Abstract
Purpose of review
This review summarizes research literature regarding mucosal immunity to HIV and SIV, with an emphasis on work published within the past 18 months.
Recent findings
Notable recent studies have focused on the pivotal events occurring within mucosal tissues during acute HIV/SIV infection that serve to establish a balance between detrimental immune activation and beneficial adaptive responses. In cervicovaginal mucosa, an early inflammatory response leads to recruitment of susceptible target cells. At this acute stage, the in vivo ratio between CD8+ effector cells and infected CD4+ T-cells may be critical for limiting viral dissemination. Acute infection is also accompanied by loss of germinal center architecture and T/B cell apoptosis in Peyer’s patches of the gastrointestinal tract. During chronic infection, mucosal CD8+ T-cells may play a role in immune control, as suggested by studies of elite controllers.
Summary
Mucosal tissues serve as the major portal of entry for HIV, and house a majority of the body’s lymphocytes, including CD4+ T-cells that are targets for infection. Recent studies have focused renewed attention on events occurring immediately after transmission, and underscore the concept that the balance between inflammation and protective immunity is established by host responses in mucosal tissues.
Keywords: gut, CTL, IgA, acute, chronic, controller
Introduction
HIV is a mucosal pathogen, and AIDS-associated diarrhea and wasting syndromes were documented during the earliest years of the epidemic [1,2]. The dramatic depletion of CD4+ T-cells in the gastrointestinal lamina propria was described in the mid-1990s [3,4]. Similar findings were reported in SIV-infected macaques, and it soon became clear that a precipitous decline in mucosal CD4+ T-cells accompanied acute infection [5–7]. A resurgence of interest in this area has led to a number of studies designed to characterize host-pathogen interactions in the gastrointestinal and genitourinary tracts, and elucidate the role of mucosal immunity in controlling HIV. This review focuses on recently published work in the field of mucosal immunology as it relates to HIV/SIV infection.
New insights on acute HIV/SIV infection: A proinflammatory cascade leads to rapid viral dissemination
Current models suggest that HIV/SIV mucosal transmission begins with seeding of tissues by a small “founder” population [8,9]. Within 1–2 weeks, infection becomes systemic, with extensive viral replication and CD4+ T-cell depletion in the intestinal lamina propria (Figure 1A). Recent work has elucidated the events leading to this rapid amplification and dissemination of virus, focusing on the role of innate inflammatory responses [10**].
Figure 1. Immune defenses in the gastrointestinal mucosa.
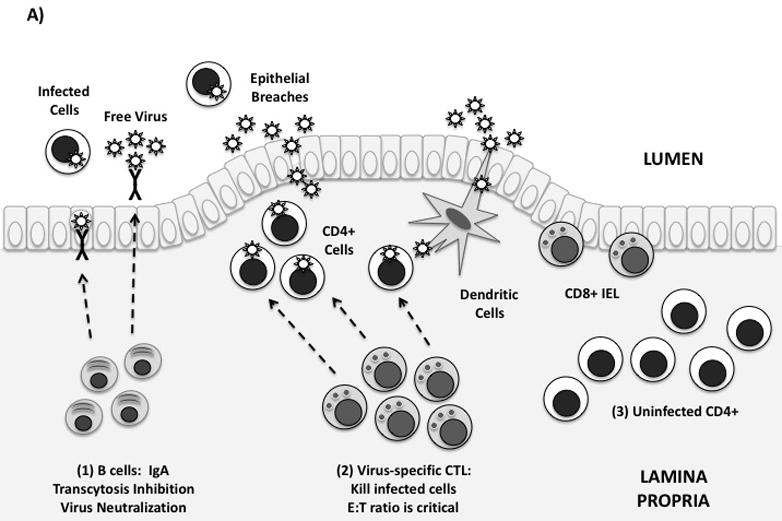
(A) Idealized mucosal defenses. Virus may enter the gut through transcytosis, direct entry via epithelial breaches, or by binding to dendritic cells. Immune defenses in the lamina propria are shown from left to right: (1) Mucosal antibodies are secreted by plasma cells in the lamina propria. IgA dimers are taken up by epithelial cells and secreted into the GI lumen. Mucosal IgA may block infection by binding and neutralizing free virus, infected cells, and/or by blocking transcytosis of virus by epithelial cells. (2) Virus-specific CTL can kill infected cells by granule exocytosis. “Polyfunctional” CTL also secrete multiple cytokines and chemokines. In order to adequately control infection, a high in vivo E:T ratio is required. (3) Uninfected CD4+ T-cells are abundant in the intestinal lamina propria, and are exquisitely sensitive to HIV infection. Th17 cells may be preferentially infected and depleted.
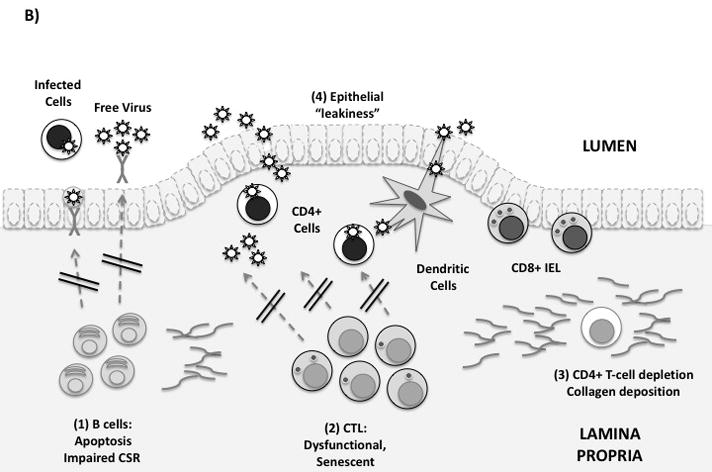
(B) Mucosal immune defenses are impaired in HIV infection. From left to right: (1) Mucosal B-cell function is affected by disruption of Peyer’s patches, induction of T/B-cell apoptosis, and by direct effects of Nef on class switching. (2) Mucosal CTL express low levels of perforin and high levels of PD-1. Free E-cadherin may ligate KLRG-1, inhibiting CTL function. (3) Lamina propria CD4+ T-cells, including Th17 cells, are rapidly infected and depleted. Collagen fibrosis impairs their reconstitution following antiretroviral therapy. (4) Local production of proinflammatory cytokines and loss of the Th17/Treg balance leads to increased epithelial permeability to microbial products. This may contribute to systemic immune activation.
Following intravaginal inoculation of macaques, Li and collagues used in situ hybridization and immunohistochemistry to generate a digital “atlas” showing the locations of SIV RNA+ cells in cervix and vagina the first 10 days post-infection [10**]. An early increase in expression of MIP-3α (CCL20) was associated with an influx of CD123+ plasmacytoid dendritic cells (pDC). These cells secreted chemokines (MIP-1α/CCL3 and MIP-1β/CCL4), attracting CCR5+CD4+ T-cells to the endocervix and leading to broad dissemination of infection. To date, similar studies of acute intra-rectal infection have not been reported.
Surprisingly, this work revealed that spreading of infection beyond the site of transmission could be interrupted by administration of an anti-inflammatory compound, glycerol monolaurate (GML) to the cervicovaginal mucosa. GML-treated macaques were completely protected from high-dose mucosal challenge with SIVmac. Thus, a naturally occurring compound can provide protection from mucosal exposure simply by inhibiting immune activation and cytokine/chemokine production [10**].
Location, location, location: in vivo E:T ratio predicts viral persistence
Previous studies have suggested that mucosal HIV/SIV-specific cytotoxic T-cells (CTL) are elicited “too little and too late” [11]. Li, Skinner and colleagues used a combination of in situ hybridization and MHC class I tetramer staining, termed “in situ tetramer/hybridization” (ISTH), to quantify infected cells and SIV-specific CTL in mucosal tissues [12**]. Significant reductions in viral load during early infection were associated with E:T ratios of ≥100 in the female reproductive tract. The authors also studied a well-established system of acute vs. persistent infection: lymphocytic choriomeningitis virus (LCMV) [12**]. The Armstrong strain of LCMV is cleared without causing disease, while clone 13 induces persistent viremia. Notably, when ISTH was used to evaluate in vivo E:T ratios, the Armstrong strain induced a ratio of 40:1 in spleen by 8 days post-infection. Although clone 13 also elicited CTL, the effective E:T ratio in tissues was <1:1. Thus, in both SIVmac and LCMV infections, in vivo E:T ratio during acute infection was inversely related to the establishment of persistent infection. The authors concluded that a vaccine capable of eliciting a cell-mediated immune response near the portal of entry “enough and soon enough” might prevent viral dissemination [12**] (Figure 1A).
Do mucosal T-cell responses matter during chronic HIV infection?
If HIV is analogous to LCMV clone 13, and the acute phase CTL response is “too little and too late”, then does a mucosal CTL response during chronic infection matter, or is it irrelevant? Two studies revealed that Gag-specific rectal CD8+ T-cell responses in chronically infected individuals were positively associated with CD4 count, and inversely related to plasma viral load [13,14*]. There was also an association between polyfunctional rectal Gag-specific CD8+ T-cell responses, lower plasma viral load, and higher blood CD4 count. Unfortunately, cross-sectional studies cannot conclusively demonstrate a protective role for these responses, so it is also possible that robust CD8+ T-cell responses are a consequence of CD4+ T-cell preservation and relatively low viremia.
Nevertheless, a contribution of mucosal responses to immune control is suggested by studies measuring rectal CD8+ T-cell responses in 28 individuals who control HIV without therapy (i.e., “Controllers”). Ferre and colleagues found that polyfunctional Gag-specific CD8+ T-cells were significantly more abundant in rectal mucosa from controllers as compared to non-controllers and patients on ART. Controllers also had relative preservation of rectal CD4+ T-cells as compared to other HIV-infected groups [15*].
Although T-cell responses in HIV controllers can be robust, the vast majority of infected individuals do not effectively control HIV. Gut CD8+ T-cells from patients with chronic HIV infection, or uninfected controls, contain very low levels of perforin [16,17]. Because gut perforin expression is increased in inflammatory conditions [18,19], this adaptation may limit inappropriate “friendly fire” that could lead to epithelial damage [16,19]. However, it might also reduce the ability of mucosal CD8+ T-cells to clear infection. Furthermore, CD8+ T-cells in lymph nodes and gastrointestinal mucosa of infected macaques express high levels of PD-1, suggesting immune exhaustion [20]. Together, these findings suggest that the mucosal CD8+ T-cell response to chronic HIV/SIV infection may be inadequate in most cases (Figure 1B).
An intriguing report suggested that damage to gut epithelium triggers CD8+ T-cell dysfunction by mediating ligation of the inhibitory receptor KLRG-1 by E-cadherin [21*]. In HIV-infected subjects, increased levels of soluble E-cadherin, an adherens junction protein, are detected in plasma, and the protein shows abnormal distribution in intestinal mucosa. In in vitro assays, HIV-specific CD8+ T-cell function was reduced in the presence of soluble E-cadherin. Of note, this study did not assess the function of CD8+ T-cells isolated from the GI tract; instead, assays were performed using PBMC with or without addition of E-cadherin.
Does a mucosal trafficking marker target CD4 T-cells for HIV infection?
Within the past several years, it has become clear that lymphocytes primed in mucosal inductive sites are “instructed” to express antigens that direct homing to mucosal effector sites. Intestinal APC synthesize retinoic acid, which “instructs” newly primed lymphocytes to express α4β7 integrin [22–24]. Integrin α4β7 mediates intestinal trafficking by interacting with mucosal addressin cell adhesion molecule-1 (MAdCAM-1), expressed on venules in the lamina propria.
Intriguingly, α4β7 may also play a unique role in HIV infection, as activated α4β7 heterodimer can bind gp120 on infected CD4+ T-cells [25*]. The gp120-α4β7 interaction, in turn, triggers activation of αLβ2 integrin (LFA-1) on the CD4+ T-cell surface. Because LFA-1 facilitates cell-cell interactions, its upregulation on infected cells may facilitate viral transfer to uninfected cells [25*]. Furthermore, most Th17 cells express α4β7; this could explain their preferential infection and depletion during acute infection [26*]. The balance between Th17 and regulatory T-cells may be critical in determining immune activation “set point”; this topic is reviewed elsewhere in this journal issue.
Another study revealed that the loss of β7high cells in blood during SIV infection closely parallels the depletion of lamina propria CD4+ T-cells [27]. This suggests that the frequency of β7high CD4+ T-cells in blood may be used as a surrogate marker to estimate loss or restoration of intestinal CD4+ T-cells, without the need for gut biopsy. It remains to be determined whether expression of the same marker on blood CD8+ T-cells can adequately substitute for the direct measurement of HIV-specific CD8+ T-cell responses in the gastrointestinal tract.
Mucosal B-cells and HIV: polyclonal activation, neutralizing IgA, and Trojan horses
It has been known for many years that HIV infection leads to destruction of the follicular dendritic cell network and involution of germinal centers. However, a recent study investigated B cells in blood and terminal ileum from patients in acute/early infection [28*]. HIV infection rapidly induced polyclonal activation of B cells in blood and gut, including those specific for influenza and autoantigens. Ileal Peyer’s patches exhibited loss of germinal centers, and over 80% of follicles contained apoptotic T and/or B cells. While not unexpected, these findings confirm that rapid and profound damage occurs to mucosal inductive sites during acute HIV infection, with important consequences for B-cell differentiation and antibody production.
The issue of “protective” mucosal antibodies in highly-exposed, persistently seronegative (HEPS) individuals remains controversial [29]. Although HEPS by definition lack circulating IgG antibodies specific for HIV, several studies have reported HIV-specific IgA in plasma or mucosal secretions from HEPS [30–36] (Figure 1A). In some cases these antibodies have been found to neutralize HIV infectivity in vitro [37,38] and/or to inhibit viral transcytosis across an epithelial monolayer [35,39]. However, other studies have failed to detect such antibodies [40–42].
A recent study described the construction of a Fab library from cervical B-cells of HEPS women, from which IgAs specific for gp41 were obtained [43**]. These antibodies blocked transcytosis of X4 and R5 HIV, and neutralized infectivity in vitro. Intriguingly, a Fab of subtype IgA1 (Fab 43) specific for the gp41 membrane-proximal region showed an unusually long CDR3 region, similar to those described for broadly neutralizing IgG antibodies. Fab 43 also showed evidence of hypermutation, suggesting affinity maturation during prolonged exposure to antigen [43**]. Given the controversies that persist in this field, it will be important to confirm these potentially exciting findings.
Is Nef a “Trojan horse” to block class-switching and evade mucosal antibodies? Several years ago, it was reported that HIV Nef can perturb B-cell signaling and blocking class-switching recombination [44] (Figure 1B). The initial study did not provide a mechanism by which Nef could enter B-cells in vivo. However, a follow-up study revealed that Nef may utilize intercellular “bridges” to enter B-cells, which are not typically infected by HIV [45**]. In lymphoid tissues, including Peyer’s patches, B-cells and macrophages are in close proximity and actin-driven cellular conduits form, apparently as a normal intercellular communication network that is ‘hijacked’ by Nef. Accumulation of Nef in germinal centers was associated with decreased expression of activation-induced cytidine deaminase (AID). The presence of Nef inhibited class switching to IgA and IgG2, and individuals harboring ΔNef viruses had higher levels of these subclasses in plasma than subjects infected with wild-type HIV [45**]. Interestingly, CD40-independent class switching outside of germinal centers was increased in HIV-infected subjects, likely contributing to nonspecific hypergammaglobulinemia.
Can mucosal CD4+ T-cells and epithelial integrity be reconstituted with HAART?
Estes, Schacker and colleagues previously established that immune activation in HIV/SIV infection is associated with disruption of lymphoid tissue architecture and deposition of collagen in lymph nodes, potentially limiting CD4+ T-cell repopulation after antiretroviral therapy (ART) [46]. Recently, these authors reported that CD4+ T-cell depletion in ileal Peyer’s patches is accompanied by even more extensive collagen deposition than in lymph nodes [47**]. ART begun during early infection led to an increase in CD4+ central memory cells in Peyer’s patches, but did not restore effector memory cells in the ileal lamina propria. Initiation of ART during chronic infection did not significantly increase CD4+ T-cells in gut inductive or effector sites. These findings strongly suggest that fibrotic damage severely disrupts the ability of GALT to support normal T-cell trafficking and survival, even following ART (Figure 1B).
Not only do CD4+ T-cells fail to fully repopulate the GI tract following ART, but recent work suggests that HIV proviral DNA persists in CD4+ T-cells from terminal ileum following up to 9.9 years of effective therapy [48*]. Viral envelope (C2-V5) sequences were determined from three sources: resting blood CD4+ T-cells, activated blood CD4+ T-cells, and ileal CD4+ T-cells. Results revealed that viral DNA sequences from these three sources were not significantly different, suggesting ongoing “cross-seeding” of blood and GALT CD4+ T-cells, rather than sequence compartmentalization between anatomical sites [48*].
On an encouraging note, a study of 23 HIV-positive subjects on long-term ART with durable viral suppression revealed CD4+ T-cell reconstitution in sigmoid colon to levels statistically equivalent to healthy controls [49*]. Unfortunately, the extent of repopulation in terminal ileum of these patients is unknown. It remains to be determined whether the differences in outcome between this and the previously cited study are related to the sites sampled (i.e., lower vs. upper GI tract) or other factors.
Macal and colleagues analyzed jejunal biopsies from 14 subjects on long-term ART, of whom 6 had minimal restoration and 8 had jejunal CD4+ T-cell percentages comparable to uninfected controls [50*]. Notably, several patients had detectable proviral DNA in mucosa despite undetectable plasma viremia. Relative gut CD4+ T-cell restoration was associated with higher percentages of CD4+ central memory cells and Th17 cells in jejunal mucosa, and with polyfunctional HIV-specific CD4+ T-cell responses in some subjects [50*]. These findings demonstrate that immune responses to HIV and other pathogens can be partially restored in jejunal mucosa following ART.
The effects of suppressive ART on gut epithelial damage were assessed in a study that evaluated electrophysiological properties, mannitol permeability, villus/crypt ratio, apoptosis and tight junction proteins in jejunal mucosa [51*]. Untreated HIV+ subjects showed significant impairment as compared to healthy controls; however, findings in ART-treated subjects were statistically equivalent to controls. These authors also measured cytokine production in jejunal biopsies, found increased TNFα, IL-2 and IL-4 in untreated HIV+ subjects, and hypothesized that TNFα overproduction may be responsible for the impaired epithelial function observed in untreated infection [51*].
In summary, these studies reveal that partial restoration of gastrointestinal CD4+ T-cells is possible in patients on long-term ART. Restoration appears to be impeded by factors contributing to immune activation and/or collagen deposition, and enhanced by durable control of viral replication. Additional studies are needed to fully assess differences in reconstitution between inductive vs. effector sites, and upper vs. lower GI tract.
Conclusions
Recent studies have highlighted the importance of mucosal defenses in determining the host-pathogen balance. Curbing immune activation at the site of transmission may abrogate viral dissemination, and the in vivo E:T ratio near the site of initial infection may also be critical in limiting viral persistence. Individuals who control HIV without therapy have unusually strong HIV-specific T-cell responses in mucosal tissues, which may limit viral replication during chronic infection. Mucosal B-cell responses are compromised by events occurring during acute infection, and class-switching may be impaired by a Nef-mediated mechanism. Finally, at least partial restoration of gut CD4+ T-cells, barrier function, and mucosal T-cell function is possible in patients on suppressive ART.
It remains to be seen how effectively these concepts will be translated into microbicide, vaccine, and/or immunotherapeutic approaches. Several gaps remain to be filled, including elucidation of the early stages of acute infection following rectal transmission; systematic comparison of the effects of HIV/SIV on the upper vs. lower gastrointestinal tract; and detailed study of immune defenses in the male and female reproductive tracts.
Acknowledgments
The author is supported by grants from the National Institutes of Health (NIH/NIAID R01-AI-057020; P01-AI-083050), the California HIV/AIDS Research Program (CHRP, grant CH05-D-606), and the Pendleton Charitable Trust.
References and recommended reading
- 1.Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kotler DP, Gaetz HP, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 3.Schneider T, Jahn HU, Schmidt W, et al. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider T, Ullrich R, Bergs C, et al. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin Exp Immunol. 1994;95:430–435. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heise C, Miller CJ, Lackner A, et al. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 6.Heise C, Vogel P, Miller CJ, et al. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 7.Veazey R, DeMaria M, Chalifoux L, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn CA, Hunter E. Viral characteristics of transmitted HIV. Curr Opin HIV AIDS. 2008;3:16–21. doi: 10.1097/COH.0b013e3282f2982c. [DOI] [PubMed] [Google Scholar]
- 9.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 10**.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. This paper reported that a naturally occurring compound can provide protection from mucosal exposure simply by inhibiting local immune activation and cytokine/chemokine production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds MR, Rakasz E, Skinner PJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Li Q, Skinner PJ, Ha SJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. This study evaluated the in vivo E:T ratio during acute infection with SIV mac and LCMV and demonstrated that a high effector-to-target ratio is associated with viral clearance, while a low ratio is associated with persistent infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchfield JW, Lemongello D, Walker DH, et al. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Critchfield JW, Young DH, Hayes TL, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. This study reported an association between polyfunctional rectal Gag-specific CD8+ T-cell responses, lower plasma viral load, and higher blood CD4 counts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Ferre AL, Hunt PW, Critchfield JW, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. Polyfunctional Gag-specific CD8+ T-cells were significantly more abundant in rectal mucosa from HIV controllers as compared to non-controllers and patients on HAART. This study suggested a role for mucosal T-cell responses in control of HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shacklett BL, Cox CA, Quigley MF, et al. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J Immunol. 2004;173:641–648. doi: 10.4049/jimmunol.173.1.641. [DOI] [PubMed] [Google Scholar]
- 17.Chott A, Gerdes D, Spooner A, et al. Intraepithelial lymphocytes in normal human intestine do not express proteins associated with cytolytic function. Am J Pathol. 1997;151:435–442. [PMC free article] [PubMed] [Google Scholar]
- 18.Ciccocioppo R, Di Sabatino A, Parroni R, et al. Cytolytic mechanisms of intraepithelial lymphocytes in coeliac disease (CoD) Clin Exp Immunol. 2000;120:235–240. doi: 10.1046/j.1365-2249.2000.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merger M, Viney JL, Borojevic R, et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51:155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velu V, Kannanganat S, Ibegbu C, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Kwon D, Streeck H, Jolin J, et al. The epithelial adherens junction protein E-cadherin inhibits HIV-1-specific CD8+ T-cell responses by binding to KLRG1. Mucosal Immunol. 2009;2:S1. An intriguing abstract suggesting that damage to gut epithelium triggers CD8+ T-cell dysfunction by mediating ligation of the inhibitory receptor KLRG-1 by E-cadherin. [Google Scholar]
- 22.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 24.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 25*.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. This study demonstrates that activated α4β7 can bind gp120 on infected CD4+ T-cells, and may facilitate viral transfer to uninfected cells. [DOI] [PubMed] [Google Scholar]
- 26*.Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. This study demonstrates that most Th17 cells express α4β7, which could explain their preferential infection and depletion during acute HIV/SIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Xu H, Gill AF, et al. Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2009;2:518–526. doi: 10.1038/mi.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Levesque MC, Moody MA, Hwang KK, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. This detailed study confirmed that rapid and profound damage occurs to mucosal inductive sites during acute HIV infection, with important consequences for B-cell differentiation and antibody production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shacklett BL. Understanding the “lucky few”: the conundrum of HIV-exposed, seronegative individuals. Curr HIV/AIDS Rep. 2006;3:26–31. doi: 10.1007/s11904-006-0005-2. [DOI] [PubMed] [Google Scholar]
- 30.Beyrer C, Artenstein AW, Rugpao S, et al. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J Infect Dis. 1999;179:59–67. doi: 10.1086/314556. [DOI] [PubMed] [Google Scholar]
- 31.Kaul R, Trabattoni D, Bwayo JJ, et al. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kaul R, Plummer F, Clerici M, et al. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15:431–432. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- 33.Mazzoli S, Lopalco L, Salvi A, et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999;180:871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoli S, Trabattoni D, Lo Caputo S, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 35.Devito C, Broliden K, Kaul R, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 36.Devito C, Hinkula J, Kaul R, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14:1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 37.Hirbod T, Kaul R, Reichard C, et al. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS. 2008;22:727–735. doi: 10.1097/QAD.0b013e3282f56b64. [DOI] [PubMed] [Google Scholar]
- 38.Hirbod T, Reichard C, Hasselrot K, et al. HIV-1 neutralizing activity is correlated with increased levels of chemokines in saliva of HIV-1-exposed uninfected individuals. Curr HIV Res. 2008;6:28–33. doi: 10.2174/157016208783571964. [DOI] [PubMed] [Google Scholar]
- 39.Belec L, Ghys PD, Hocini H, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 40.Skurnick JH, Palumbo P, De Vico A, et al. Correlates of non-transmission in United States women at high risk of HIV-1 transmission through sexual exposure. Journal of Infectious Diseases. 2002;185:428–438. doi: 10.1086/338830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorrell L, Hessell AJ, Wang M, et al. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. AIDS. 2000;14:1117–1122. doi: 10.1097/00002030-200006160-00008. [DOI] [PubMed] [Google Scholar]
- 42.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol. 2006;72:1–17. doi: 10.1016/j.jri.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 43**.Tudor D, Derrien M, Diomede L, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. This study described the construction of a Fab library from cervical B-cells of HEPS women, from which IgAs specific for gp41 were obtained. These antibodies blocked transcytosis of X4 and R5 HIV, and neutralized infectivity in vitro. [DOI] [PubMed] [Google Scholar]
- 44.Qiao X, He B, Chiu A, et al. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 45**.Xu W, Santini PA, Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. This study demonstrated that Nef can utilize intercellular “bridges” to enter mucosal B-cells. Accumulation of Nef in mucosal inductive sites was associated with reduced class-switching to IgG and IgA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Estes J, Baker JV, Brenchley JM, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–464. doi: 10.1086/590112. This study reported that CD4+ T-cell depletion in ileal Peyer’s patches is accompanied by more extensive collagen deposition than in lymph nodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis. 2008 doi: 10.1086/527324. This study reported that HIV proviral DNA persists in CD4+ T-cells from terminal ileum following up to 9.9 years of effective therapy. [DOI] [PubMed] [Google Scholar]
- 49*.Sheth PM, Chege D, Shin LY, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008;1:382–388. doi: 10.1038/mi.2008.23. This study of 23 HIV-positive subjects on long-term ART revealed CD4+ T-cell reconstitution in sigmoid colon to levels statistically equivalent to healthy controls. [DOI] [PubMed] [Google Scholar]
- 50*.Macal M, Sankaran S, Chun TW, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. In this study, relative CD4+ T-cell restoration was associated with higher percentages of CD4+ central memory cells and Th17 cells in jejunal mucosa. [DOI] [PubMed] [Google Scholar]
- 51*.Epple HJ, Schneider T, Troeger H, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58:220–227. doi: 10.1136/gut.2008.150425. This study demonstrated restoration of epithelial barrier function in patients on long-term ART, and suggested that TNFα overproduction may be responsible for the impaired epithelial function observed in untreated infection. [DOI] [PubMed] [Google Scholar]


