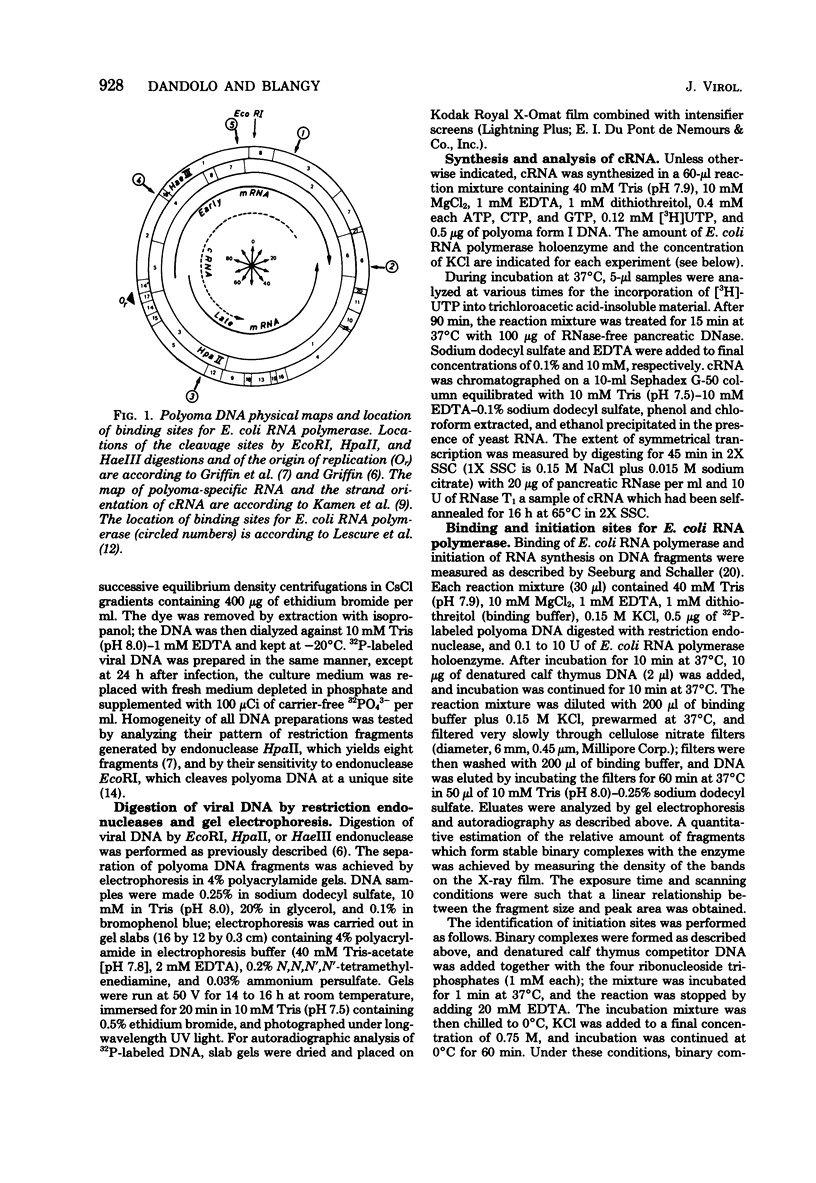
- Griffin B. E. Fine structure of polyoma virus DNA. J Mol Biol. 1977 Dec 5;117(2):447–471. doi: 10.1016/0022-2836(77)90137-1. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz P., Stern R., Ghosh P. K., Weissman S. M. Specificity of initiation of transcription of simian virus 40 DNA I by Escherichia coli RNA polymerase: identification and localization of five sites for initiation with [gamma-32P]ATP. J Virol. 1977 May;22(2):430–445. doi: 10.1128/jvi.22.2.430-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure B., Oudet P., Chambon P., Yaniv M. Transcription of polyoma virus DNA in vitro. Localization of Escherichia coli RNA polymerase initiation sites. J Mol Biol. 1976 Nov;108(1):83–97. doi: 10.1016/s0022-2836(76)80096-4. [DOI] [PubMed] [Google Scholar]
- Lindstrom D. M., Dulbecco R. Strand orientation of simian virus 40 transcription in productively infected cells. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1517–1520. doi: 10.1073/pnas.69.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Effects of supercoiling on transcription from bacteriophage PM2 deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3164–3169. doi: 10.1021/bi00712a025. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. The binding of RNA polymerase to DNA. J Mol Biol. 1966 Oct 28;21(1):83–114. doi: 10.1016/0022-2836(66)90081-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Dhar R., Weissman S. M., Lebowitz P., Lewis A. M., Jr Preferred site for initiation of RNA transcription by Escherichia coli RNA polymerase within the simian virus 40 DNA segment of the nondefective adenovirus-simian virus 40 hybrid viruses Ad2 + ND 1 and Ad2 + ND 3 . J Virol. 1973 May;11(5):682–693. doi: 10.1128/jvi.11.5.682-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain B. S., Weissman S. M., Dhar R., Pan J. The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 1. The sequence of the late strand transcript. Nucleic Acids Res. 1974 Apr;1(4):577–594. doi: 10.1093/nar/1.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]