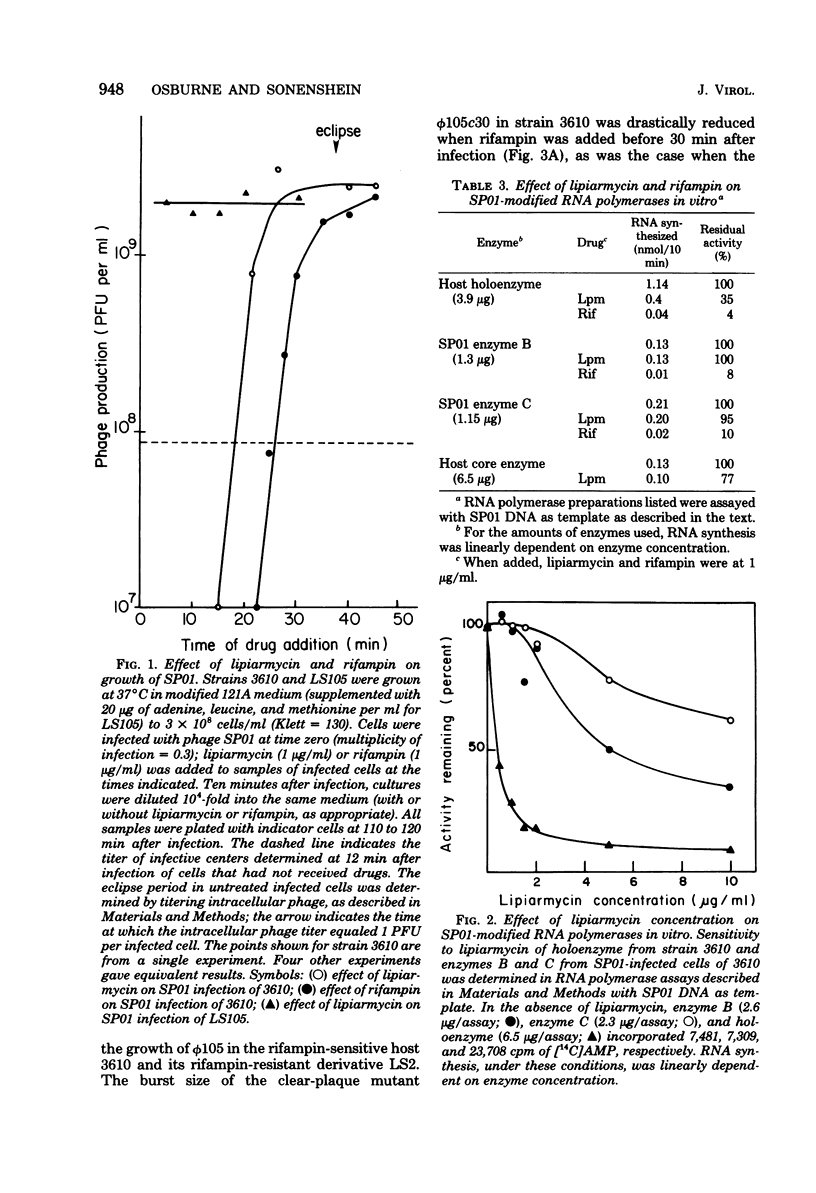
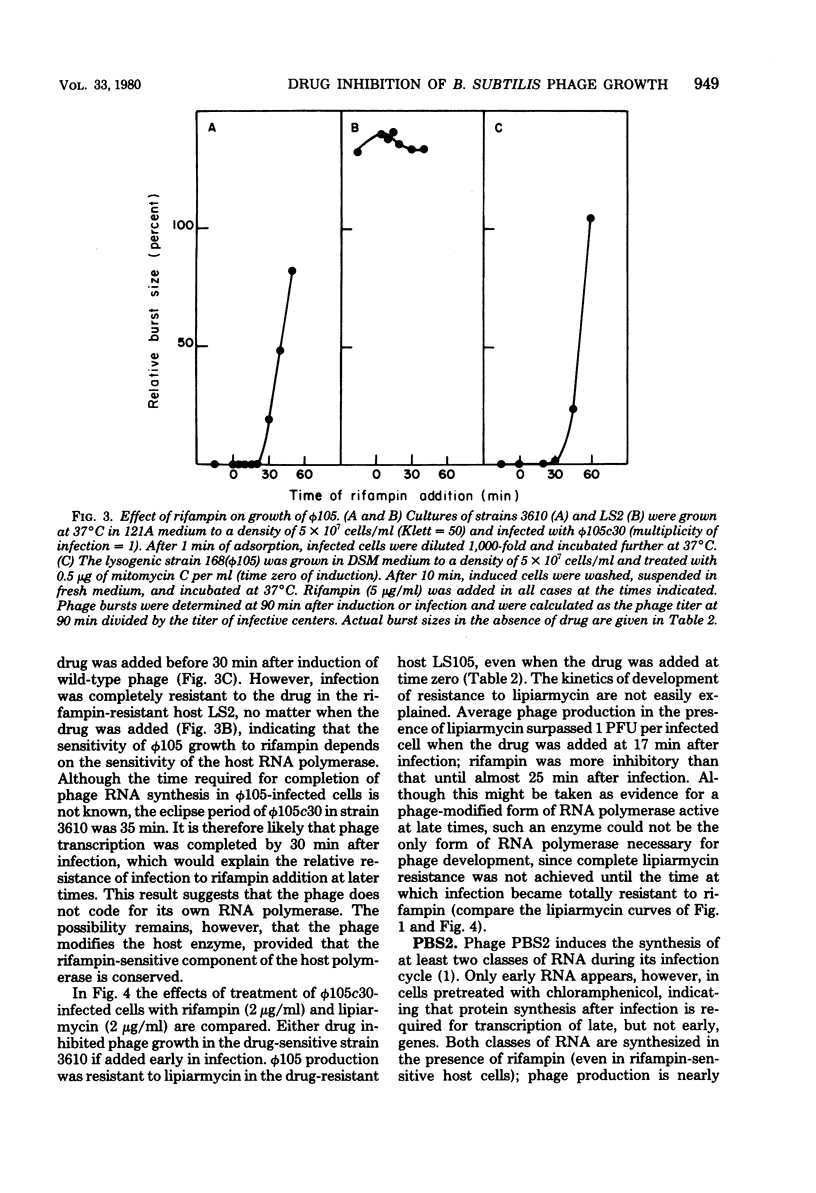
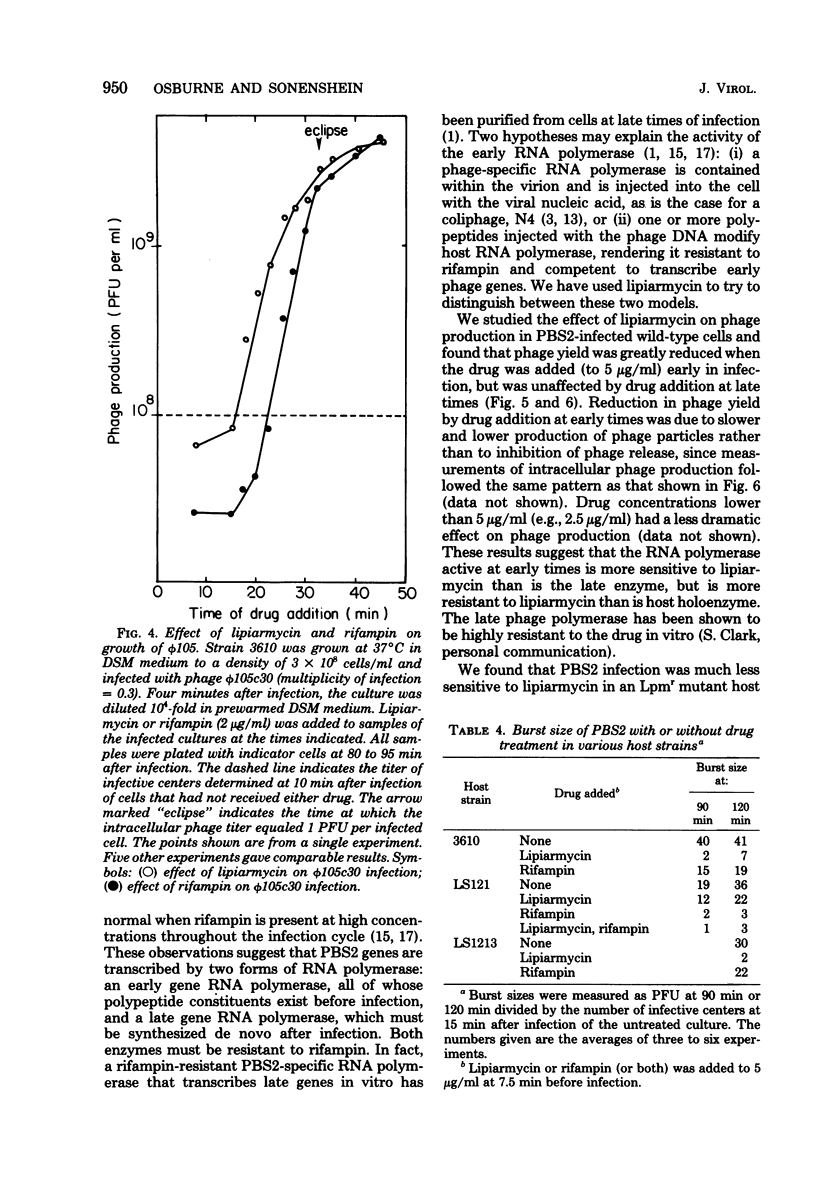
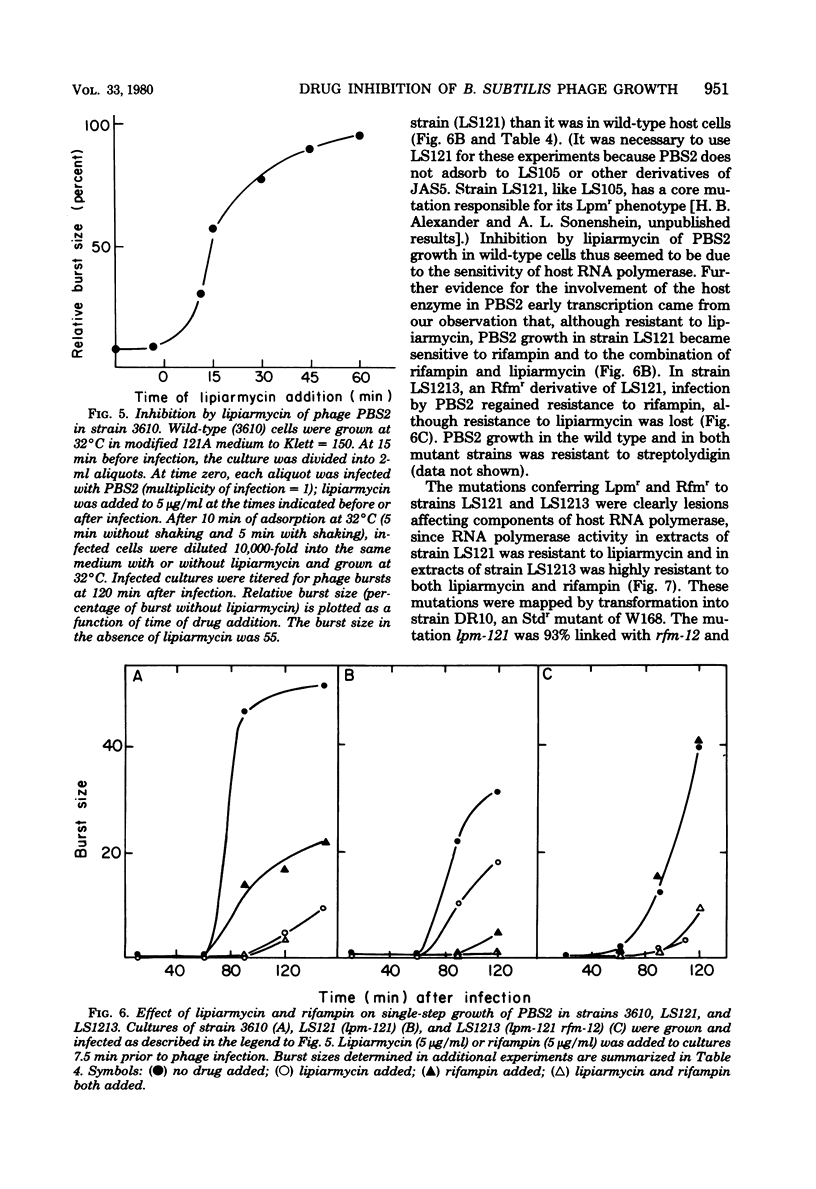
Abstract
We have used lipiarmycin, a specific inhibitor of initiation of transcription, to study the role of host RNA polymerase in the transcription programs of various phages of Bacillus subtilis. Unlike rifampin, lipiarmycin preferentially inhibits transcription dependent on the sigma subunit of RNA polymerase because it inactivates holoenzyme at a much greater rate than it does core enzyme. With phage SP01, addition of lipiarmycin at a middle-to-late time of infection did not inhibit phage production even though phage production was sensitive to addition of rifampin at that time. This result is consistent with the notion that unmodified host RNA polymerase holoenzyme becomes dispensable after transcription of early classes of SP01 genes, even though host core enzyme is required for synthesis of all classes of phage RNA. SP01-modified forms of RNA polymerase, which lack sigma subunit but contain phage-coded polypeptides and are able to transcribe middle and late genes, were resistant to lipiarmycin in vitro. For phage phi 105, phage development was sensitive to both lipiarmycin and rifampin in wild-type cells and resistant to both drugs in resistant mutant cells, leading to the conclusion that the activity of host holoenzyme was required for phage RNA synthesis. Growth of phage PBS2, which was resistant to rifampin, was sensitive to the addition of lipiarmycin at early times of infection of a wild-type host strain. In a lipiarmycin-resistant mutant host, PBS2 growth was resistant to lipiarmycin. This result suggests that host holoenzyme plays a previously unanticipated role in transcription of PBS2 genes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark S. Transcriptional specificity of a multisubunit RNA polymerase induced by Bacillus subtilis bacteriophage PBS2. J Virol. 1978 Jan;25(1):224–237. doi: 10.1128/jvi.25.1.224-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronelli C., White R. J., Lancini G. C., Parenti F. Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot (Tokyo) 1975 Apr;28(4):253–259. doi: 10.7164/antibiotics.28.253. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Zivin R., Rothman-Denes L. B. Novel template requirements of N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3220–3224. doi: 10.1073/pnas.75.7.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Ohlsson-Wilhelm B. M., Geiduschek E. P. Transcription during bacteriophage SPO1 development: mutations affecting the program of viral transcription. J Mol Biol. 1971 Apr 28;57(2):301–317. doi: 10.1016/0022-2836(71)90348-2. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. Reconstitution studies show that rifampicin resistance is determined by the largest polypeptide of Bacillus subtilis RNA polymerase. J Biol Chem. 1977 Dec 25;252(24):9024–9031. [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. beta' subunit of bacterial RNA polymerase is responsible for streptolydigin resistance in Bacillus subtilis. Nature. 1978 Apr 27;272(5656):837–839. doi: 10.1038/272837a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Osburne M. S., Sonenshein A. L. Behavior of a temperate bacteriophage in differentiating cells of Bacillus subtilis. J Virol. 1976 Jul;19(1):26–35. doi: 10.1128/jvi.19.1.26-35.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Tjian R., Nelson J., Losick R. In vitro transcription of a late class of phage SP01 genes. Nature. 1975 Sep 18;257(5523):248–251. doi: 10.1038/257248a0. [DOI] [PubMed] [Google Scholar]
- Pesce A., Casoli C., Schito G. C. Selectivity of transcription and structure of coliphage N4 virion-associated RNA polymerase. Biochem Biophys Res Commun. 1978 Jun 14;82(3):1040–1048. doi: 10.1016/0006-291x(78)90888-4. [DOI] [PubMed] [Google Scholar]
- Price A. R., Frabotta M. Resistance of bacteriophage PBS2 infection to rifampicin, an inhibitor of Bacillus subtilis RNA synthesis. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1578–1585. doi: 10.1016/0006-291x(72)90894-7. [DOI] [PubMed] [Google Scholar]
- Reeve J. N. Bacteriophage infection of minicells: a general method for identification of "in vivo" bacteriophage directed polypeptide biosynthesis. Mol Gen Genet. 1977 Dec 14;158(1):73–79. doi: 10.1007/BF00455121. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Takahashi I. The synthesis of nucleic acids in Bacillus subtilis infected with phage PBS 1. Can J Biochem. 1973 Sep;51(9):1219–1224. doi: 10.1139/o73-161. [DOI] [PubMed] [Google Scholar]
- Sergio S., Pirali G., White R., Parenti F. Lipiarmycin, a new antibiotic from Actinoplanes III. Mechanism of action. J Antibiot (Tokyo) 1975 Jul;28(7):543–549. doi: 10.7164/antibiotics.28.543. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Dean D. H., Halvorson H. O. Low-frequency specialized transduction with Bacillus subtilis bacteriophage phi 105. Virology. 1974 Dec;62(2):393–403. doi: 10.1016/0042-6822(74)90401-2. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Alexander H. B. Initiation of transcription in vitro inhibited by lipiarmycin. J Mol Biol. 1979 Jan 5;127(1):55–72. doi: 10.1016/0022-2836(79)90459-5. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Alexander H. B., Rothstein D. M., Fisher S. H. Lipiarmycin-resistant ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):73–79. doi: 10.1128/jb.132.1.73-79.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Talkington C., Pero J. Restriction fragment analysis of the temporal program of bacteriophage SPO1 transcription and its control by phage-modified RNA polymerases. Virology. 1977 Dec;83(2):365–379. doi: 10.1016/0042-6822(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Tijan R., Pero J. Bacteriophage SP01 regulatory proteins directing late gene transcription in vitro. Nature. 1976 Aug 26;262(5571):753–757. doi: 10.1038/262753a0. [DOI] [PubMed] [Google Scholar]



