Abstract
The progression from recreational drug use to drug addiction impacts multiple neurobiological processes and can be conceptualized as a transition from positive to negative reinforcement mechanisms driving both drug-taking and drug-seeking behaviors. Neurobiological mechanisms for negative reinforcement, defined as drug taking that alleviates a negative emotional state, involve changes in the brain reward system and recruitment of brain stress (or antireward) systems within forebrain structures, including the extended amygdala. These systems are hypothesized to be dysregulated by excessive drug intake and to contribute to allostatic changes in reinforcement mechanisms associated with addiction. Points of intersection between positive and negative motivational circuitry may further drive the compulsivity of drug addiction but also provide a rich neurobiological substrate for therapeutic intervention.
Keywords: addiction, amygdala, corticotropin-releasing factor, drug dependence, dynorphin, extended amygdala, neuroadaptation, norepinephrine, opponent process, stress
Drug addiction: recreational drug use versus dependence
Drug addiction (also known as ‘substance dependence’ according to the Diagnostic and Statistical Manual of Mental Disorders [DSM] [1]) is a chronic, relapsing disorder that has been characterized by a compulsion to seek and take drugs, loss of control over drug intake; and an emergence of a negative emotional state (e.g., dysphoria, anxiety and irritability) that defines a motivational withdrawal syndrome when access to the drug is prevented [2]. The occasional, limited, recreational use of a drug is clinically distinct from escalated drug use, loss of control over drug intake and the emergence of compulsive drug-seeking behavior that characterize addiction. The critical nature of the distinction between drug abuse and dependence has been illustrated by data showing that approximately 15.6% (29 million) of the US adult population will go on to engage in nonmedical (illicit) drug use at some point in their lives, with approximately 2.9% (5.4 million) transitioning to DSM-defined substance dependence as a result of chronic illicit drug use [3,4]. Regarding alcohol, approximately 51% (120 million) of people over the age of 12 years are drinkers, and of these current users, 7.7% (18 million) have met the criteria for substance abuse or dependence. For nicotine, in 2007, approximately 28.6% (70.9 million) North Americans aged 12 years or older were current users (having used in the previous month) of a tobacco product, with 24.2% of the population (60.1 million) being current cigarette smokers and 3.2% (8.1 million) using smokeless tobacco [5].
While most of the early studies on the neurobiology of drug addiction focused on the acute pharmacological effects of drug exposure, attention is now shifting to chronic administration models and investigations into the long-term neuroadaptive changes in the brain that may predispose individuals to relapse. Overall, the purpose of current drug abuse research is to decipher the genetic/epigenetic, biochemical, cellular and circuitry mechanisms that mediate the transition from recreational, controlled drug use to the uncontrolled drug-taking and drug-seeking behaviors endemic to addicted populations. Recent interest has also been focused on long-term plasticity that has the potential to remain long after drug use has terminated, which may emphasize the persistence of relapse well into the abstinent period.
The overall hypothesis argued here is that drug addiction represents a detachment from homeostatic brain regulatory mechanisms that control the normal everyday emotional states of the animal. More specifically, two components are hypothesized to comprise the break from homeostasis: underactivation of brain reward transmitter function and recruitment of brain stress (or antireward) systems. These systems (reward and stress) interact at all stages of the addiction cycle to potentiate the dysregulation. In addition, drug addiction, similar to other chronic physiological disorders such as high blood pressure, worsens over time, is subject to significant environmental influences and leaves a residual neuroadaptive trace that allows rapid ‘re-addiction’ even years after detoxification and abstinence. These characteristics of drug addiction imply more than just a homeostatic dysregulation of hedonic function and executive function, and rather a dynamic break with homeostasis of these systems toward a new set point.
Motivation: positive & negative reinforcement mechanisms
The motivation for pathological drug seeking involves two distinct sources of reinforcement that distinguish initial drug use from drug addiction – positive and negative reinforcement. Positive reinforcement occurs when presentation of a stimulus increases the probability of response, and usually refers to the generation of a positive hedonic (rewarding) state. Support for a rewarding effect of drugs of abuse comes from the observation that nondependent animals will work to obtain drugs even in the absence of an imposed motivational state (e.g., food or water deprivation, or drug withdrawal), and this formed the foundational basis for early theoretical positions regarding incentive motivation [6] and more recently, incentive salience [7]. From this, one prominent conceptual framework for addiction is that drugs usurp brain incentive salience systems [8,9], leading to a narrowing of the behavioral repertoire towards the goal of obtaining and using drugs.
By contrast, negative reinforcement involves the use of drugs to either self-medicate an existing aversive state or to alleviate negative emotional symptoms induced by drug withdrawal (including dysphoria, anxiety, irritability and sleep disturbances). In their theory of opponent processes, Solomon and Corbit postulated that hedonic, affective or emotional states, once initiated, are automatically tempered by the CNS via mechanisms that reduce the intensity of hedonic feelings [10]. From a drug-taking perspective (using brain motivational systems as an example), the initial positive hedonic response resultant from drug use – a-process – is hypothesized to be opposed or counteracted by a negative hedonic response – b-process – for the purpose of homeostatic balance within brain motivational systems. This affect control system was conceptualized as a single negative feedback or opponent process loop that opposes the stimulus-aroused affective state in order to suppress or reduce departures from hedonic homeostasis [10–12].
More recently, the opponent process theory has been expanded for the basis of studying the neurobiology of drug addiction from a neurocircuitry perspective. An allostatic model of brain motivational systems has been proposed to explain the persistent changes in motivation that are associated with drug dependence in addicted states [13,14]. In this formulation, addiction is conceptualized as a cycle of increasing dysregulation of brain reward–antireward systems that results in the generation and potentiation of a negative emotional state, which contributes to the compulsive seeking and use of drugs, even in the face of adverse consequences. Normal counteradaptive processes, such as the opponent b-process, that are part of the homeostatic limitation of reward function fail to return to within the natural homeostatic range, leading to a state of pathology.
These counteradaptive operations are hypothesized to be mediated by two processes – within-system neuroadaptations and between-system neuroadaptations [15]. In a within-system opponent neuroadaptation, “the primary cellular response element to the drug would itself adapt to neutralize the drug’s effects; persistence of the opposing effects after the drug disappears would produce the withdrawal response” [15]. Thus, a within-system opponent neuroadaptation is a biochemical or cellular change within a given reward circuit designed to curtail overactivity of hedonic processing associated with drug use, resulting in decreased reward function.
In a between-system neuroadaptation, neurochemical systems other than those involved in the initial positive rewarding effects of drugs of abuse are recruited (and possibly dysregulated) following chronic activation of the reward system [15]. Thus, a between-system neuroadaptation is a circuitry change in which another distinct neural substrate (in this case, an antireward circuit) is activated by the reward circuit and exerts opposing actions in order to limit reward function. In this article, we discuss competing neuroadaptive changes in the brain reward systems (within-system adaptations) and brain stress systems (between-system adaptations), both of which contribute to the pathology of dysregulated motivational systems in addiction.
Transition to addiction: homeostasis versus allostatic dysregulation
The transitional development of aversive or negative emotional states that drive negative reinforcement in addiction has been defined as the ‘dark side’ of drug addiction [14]. Drug-associated negative emotional states consist of key motivational elements, such as chronic irritability, emotional pain, dysphoria and loss of motivation for natural rewards (including career- and family-based rewards). Two distinct processes are hypothesized to form the neurological basis of this state: loss of function in the reward systems (within-system neuroadaptations) and recruitment of brain stress or antireward systems (between-system neuroadaptations) [2,15]. As addiction develops, brain stress systems such as corticotropin-releasing factor (CRF), norepinephrine and dynorphin are recruited, producing aversive or stress-like states [16–18]. At the same time, within the positive motivational circuits of the ventral striatum-extended amygdala, reward function is diminished. The combination of decreases in reward neurotransmitter function and potentiation of brain stress systems provides a powerful source of negative reinforcement that contributes to compulsive drug-seeking behavior and long-term dependence.
Allostasis was originally conceptualized to explain the persistent morbidity of arousal and autonomic function associated with chronic stress and is defined as ‘stability through change’, in which continuous readjustment of all parameters occurs toward a new set point [19]. The state of chronic regulatory system deviation from its normal (homeostatic) operating level can be defined as an ‘allostatic state’. Thus, the very physiological mechanism that generates rapid responsiveness to environmental challenge becomes the very source of pathology if adequate time or resources are not available to dampen the response.
Two components are hypothesized to adjust to the challenges of the brain produced by drugs of abuse to produce an allostatic-like state; underactivation of brain reward transmitters and circuits and recruitment of the brain stress (or antireward) systems. Repeated challenges, such as with drugs of abuse, lead to attempts of the brain via biochemical, cellular and neurocircuitry changes to maintain stability, but at a cost. For the drug addiction framework elaborated here, the residual deviation from normal brain reward threshold regulation is termed an ‘allostatic state’, and the weight on the system or cost leading to sustained pathology is the ‘allostatic load’ [20]. The allostatic state represents a combination of a chronic elevation of the reward set point fueled from the opponent process, motivational perspective by decreased function of reward circuits and recruitment of brain stress systems, both of which lead to the compulsivity of drug seeking and drug taking. What follows is a review of the neurobiology underlying positive and negative reinforcement mechanisms, and a discussion of how specific neuroadaptations within and between these systems may support the persistence of drug addiction.
Neurobiology of reward & positive reinforcement
Comprehension of a brain reward system was greatly facilitated by the discovery of electrical brain stimulation reward by Olds and Milner [21]. Brain stimulation reward involves widespread neurocircuitry throughout the brain, but the most sensitive sites include the trajectory of the medial forebrain bundle that connects the ventral tegmental area with the basal forebrain (Figure 1) [21–23]. All drugs of abuse acutely decrease brain stimulation reward thresholds (i.e., increase or facilitate reward) [24], and when administered chronically, increase reward thresholds (decrease reward) during withdrawal. Although much emphasis was initially focused on the role of the ascending monoamine systems, particularly the dopamine system, in the medial forebrain bundle in mediating brain stimulation reward, other nondopaminergic systems in the medial forebrain bundle clearly play a key role [25–27]. Indeed, the role of dopamine is hypothesized to be more indirect. Many studies suggest that activation of the mesolimbic dopamine system attaches incentive salience to stimuli in the environment [7,28,29] to drive the performance of goal-directed behavior [30] or activation in general [31,32], and work concerning the acute reinforcing effects of drugs of abuse supports this hypothesis.
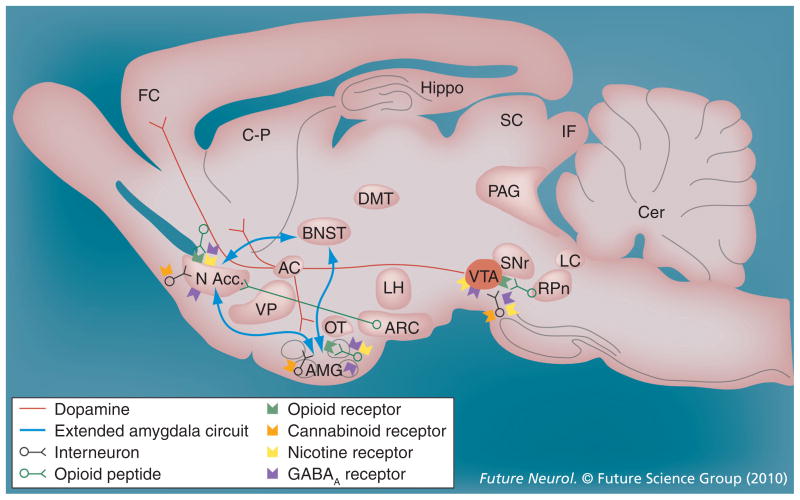
Figure 1. Neurotransmitter pathways and receptor systems implicated in the acute reinforcing effects of drugs of abuse.
Sagittal rodent brain section. Cocaine and amphetamines increase dopamine levels in the nucleus accumbens and amygdala via direct actions on dopamine terminals. Opioids activate endogenous opioid receptors in the ventral tegmental area, nucleus accumbens and amygdala. Opioids also facilitate the release of dopamine in the nucleus accumbens via actions either in the ventral tegmental area or nucleus accumbens, but are also hypothesized to activate elements independent of the dopamine system. Alcohol activates GABAA receptors or enhances GABA release in the ventral tegmental area, nucleus accumbens and amygdala. Alcohol is also hypothesized to facilitate the release of opioid peptides in the ventral tegmental area, nucleus accumbens and central nucleus of the amygdala. Alcohol facilitates the release of dopamine in the nucleus accumbens via an action either in the ventral tegmental area or nucleus accumbens. Nicotine activates nicotinic acetylcholine receptors in the ventral tegmental area, nucleus accumbens and amygdala, either directly or indirectly, via actions on interneurons. Cannabinoids activate cannabinoid CB1 receptors in the ventral tegmental area, nucleus accumbens and amygdala. Cannabinoids facilitate the release of dopamine in the nucleus accumbens via an unknown mechanism, either in the ventral tegmental area or nucleus accumbens. The blue arrows represent the interactions within the extended amygdala system hypothesized to play a key role in psychostimulant reinforcement. The medial forebrain bundle represents ascending and descending projections between the ventral forebrain (nucleus accumbens, olfactory tubercle and septal area) and the ventral midbrain (ventral tegmental area; not shown in figure for clarity).
AC: Anterior commissure; AMG: Amygdala; ARC: Arcuate nucleus; BNST: Bed nucleus of the stria terminalis; Cer: Cerebellum; C-P: Caudate-putamen; DMT: Dorsomedial thalamus; FC: Frontal cortex; Hippo: Hippocampus; IF: Inferior colliculus; LC: Locus coeruleus; LH: Lateral hypothalamus; MFB: Medial forebrain bundle; N Acc.: Nucleus accumbens; OT: Olfactory tract; PAG: Periaqueductal gray; RPn: Reticular pontine nucleus; SC: Superior colliculus; SNr: Substantia nigra pars reticulata; VP: Ventral pallidum; VTA: Ventral tegmental area.
Positive reinforcement for drugs of abuse occurs when the presentation of a drug increases the probability of a response to obtain the drug and, as previously noted, usually refers to the generation of a positive hedonic (rewarding) state. Animal models of the positive reinforcing or rewarding effects of drugs, in the absence of withdrawal or deprivation, are extensive and well validated and include intravenous drug self-administration, conditioned place preference and brain stimulation reward [33].
The acute reinforcing effects of drugs of abuse are mediated by connections of the medial forebrain bundle reward system with primary contributions from the ventral tegmental area, nucleus accumbens and amygdala. A substantial amount of evidence supports the hypothesis that psychostimulant drugs dramatically activate the mesolimbic dopamine system (projections from the ventral tegmental area to the nucleus accumbens) during limited-access drug self-administration and that this mechanism is critical for mediating the rewarding effects of cocaine, amphetamines and nicotine. However, evidence supports both dopamine-dependent and -independent neural substrates for opioid and alcohol reward [13,34,35]. Serotonin systems, particularly those involving serotonin 5-HT1B receptor activation in the nucleus accumbens, have also been implicated in the acute reinforcing effects of psychostimulant drugs, while μ-opioid receptors in both the nucleus accumbens and ventral tegmental area mediate the reinforcing effects of opioids. Opioid peptides in the ventral striatum and amygdala have been hypothesized to mediate the acute reinforcing effects of ethanol self-administration, largely based on the effects of opioid antagonists. Inhibitory GABA systems are activated both pre- and post-synaptically in the amygdala by ethanol at intoxicating doses, and GABA receptor antagonists block ethanol self-administration (for comprehensive reviews, see [34,35]). Altogether, the neurochemical mechanisms underlying the rewarding effects of drugs of abuse stand in sharp contrast to the distinct systems recruited during excessive drug use and withdrawal.
Recruitment of brain stress systems in addiction
Corticotropin-releasing factor
A common response to acute withdrawal and protracted abstinence from all major drugs of abuse in humans is the manifestation of anxiety-like or aversive-like states, and animal models have revealed anxiety-like or aversive-like responses to all major drugs of abuse during acute withdrawal [36–40]. CRF is a 41-amino acid polypeptide that regulates hormonal, sympathetic and behavioral responses to stressors. Substantial CRF-like immuno-reactivity and CRF1-receptor density are present in the neocortex, extended amygdala, medial septum, hypothalamus, thalamus, cerebellum, and autonomic midbrain and hindbrain nuclei [41,42]. Both systemic administration of a CRF1-receptor antagonist and direct intracerebral administration of a peptide CRF-receptor antagonist decreased drug withdrawal-induced anxiety-like or aversive responses [43–50]. Moreover, intracerebral administration of peptidergic CRF-receptor antagonists has localized the anxiety-like or aversive effects to the central nucleus of the amygdala [44,51]. Importantly, CRF antagonists injected intracerebroventricularly or systemically also blocked the potentiated anxiety-like responses to stressors observed during extended abstinence from chronic ethanol drinking [52,53].
Chronic administration of drugs of abuse, either via self-administration or passive (noncontingent) administration, increases extracellular CRF within the extended amygdala measured by in vivo microdialysis [54]. Continuous access to intravenous self-administration of cocaine for 12 h increased extracellular CRF in the central nucleus of the amygdala [55]. Opioid withdrawal following chronic morphine exposure in rats also increased extracellular CRF in the central nucleus of the amygdala [56]. Both acute nicotine administration and withdrawal from chronic nicotine elevated CRF extrahypothalamically in the basal forebrain [57]. Increased CRF-like immunoreactivity has been observed in adult rats following exposure to nicotine during adolescence and has been linked to an anxiety-like phenotype [58]. Extracellular CRF is also increased in the central nucleus of the amygdala during precipitated withdrawal from chronic nicotine [50]. During acute (2–12 h) ethanol withdrawal, an increase in extracellular CRF occurs within the central nucleus of the amygdala and bed nucleus of the stria terminalis of ethanol-dependent rats [59–61]. Precipitated withdrawal from chronic cannabinoid exposure also increased CRF in the central nucleus of the amygdala [62]. Overall, these results demonstrate that all major drugs of abuse produce a dramatic increase in extracellular levels of CRF measured by in vivo microdialysis during acute withdrawal after chronic drug administration.
The ability of neuropharmacological agents to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal would predict the motivational effects of these agents in animal models given extended access to drugs. Animal models of extended access involve exposure of the animals to extended sessions of intravenous self-administration of drugs (i.e., several hours/day vs 1 h/day) and passive, intermittent alcohol vapor exposure (14 h on/10 h off). Animals are then tested for self-administration at various time-points into withdrawal, ranging from 2 to 6 h for ethanol to several days with nicotine. CRF antagonists selectively blocked the increased self-administration of drugs associated with extended access to intravenous self-administration of cocaine [63], nicotine [50] and heroin [64]. CRF antagonists also blocked the increased self-administration of ethanol in dependent rats [48]. Evidence that specific sites within the brain mediate these CRF antagonist actions has focused on the central nucleus of the amygdala. Intracerebroventricular administration of the CRF-receptor antagonist D-Phe CRF12–41 blocked the dependence-induced increase in ethanol self-administration during both acute withdrawal and protracted abstinence [65,66]. When administered directly into the central nucleus of the amygdala, lower doses of D-Phe CRF12–41 blocked ethanol self-administration in ethanol-dependent rats [59].
In humans, two single nucleotide polymorphisms (SNPs) in the CRF1 receptor gene (crhr1) were associated with binge drinking in adolescents and excessive drinking in adults [67]. Moreover, homozygosity at one of these SNPs (rs1876831, C allele) was associated with heavy drinking in relation to stressful life events in adolescents [68].
These data suggest an important role for CRF, primarily within the central nucleus of the amygdala, in mediating the increased self-administration associated with dependence and suggest that CRF in the basal forebrain may also play an important role in the development of the aversive motivational effects that drive the increased drug-seeking associated with cocaine, heroin and nicotine withdrawal.
Norepinephrine
Norepinephrine is a well-established neurotransmitter with widespread distribution throughout the brain and hypothesized functions in mediating arousal, attention and stress in both normal and pathological states [69]. Cell bodies for the brain norepinephrine systems originate in the locus coeruleus and brainstem. The locus coeruleus is the source of a dorsal noradrenergic pathway to the cortices and hippocampus, and the brainstem projections converge in the ventral noradrenergic bundle to innervate the basal forebrain and hypothalamus. Norepinephrine is released in the brain during stress [69,70], and the projections from the locus coeruleus are hypothesized to play a key role in maintaining attentional homeostasis [71]. By contrast, the projections from the brainstem via the ventral bundle to the extended amygdala mediate behavioral responses to stressors [72,73].
Norepinephrine has long been hypothesized to be activated during withdrawal from drugs of abuse. Opioids decrease firing of noradrenergic neurons in the locus coeruleus, and in turn, the locus coeruleus is activated during opioid withdrawal [74]. Substantial evidence also suggests that in both animals and humans, central noradrenergic systems are activated during acute withdrawal from ethanol and this adaptation may have motivational significance. Alcohol withdrawal in humans is associated with activation of noradrenergic function, as measured from cerebrospinal fluid [75–77]. In animals, evidence implicates a role for norepinephrine systems in ethanol self-administration and in the increased self-administration associated with dependence. Voluntary ethanol consumption was decreased by both selective pharmacological and neurotoxin-specific disruption of noradrenergic function [78–81]. Mice with reduced levels of brain norepinephrine via knockout of the dopamine β-hydroxylase gene also have a reduced preference for ethanol [82].
In studies using animal models of dependence, noradrenergic drugs can block the aversive stimulus effects of opioid withdrawal [39]. Moreover, the α1 noradrenergic receptor antagonist prazosin blocked the increased drug intake associated with ethanol dependence [83], extended access to cocaine [84] and extended access to opioids [85]. Thus, converging data suggest that disruption of noradrenergic function blocks ethanol reinforcement, that noradrenergic neurotransmission is enhanced during drug withdrawal and that nor-adrenergic antagonists can block the increased drug self-administration associated with acute withdrawal in dependent animals.
Perhaps more intriguing is the prominent reciprocal interaction between CNS CRF and nor-epinephrine systems. Conceptualized as a feed-forward system at multiple levels of the pons and basal forebrain, CRF activates norepinephrine, and norepinephrine in turn activates CRF [72]. Much pharmacological, physiological and anatomical evidence supports an important role for a CRF–norepinephrine interaction in the locus coeruleus in response to stressors [86–88]. Moreover, evidence also indicates that norepinephrine stimulates CRF release in the paraventricular nucleus of the hypothalamus [89], bed nucleus of the stria terminalis and central nucleus of the amygdala. Such feed-forward systems may have powerful functional significance for the mobilization of an organism in response to environmental challenge, but such a mechanism may also be particularly vulnerable to pathology [72].
Dynorphin
Dynorphins are opioid peptides that are derived from the prodynorphin precursor, contain the leucine (leu)-enkephalin sequence at the N-terminal portion of the molecule, and are the presumed endogenous ligands for the κ-opioid receptor [90]. Dynorphins have widespread distribution throughout the CNS [91] and play a role in a wide variety of physiological systems, including neuroendocrine regulation, pain regulation, motor activity, cardiovascular function, respiration, temperature regulation, feeding behavior and stress responsivity [92]. Dynorphin has long been hypothesized to mediate negative emotional states, since κ-receptor agonists produce place aversions [93], depression and dysphoria in humans [94]. κ-receptor agonists also increase brain stimulation reward thresholds [95]. Dynorphin inhibits dopamine release, both via the origins and terminals of the mesolimbic dopamine system, and this effect has been hypothesized to contribute to the aversive effects of dynorphin [96–98].
Substantial evidence suggests that dynorphin peptide and dynorphin gene expression are activated in the striatum and ventral striatum during acute and chronic administration of cocaine [99–102]. Chronic ethanol exposure produces a decrease in κ-opioid receptors in the nucleus accumbens [103] and an increase in dynorphin B expression in the nucleus accumbens [104], providing evidence for an upregulation of dynorphin activity in ethanol dependence. Opiate withdrawal has been shown to increase dynorphin levels in both the amygdala [105] and nucleus accumbens [106].
Direct support for the hypothesis that dynorphin is one of the negative emotional systems recruited in dependence is the observation that the κ-receptor antagonist nor-binaltorphimine, when injected intracerebroventricularly or systemically, blocked ethanol self-administration in dependent, but not nondependent, animals [107,108]. κ-receptor-knockout mice also drank less ethanol in a two-bottle choice test using escalating doses of ethanol [109]. κ-receptor antagonist administration had no effect on baseline, limited-access cocaine or heroin self-administration in primates [110,111], but systemic administration of nor-binaltorphimine selectively blocked the increased cocaine self-administration associated with extended access, suggesting a motivational role for the dynorphin system in cocaine dependence [112]. Intracerebroventricular dynorphin A treatment decreased heroin-stimulated dopamine release and significantly increased heroin self-administration in daily 5 h sessions, whereas a κ-receptor antagonist had the opposite effects [113].
Stress also increases dynorphin activity [114] and suggests a potential interaction of dynorphin with CRF systems. Forced swim stress and inescapable footshock produced place aversions in mice that were blocked by a κ-receptor antagonist and dynorphin gene knockout, and CRF was hypothesized to produce its aversive effects via dynorphin activation [115]. Blockade of dynorphin activity, either via κ-receptor antagonism or prodynorphin gene disruption, blocked both stress-induced reinstatement of cocaine-induced place preference [116] and stress-induced reinstatement of cocaine-seeking behavior [117]. Evidence also shows that reinstatement of drug-seeking behavior via κ-opioid receptor activation is mediated by CRF [118]. Thus, the dynorphin–κ-opioid receptor system mimics stressor exposure in animals in producing aversive effects and inducing drug-seeking behavior, and this aversive response may involve reciprocal interactions with nucleus accumbens dopamine and/or extrahypothalamic brain CRF systems.
In humans, a repeat polymorphism in the dynorphin gene promoter is thought to regulate transcriptional activation in proportion to the number of repeats [119]. Alleles with longer repeats have been associated with the risk of methamphetamine dependence in a Japanese population [120]. An association has also been found between the number of repeats and both opioid dependence [121] and cocaine/alcohol codependence [122] in African–Americans. However, in comparison with these findings, Kreek and colleagues found a significant association between cocaine dependence and a risk SNP allele (rs910079, C allele) that resulted in reduced striatal preprodynorphin expression in Caucasians [123]. It is not known how these mRNA changes translate into dynorphin peptide levels. However, these data emphasize the importance of distinguishing and understanding the neurobiological effects of a potential genetic predisposition to addiction versus behavioral modifications resulting from drug-induced neuroadaptations.
Neuroadaptations within reward & brain stress systems in addiction
Based on their pharmacological properties, drugs of abuse elicit strong physiological responses that can produce rapid and long-lasting neuronal plasticity. Furthermore, subjective responses to drugs also act as visceral cues that promote drug-related associations with the environment [8]. Altogether, these neuroadaptations are hypothesized to underlie the development and maintenance of escalated drug intake and a heightened propensity for relapse. However, determining precisely which drug-induced neuroadaptations in turn drive specific components (e.g., drug taking vs drug seeking) within the multifaceted psychopathology of dependence is critically important [124]. In addition, as addiction can be viewed as a transitional process whereby recreational drug use eventually transitions to dependence, distinguishing which neuroadaptive changes are associated with which stage (early abuse vs late-stage dependence) is useful. For example, it needs to be asked whether the neurobiological changes elicited by casual drug use are simply magnified in the dependent state, or whether the transition to addiction is defined by the recruitment of entirely new neuroadaptations within separate neuronal circuits.
Within-system neuroadaptations in the reward system
During the transition to drug dependence, neural substrates in the ventral striatum important for the acute reinforcing effects of drugs of abuse, such as dopamine and opioid peptides (see previously), become compromised and contribute to an attenuation of positive reinforcement potential. In this state, drugs are presumably taken (sometimes in increasing amounts) in an attempt to restore the decreased function of positive reward systems. Neurobiological evidence for within-system neuroadaptations comes from the observation that chronic administration of all drugs of abuse decreases the function of the mesolimbic dopamine circuit. Decreases in dopaminergic and serotonergic neurotransmission in the nucleus accumbens occur during drug withdrawal in animals [125–127], and blunted firing of dopamine neurons in the ventral tegmental area has also been observed during withdrawal from opioids, nicotine and ethanol [128]. Pretreatment with dopamine receptor agonists can alleviate withdrawal-induced symptoms of decreased reward [129,130]. A definitive relationship between negative affective coding and diminished dopamine neuron activity was recently established by Liu et al., who generated a place aversion in animals by directly inactivating mesocortical A10 dopamine neurons [131].
Between-system neuroadaptations in the extended amygdala
Recent neuroanatomical data and new functional observations have provided support for the hypothesis that the neuroanatomical substrates for many of the altered motivational effects of drug dependence may involve a common neural circuitry that exists as a separate entity within the basal forebrain, termed the ‘extended amygdala’ [132]. The extended amygdala represents a macrostructure composed of several basal forebrain structures, including the bed nucleus of the stria terminalis, central medial amygdala and a transition zone in the posterior part of the medial nucleus accumbens (i.e., posterior shell) [133,134]. These structures have similarities in morphology, immunohistochemistry, and connectivity [132] and receive afferent connections from limbic cortices, the hippocampus, basolateral amygdala, midbrain and lateral hypothalamus. The efferent connections from this complex include the posterior medial (sublenticular) ventral pallidum, ventral tegmental area, various brainstem projections and, perhaps most intriguingly from a functional point of view, a considerable projection to the lateral hypothalamus [135].
Key elements of the extended amygdala include not only neurotransmitters associated with the positive reinforcing effects of drugs of abuse (e.g., dopamine), but also major components of the brain-stress systems associated with the negative reinforcement mechanisms hypothesized to underlie dependence [136]. Several neurotransmitters localized to the extended amygdala, such as CRF, norepinephrine and dynorphin, are activated during stress, in anxiety-like states, and during drug withdrawal in dependent animals. More importantly, antagonists of these neurochemical systems selectively block drug self-administration in dependent animals, suggesting a key role for these neurotransmitters in the extended amygdala in the negative reinforcement associated with drug dependence.
Role of positive & negative reinforcement mechanisms in drug dependence
The brain reward system is thus implicated in both the positive reinforcement produced by exposure to drugs of abuse and the negative reinforcement produced by drug dependence and is mediated by the ventral striatum and extended amygdala, respectively. Neuropharmacological studies in animal models of addiction have provided evidence for the dysregulation of selective neurochemical mechanisms in specific positive reinforcement (reward) neurochemical systems in the ventral striatum (dopamine, opioid peptides and GABA). Recruitment of brain-stress systems (i.e., CRF, dynorphin and norepinephrine) also occurs in the extended amygdala, which drives the negative motivational states associated with drug abstinence. The changes in reward and stress systems are hypothesized to not only contribute to the increased motivation to take drugs in dependence, but also to remain outside of a homeostatic state following acute dependence, and as such may contribute to the vulnerability for relapse in addiction (Figure 2).
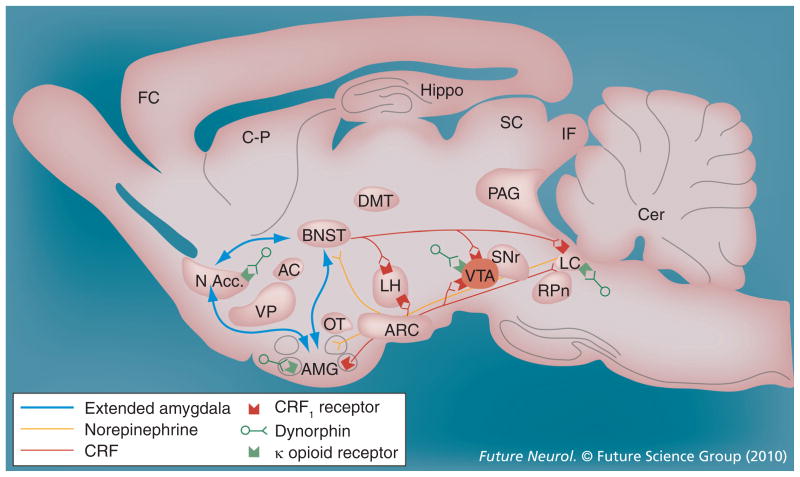
Figure 2. Recruitment of extended amygdala circuitry regulating the negative reinforcement mechanisms underlying drug addiction.
Neurotransmitter systems impacting the extended amygdala, such as CRF, norepinephrine and dynorphin/κ-opioid receptor systems, are potentiated during stress, in anxiety-like states and during drug withdrawal in dependent animals. AC: Anterior commissure; AMG: Amygdala; ARC: Arcuate nucleus; BNST: Bed nucleus of the stria terminalis; Cer: Cerebellum; C-P: Caudate-putamen; CRF: Corticotropin-releasing factor; DMT: Dorsomedial thalamus; FC: Frontal cortex; Hippo: Hippocampus; IF: Inferior colliculus; LC: Locus coeruleus; LH: Lateral hypothalamus; MFB: Medial forebrain bundle; N Acc.: Nucleus accumbens; OT: Olfactory tract; PAG: Periaqueductal gray; RPn: Reticular pontine nucleus; SC: Superior colliculus; SNr: Substantia nigra pars reticulata; VP: Ventral pallidum; VTA: Ventral tegmental area.
Within- & between-system neuroadaptations: points of intersection
Coinciding with diminished dopamine levels in the nucleus accumbens, chronic administration of cocaine also reduces inhibitory G protein subunits Giα and Goα in the nucleus accumbens [137–139]. Methamphetamine and heroin abusers showed decreases in Giα in the nucleus accumbens [140], and unpredictable stress also decreased Giα levels [141]. Inactivation and downregulation of inhibitory G protein α subunits with pertussis toxin injected into the nucleus accumbens increased cocaine and heroin self-administration under limited access conditions (3 h/day) [142]. In this case, rats receiving injections of pertussis toxin bilaterally into the nucleus accumbens demonstrated a prolonged increase in cocaine and heroin self-administration and an upward shift of dose–response functions. Extended access to drug availability also produces increases in self-administration and shifts upward of the dose–response function for cocaine with repeated sessions [143], identical to that produced by injections of pertussis toxin into the nucleus accumbens [142].
Paralleling reductions in inhibitory G protein levels, chronic exposure to a wide variety of drugs of abuse upregulates cAMP formation, cAMP-dependent protein kinase A (PKA) activity and PKA-dependent protein phosphorylation in the nucleus accumbens [137,144,145]. In turn, a variety of interventions that tonically activate the nucleus accumbens cAMP–PKA signaling system produce escalated drug self-administration and/or enhanced drug-seeking behavior. In addition to pertussis toxin-mediated downregulation of inhibitory G protein subunits, these manipulations include micro-injection of the PKA activator Sp-adenosine 3′,5′-cyclic monophosphorothioate triethyl-ammonium salt (Sp-cAMPS) [146] and micro-injection of the stimulatory G protein Gsα activator cholera toxin [147]. By contrast, micro-injection of the PKA inhibitor Rp-adenosine 3′,5′-cyclic monophosphorothioate triethyl-ammonium salt (Rp-cAMPS) reduces cocaine intake [146]. Thus, upregulation of a post-synaptic Gs–cAMP–PKA signaling pathway in the nucleus accumbens may constitute a critical neuroadaptation that is central to the establishment and maintenance of the addicted state (Figure 3) [148].
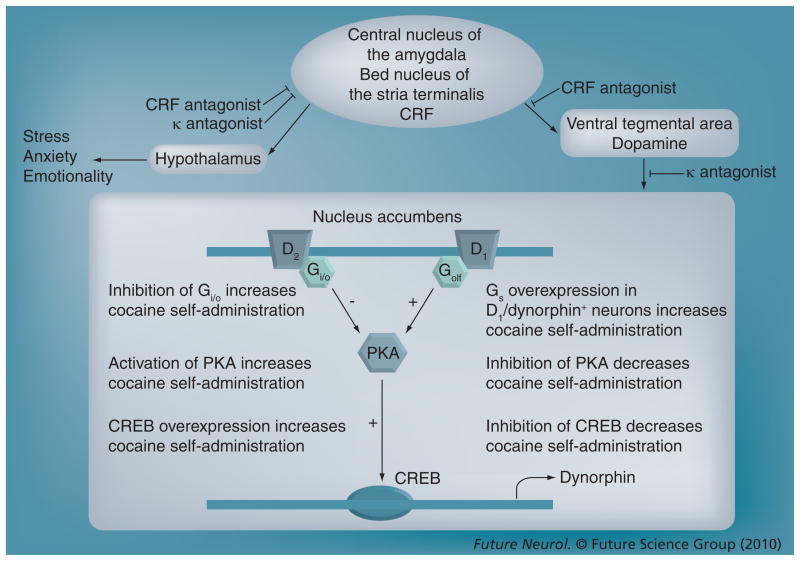
Figure 3. Regulation of the mesoaccumbens dopamine circuit by the extended amygdala and potential points of therapeutic intervention for drug addiction.
Cocaine self-administration depletes dopamine levels in the nucleus accumbens but results in a compensatory upregulation of the PKA–CREB–dynorphin signaling pathway. Importantly, mimicking these adaptations in rodent models results in an escalation of drug self-administration. The mesoaccumbens dopamine circuit comprises midbrain ventral tegmental area dopamine neurons that project to the nucleus accumbens in the basal forebrain. This system also receives afferent stimulation from the extended amygdala (central nucleus of the amygdala and bed nucleus of the stria terminalis). Escalated drug intake is associated with increased CRF release in the extended amygdala, and CRF-receptor antagonism within the extended amygdala may suppress the activation of the mesoaccumbens system during drug self-administration. In addition, given the projection from the extended amygdala to hypothalamic nuclei that regulate emotionality, CRF antagonism may act to reduce the stress and anxiety associated with escalated drug intake. Dynorphin acts at presynaptic κ-opioid receptors located on ventral tegmental area projection neurons to inhibit dopamine release, and upregulation of dynorphin release following cocaine self-administration is a possible mechanism underlying decreased reward during withdrawal. Blockade of nucleus accumbens κ-opioid receptors may restore both dopamine levels and the hedonic set point.
CREB: cAMP response-element binding protein; CRF: Corticotropin-releasing factor; D1: Dopamine D1 receptor; D2: Dopamine D2 receptor; G: G-protein; Gi/o: Inhibitory G-protein; Gs: Stimulatory G protein PKA: Protein kinase A.
The activation of dynorphin systems in the nucleus accumbens has long been associated with stimulation of the dopamine system by cocaine and amphetamine. Activation of stimulatory G-protein-coupled dopamine D1 receptors stimulates a cascade of events (including increased cAMP–PKA activity) that ultimately leads to cAMP response-element binding protein (CREB) phosphorylation and subsequent alterations in gene expression, most notably the transcription of prodynorphin mRNA. Subsequent activation of dynorphin systems could contribute to the dysphoric syndrome associated with cocaine dependence and also feedback to decrease dopamine release [149]. In fact, data suggest that activation of dynorphin systems mediates stress responses and is aversive [115,116]. Recently, Self and colleagues provided further evidence for this hypothesis by overexpressing constitutively active Gsα subunits in dynorphin-positive striatal neurons [150]. This mouse line exhibits a pronounced upregulation of dynorphin expression in the nucleus accumbens and increased levels of cocaine self-administration. Such enhanced dynorphin action in the nucleus accumbens could represent a convergence point of within (diminished proponent)- and between (intensified opponent)-system neuroadaptations (Figure 3).
Cross-sensitization between the effects of stress and dopamine circuitry may represent another point of intersection between proponent and opponent systems acting together to drive drug dependence. Although reduced mesoaccumbens dopamine function is linked to decreased reward in drug withdrawal, it may also set the stage for heightened sensitivity to stimuli that increase dopamine levels in the accumbens, including stress [151]. CRF directly stimulates dopamine terminals in the nucleus accumbens [152], and cocaine-induced dopamine release in the nucleus accumbens is blocked by CRF1-receptor antagonism [153]. In turn, mesolimbic dopamine influences CRF release [154] and CRF-mediated behaviors in the extended amygdala [155]. Interestingly, animals predisposed to amphetamine self-administration display more robust stress-induced increases in accumbens dopamine levels [156], suggesting that crosstalk between stress and dopamine systems may make individuals initially more sensitive to illicit drug exposure. Comparatively, in the postdependent state, models of stress-induced reinstatement to drug-seeking behavior have been generated in order to explore the neurobiological mechanisms of relapse [157]. Use of this paradigm has identified a central amygdala–VTA motive circuit that is essential for this behavior [158]. In addition to its effects in the central amygdala, CRF also acts within this circuit at the level of the VTA to drive stress-induced reinstatement along with other local neuroadaptations in cocaine-experienced, but not naive, animals [159]. Rats with extended access to cocaine self-administration were also more susceptible to reinstatement elicited by stress or central CRF infusion versus limited-access animals [160]. It should be noted that these experiments used foot-shock stressors to induce drug-seeking behavior and it would be useful to extend this model to more natural forms of stress [161].
Drug withdrawal-induced neuroadaptations
Relapse vulnerability is a defining feature of drug addiction and can persist for several months of abstinence from the drug [162]. Enhanced drug cravings may underlie the potential for relapse in humans and can be elicited by exposure to drug-associated contextual cues [163,164]. The ability of contextual cues to elicit relapse behavior can be modeled in rodents by re-exposure to the environment where drugs were self-administered on prior occasions [165]. In this model, drug-seeking behavior increases time-dependently as the withdrawal period progresses [166,167], a phenomenon hypothesized to prolong vulnerability to relapse. Similarly, a forced period of abstinence between drinking bouts increases alcohol intake in rats, a phenomenon known as the alcohol deprivation effect [168]. Moreover, multiple cycles of drinking and abstinence lead to excessive alcohol drinking, which can be attenuated by the anticraving drug acamprosate [169].
Chronic drug use produces numerous neurobiological changes involved in regulating drug taking and seeking, but relatively few changes have been shown to persist or arise during protracted withdrawal concomitant with increased drug-seeking behavior. Such changes include withdrawal time-dependent increases in extracellular dopamine levels, glutamate receptor subunits and ERK activation in the amygdala [166,170,171] as well as increases in brain-derived neurotrophic factor (BDNF) levels in several limbic brain regions [172] following chronic cocaine self-administration. In ethanol-dependent animals, protracted abstinence leads to increased gene expression of glutamate receptor subunits and several ERK family members in the prefrontal cortex [66], in addition to increased CRF levels in the amygdala [173]. These findings underscore the notion that withdrawal from drug self-administration is a dynamic state characterized by the emergence of late-forming changes in brain function that could exacerbate the propensity for renewed drug use during abstinence.
Conclusion
Decreases in dopamine function in the mesolimbic dopamine system and increases in brain stress neurotransmission in the extended amygdala both appear to contribute to the compulsivity associated with the development and maintenance of drug addiction. Decreased dopamine neuron firing and decreased basal release of dopamine may underlie some of the withdrawal symptoms encountered during abstinence. By comparison, extrahypothalamic CRF, norepinephrine and dynorphin systems play a role in mediating the anxiety-like effects and intense dysphoria of drug withdrawal. Both within- and between-system neuroadaptations may contribute to the escalated drug taking and intensified drug seeking associated with addiction. Many of these effects have been localized to the ventral striatum and extended amygdala regions, including the bed nucleus of the stria terminalis and central nucleus of the amygdala. Although the precise neurobiological mechanisms underlying the transition from recreational drug use to addiction are still being uncovered, continuing investigations into the specific functions of drug-induced neuroadaptations within stages of the addicted state may reveal new candidate mechanisms for this process. Finally, how reward systems are modulated by other known brain neurochemical systems localized to the extended amygdala (e.g., neuropeptide Y, vasopressin, orexin and substance P), how these systems interact with emotional pain pathways (in which the extended amygdala participates to convey the emotional valence), and how individuals differ at the molecular genetic (or epigenetic) level of analysis to convey loading on these circuits remain challenges for future research.
Future perspective: epigenetic mechanisms as possible long-term mediators of addiction
Although certain gene expression changes are known to alter an animal’s motivation for drugs, the precise molecular mechanisms underlying the persistence of this relationship are largely undefined. In contrast to the multitude of short-term effects of abused drugs on neuronal signaling, recent efforts have been focused on revealing and functionally understanding the mechanisms for near-permanent molecular neuroadaptations that result from chronic drug exposure. One potential source for such persistent brain changes is epigenetics (meaning ‘upon the genome’), which describes new, stable changes in gene expression resulting from chromatin modifications. Although epigenetic mechanisms are a normal component of dynamic brain plasticity throughout the human lifespan [174], specific epigenetic alterations have also been described in several human neurological and psychiatric disorders, from Rett syndrome [175] to depression [176].
Gene expression is gated by the net sum of enzymatic regulation of protein scaffolds surrounding the DNA, and even the DNA itself. These factors include covalent histone modifications (including methylation, acetylation, phosphorylation and ubiquitination), as well as direct methylation of DNA nucleotides. Alterations in chromatin architecture can facilitate or inhibit the transcriptional potential of specific genes by gating the accessibility of transcription factors. A primary drug-induced chromatin modification is histone acetylation, which often corresponds to an increase in transcription of the associated gene. Absolute levels of histone acetylation are maintained by the balance of enzymatic activities of histone acetyl-transferases (HATs; which add acetyl groups to histone proteins) and histone deacetylases (HDACs; which cleave acetyl groups from histones). In the nucleus accumbens of rats, chronic (but not acute) cocaine exposure leads to a long-term hyperacetylation of the gene promotors for BDNF and Cdk5 [177], two proteins known to be upregulated by cocaine exposure, and in the case of BDNF, to produce increases in cocaine self-administration and cocaine-seeking behavior during withdrawal [178]. Interestingly, modifying total HDAC activity in the nucleus accumbens made cocaine either more rewarding (HDAC inhibition) or less rewarding (HDAC5 overexpression using viral-mediated gene transfer) to rats as measured by conditioned place preference (in which an animal learns to associate the rewarding effects of cocaine with a specific environment). An important question to be answered is whether manipulation of HDAC activity modifies an animal’s volitional self-administration of cocaine or other drugs of abuse. In addition to its effects on cocaine reward, loss of HDAC5 function causes an exacerbation of both chronic social defeat stress [179] and chronic neuropathic pain [180]. Therefore, epigenetic modifications could act through both positive and negative motivational systems in order to influence drug dependence.
Many of the initial studies regarding epigenetic influences on drug reward have been limited to the ventral striatum, although stable changes in gene expression in other brain regions could also exacerbate certain aspects of drug dependence. For example, alcoholism is often associated with the emergence of negative emotional states (such as anxiety and depression), which could be exacerbated by dysregulated signaling in the extended amygdala. Recent investigations by Pandey and colleagues propose chromatin remodeling as a key mechanism whereby negative reinforcement mechanisms may drive excessive alcohol drinking [181]. Anxiety-like behavior associated with withdrawal from chronic alcohol exposure was linked to increased HDAC activity in the central nucleus of the amygdala and decreased acetylation of the gene coding for neuropeptide Y, an anxiolytic (antistress) neurotransmitter present in high levels within the amygdala. In turn, inhibition of HDAC activity prevented ethanol withdrawal-induced anxiety-like behavior.
The ultimate effects of gene modification are highly dependent on the brain region and genetic substrate and may differentially regulate responsiveness to distinct drugs of abuse when manipulated systemically. As indicated above, global HDAC inhibition reduced ethanol withdrawal-induced anxiety-like behavior but potentiated cocaine reward. Further elucidation of epigenetic mechanisms within motivational circuits will shed light on how these systems respond to and change after chronic drug exposure to promote a reconstruction of gene expression, and may also provide an additional biochemical end point to determine the potential efficacy of new therapeutics for dependence.
Executive summary.
Drug addiction: recreational drug use versus dependence
Addiction is a chronic, relapsing disorder that represents a significant progression from casual drug use.
Current investigations are focused on the study of long-term neuroadaptational changes caused by chronic drug exposure.
Motivation: positive & negative reinforcement mechanisms
Both positive and negative reinforcement mechanisms underlie the motivation for drug seeking and use.
Drugs of abuse usurp brain reward systems, leading to an obsession with obtaining and using drugs despite adverse consequences and to the exclusion of obtaining other rewards.
A negative emotional state develops with chronic use, and drugs are subsequently taken to alleviate negative motivational symptoms associated with withdrawal.
Transition to addiction: homeostasis versus allostasis
As addiction develops, normal homeostatic brain regulatory functions that control reward systems may become dysregulated, leading to the establishment of a new hedonic set point, termed allostasis.
Neurobiology of reward & positive reinforcement
All drugs of abuse acutely decrease brain reward thresholds.
Dopamine and opioid peptide systems are responsible for mediating the acute, positive reinforcing effects of drugs of abuse.
Recruitment of brain stress systems in addiction
Chronic drug use leads to the recruitment of brain stress neurotransmitter systems, including corticotropin-releasing factor, norepinephrine and dynorphin.
Activation of these systems drives the dysphoria associated with drug withdrawal, and blockade of these systems reduces excessive drug self-administration in dependent, but not in nondependent, animals.
Neuroadaptations within reward & stress systems in addiction
Within-system neuroadaptations in the reward circuit following chronic drug use include diminished dopamine neurotransmission in the nucleus accumbens.
Brain stress system neurotransmitters localized to the extended amygdala are activated during drug withdrawal in dependent animals, representing a between-system neuroadaptation associated with escalating drug use.
Upregulation of the protein kinase A/cAMP response-element binding protein/dynorphin system in the nucleus accumbens and cross-sensitization between stress and mesocortical dopamine systems may represent points of convergence of within- and between-system neuroadaptations responsible for the establishment and maintenance of the addicted state.
Drug withdrawal-induced neuroadaptations
Neuroadaptations that persist or arise during drug withdrawal may underlie the propensity for renewed drug seeking and use during protracted abstinence.
Future perspective
Epigenetic neuroadaptations may represent one possible mechanism responsible for the persistence of addicted states. The reconstruction of gene expression in reward and stress systems following drug exposure could facilitate the generation of an allostatic state conducive to long-term drug dependence.
Acknowledgments
The authors would like to thank Michael Arends for editorial assistance.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors were financially supported by the Pearson Center for Alcoholism and Addiction Research and NIH grants: DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases; DA04043, DA04398 and DA023957 from the National Institute on Drug Abuse; and AA006420 and AA08459 from the National Institute on Alcohol Abuse and Alcoholism. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.American Psychiatric Association. Diagnositc and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC, USA: 2000. [Google Scholar]
- 2▪▪.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. Theoretical review arguing that drug addiction results from a dysregulation of reward homeostasis. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Statistics; MD, USA: 2008. [Google Scholar]
- 6.Bindra D. A Theory of Intelligent Behavior. Wiley; NY, USA: 1976. [Google Scholar]
- 7.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 8▪.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. Hypothesizes that the persistence of drug addiction results from a perturbation of reward-related learning and memory processes. [DOI] [PubMed] [Google Scholar]
- 9.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 10.Solomon RL, Corbit JD. An opponent-process theory of motivation: 1 Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 11.Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- 12.Poulos CX, Cappell H. Homeostatic theory of drug tolerance: a general model of physiological adaptation. Psychol Rev. 1991;98:390–408. doi: 10.1037/0033-295x.98.3.390. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 18.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. In: McGinty JF, editor. Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse. New York Academy of Sciences; NY, USA: 1999. pp. 486–498. [DOI] [PubMed] [Google Scholar]
- 19.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley and Sons; UK: 1988. pp. 629–649. [Google Scholar]
- 20.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 21.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 22.Koob GF, Winger GD, Meyerhoff JL, Annau Z. Effects of D-amphetamine on concurrent self-stimulation of forebrain and brain stem loci. Brain Res. 1977;137:109–126. doi: 10.1016/0006-8993(77)91015-0. [DOI] [PubMed] [Google Scholar]
- 23.Simon H, Stinus L, Tassin JP, et al. Is the dopaminergic mesocorticolimbic system necessary for intracranial self-stimulation? Biochemical and behavioral studies from A10 cell bodies and terminals. Behav Neural Biol. 1979;27:125–145. doi: 10.1016/s0163-1047(79)91745-x. [DOI] [PubMed] [Google Scholar]
- 24.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- 25.Hernandez G, Hamdani S, Rajabi H, et al. Prolonged rewarding stimulation of the rat medial forebrain bundle: neurochemical and behavioral consequences. Behav Neurosci. 2006;120:888–904. doi: 10.1037/0735-7044.120.4.888. [DOI] [PubMed] [Google Scholar]
- 26.Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- 27.Miliaressis E, Emond C, Merali Z. Re-evaluation of the role of dopamine in intracranial self-stimulation using in vivo microdialysis. Behav Brain Res. 1991;46:43–48. doi: 10.1016/s0166-4328(05)80095-6. [DOI] [PubMed] [Google Scholar]
- 28.Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- 29.Miliaressis E, Le Moal M. Stimulation of the medial forebrain bundle: behavioral dissociation of its rewarding and activating effects. Neurosci Lett. 1976;2:295–300. doi: 10.1016/0304-3940(76)90163-4. [DOI] [PubMed] [Google Scholar]
- 30.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 31.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- 33.Shippenberg TS, Koob GF. Recent advances in animal models of drug addiction and alcoholism. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams and Wilkins; PA, USA: 2002. pp. 1381–1397. [Google Scholar]
- 34.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 35.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 36.Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- 37.Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the ‘anxiogenic-like’ effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 38.Schulteis G, Stinus L, Risbrough VB, Koob GF. Clonidine blocks acquisition but not expression of conditioned opiate withdrawal in rats. Neuropsychopharmacology. 1998;19:406–416. doi: 10.1016/S0893-133X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 39.Harris GC, Aston-Jones G. β-Adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- 40.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- 41.Charlton BG, Ferrier IN, Perry RH. Distribution of corticotropin-releasing factor-like immunoreactivity in human brain. Neuropeptides. 1987;10:329–334. doi: 10.1016/s0143-4179(87)90083-7. [DOI] [PubMed] [Google Scholar]
- 42.Swanson LW, Sawchenko PE, Rivier J, Vale W. The organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 43.Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- 44.Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- 45.Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the ‘anxiogenic’ response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 46.Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, α-helical CRF9–41, reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- 50.George O, Ghozland S, Azar MR, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 52.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 54.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 56.Weiss F, Ciccocioppo R, Parsons LH, et al. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. In: Jenab V, editor. Quinones – The Biological Basis of Cocaine Addiction. New York Academy of Sciences; NY, USA: 2001. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 57.Matta SG, Valentine JD, Sharp BM. Nicotinic activation of CRH neurons in extrahypothalamic regions of the rat brain. Endocrine. 1997;7:245–253. doi: 10.1007/BF02778147. [DOI] [PubMed] [Google Scholar]
- 58.Slawecki CJ, Thorsell AK, Khoury AE, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 59▪.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. Demonstrates that blockade of corticotropin-releasing factor receptors specifically within the central nucleus of the amygdala reduces the increased alcohol drinking associated with dependence without altering alcohol intake in nondependent animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merlo-Pich E, Lorang M, Yeganeh M, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez de Fonseca F, Carrera MRA, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 63.Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenwell TN, Funk CK, Cottone P, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 66.Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 67.Treutlein J, Kissling C, Frank J, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 68.Blomeyer D, Treutlein J, Esser G, et al. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 69.Morilak DA, Barrera G, Echevarria DJ, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Maynert EW, Levi R. Stress-induced release of brain norepinephrine and its inhibition by drugs. J Pharmacol Exp Ther. 1964;143:90–95. [PubMed] [Google Scholar]
- 71.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 72.Koob GF. Corticotropin-releasing factor, norepinephrine and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 73.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 74.Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 75.Borg S, Czamecka A, Kvande H, Mossberg D, Sedvall G. Clinical conditions and concentrations of MOPEG in the cerebrospinal fluid of male alcoholic patients during withdrawal. Alcohol Clin Exp Res. 1985;9:103–108. doi: 10.1111/j.1530-0277.1983.tb05496.x. [DOI] [PubMed] [Google Scholar]
- 76.Borg S, Kvande H, Sedvall G. Central norepinephrine metabolism during alcohol intoxication in addicts and healthy volunteers. Science. 1981;213:1135–1137. doi: 10.1126/science.7268421. [DOI] [PubMed] [Google Scholar]
- 77.Fujimoto A, Nagao T, Ebara T, Sato M, Otsuki S. Cerebrospinal fluid monoamine metabolites during alcohol withdrawal syndrome and recovered state. Biol Psychiatry. 1983;18:1141–1152. [PubMed] [Google Scholar]
- 78.Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol: I Suppression of ethanol consumption in laboratory rats following dopamine-β-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- 79.Brown ZW, Amit Z. The effects of selective catecholamine depletions by 6-hydroxydopamine on ethanol preference in rats. Neurosci Lett. 1977;5:333–336. doi: 10.1016/0304-3940(77)90109-4. [DOI] [PubMed] [Google Scholar]
- 80.Davis WM, Werner TE, Smith SG. Reinforcement with intragastric infusions of ethanol: blocking effect of FLA 57. Pharmacol Biochem Behav. 1979;11:545–548. doi: 10.1016/0091-3057(79)90038-8. [DOI] [PubMed] [Google Scholar]
- 81.Ventura R, De Carolis D, Alcaro A, Puglisi-Allegra S. Ethanol consumption and reward depend on norepinephrine in the prefrontal cortex. Neuroreport. 2006;17:1813–1817. doi: 10.1097/01.wnr.0000239964.83566.75. [DOI] [PubMed] [Google Scholar]
- 82.Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker BM, Rasmussen DD, Raskind MA, Koob GF. α1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wee S, Mandyam CD, Lekic DM, Koob GF. α1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The α1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. In: Tache Y, Rivier C, editors. Corticotropin-Releasing Factor and Cytokines: Role in the Stress Response. New York Academy of Sciences; NY, USA: 1993. pp. 173–188. [DOI] [PubMed] [Google Scholar]
- 87.Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- 88.Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 89.Alonso G, Szafarczyk A, Balmefrezol M, Assenmacher I. Immunocytochemical evidence for stimulatory control by the ventral noradrenergic bundle of parvocellular neurons of the paraventricular nucleus secreting corticotropin-releasing hormone and vasopressin in rats. Brain Res. 1986;397:297–307. doi: 10.1016/0006-8993(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 90.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the κ opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 91.Watson SJ, Khachaturian H, Akil H, Coy DH, Goldstein A. Comparison of the distribution of dynorphin systems and enkephalin systems in brain. Science. 1982;218:1134–1136. doi: 10.1126/science.6128790. [DOI] [PubMed] [Google Scholar]
- 92.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 93.Mucha RF, Herz A. Motivational properties of κ and μ opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 94.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 95.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of κ-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 96.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. κ-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- 100.Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 101.Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 102.Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates κ and μ, but not δ, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 103.Rosin A, Lindholm S, Franck J, Georgieva J. Downregulation of κ opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett. 1999;275:1–4. doi: 10.1016/s0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- 104.Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- 105.Rattan AK, Koo KL, Tejwani GA, Bhargava HN. The effect of morphine tolerance dependence and abstinence on immunoreactive dynorphin (1–13) levels in discrete brain regions, spinal cord, pituitary gland and peripheral tissues of the rat. Brain Res. 1992;584:207–212. doi: 10.1016/0006-8993(92)90896-h. [DOI] [PubMed] [Google Scholar]
- 106.Turchan J, Lason W, Budziszewska B, Przewlocka B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31:24–28. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 107.Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. κ-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kovacs KM, Szakall I, O’Brien D, et al. Decreased oral self-administration of alcohol in κ-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29:730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- 110.Negus SS. Effects of the κ opioid agonist U50,488 and the κ opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- 111.Negus SS, Henriksen SJ, Mattox A, et al. Effect of antagonists selective for μ, δ and κ opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther. 1993;265:1245–1252. [PubMed] [Google Scholar]
- 112.Wee S, Orio L, Ghirmai S, Cashman J, Koob GF. Inhibition of κ opioid receptors attenuates the increased motivation for cocaine in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xi ZX, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by κ opioid receptors: an in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther. 1998;284:151–161. [PubMed] [Google Scholar]
- 114.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 115.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McLaughlin JP, Marton-Popovici M, Chavkin C. κ opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel κ opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 118.Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. κ agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- 119.Zimprich A, Kraus J, Woltje M, et al. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- 120.Nomura A, Ujike H, Tanaka Y, et al. Genetic variant of prodynorphin gene is risk factor for methamphetamine dependence. Neurosci Lett. 2006;400:158–162. doi: 10.1016/j.neulet.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 121.Ray R, Doyle GA, Crowley JJ, et al. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr Genet. 2005;15:295–298. doi: 10.1097/00041444-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 122.Williams TJ, LaForge KS, Gordon D, et al. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict Biol. 2007;12:496–502. doi: 10.1111/j.1369-1600.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 123.Yuferov V, Ji F, Nielsen DA, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124▪.Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. Argues for the importance of meticulously studying the precise functions of drug-induced neuroadaptations in animal models across the addiction timeline. [DOI] [PubMed] [Google Scholar]
- 125.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 126.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 127.Weiss F, Parsons LH, Schulteis G, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- 129.Markou A, Koob GF. Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology. 1992;7:213–224. [PubMed] [Google Scholar]
- 130.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 133.Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- 134.Heimer L, Alheid G. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function. Plenum Press; NY, USA: 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 135.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 136.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 137.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- 138.Striplin CD, Kalivas PW. Robustness of G protein changes in cocaine sensitization shown with immunoblotting. Synapse. 1993;14:10–15. doi: 10.1002/syn.890140103. [DOI] [PubMed] [Google Scholar]
- 139.Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi α and Go α in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 140.McLeman ER, Warsh JJ, Ang L, et al. The human nucleus accumbens is highly susceptible to G protein down-regulation by methamphetamine and heroin. J Neurochem. 2000;74:2120–2126. doi: 10.1046/j.1471-4159.2000.0742120.x. [DOI] [PubMed] [Google Scholar]
- 141.Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 142.Self DW, Terwilliger RZ, Nestler EJ, Stein L. Inactivation of Gi and Go proteins in nucleus accumbens reduces both cocaine and heroin reinforcement. J Neurosci. 1994;14:6239–6247. doi: 10.1523/JNEUROSCI.14-10-06239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 144.Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–2213. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- 145.Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Whisler K, Hall S, Edwards S, Akkentli F, Self DW. Tonic activation of NAc PKA signaling facilitates D2 receptor-mediated relapse to cocaine seeking and locomotor activity. Abstr Soc Neurosci. 2003;29:420.417. [Google Scholar]
- 148.Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 150.Larson EB, Whisler K, Smagula C, et al. Preferential over-expression of Gs-α in direct and indirect striatal output pathways differentially alters cocaine-taking and -seeking behaviors. Soc Neurosci Abstr. 2009;35 (Abstract 649.11) [Google Scholar]
- 151.Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- 152.Pan JT, Lookingland KJ, Moore KE. Differential effects of corticotropin-releasing hormone on central dopaminergic and noradrenergic neurons. J Biomed Sci. 1995;2:50–56. doi: 10.1007/BF02257925. [DOI] [PubMed] [Google Scholar]
- 153.Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- 154.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rouge-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- 157.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang B, Shaham Y, Zitzman D, et al. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mantsch JR, Baker DA, Francis DM, et al. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yap JJ, Miczek KA. Stress and rodent models of drug addiction: role of VTA–accumbens–PFC–amygdala circuit. Drug Discov Today Dis Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 163.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 164.Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict Behav. 2006;31:1881–1894. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 165.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 166.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 167.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- 169.Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- 170.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 171▪▪.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. Impressive study demonstrating the role of extracellular signal-regulated kinase signaling in the central nucleus of the amygdala in mediating the withdrawal time-dependent increase in cocaine-seeking behavior in rats. [DOI] [PubMed] [Google Scholar]
- 172.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 174.Siegmund KD, Connor CM, Campan M, et al. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS ONE. 2007;2(9):E895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 176.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 177.Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 178▪.Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. Demonstrates how early drug-induced neuroadaptations can have long-lasting effects on drug-seeking behavior. [DOI] [PubMed] [Google Scholar]
- 179.Renthal W, Maze I, Krishnan V, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 180.Renthal W, Terzi D, Olson EN, Nestler EJ, Zachariou V. Histone deacetylase 5 controls analgesic responsiveness to tricyclic antidepressants in a mouse model of neuropathic pain. Soc Neurosci Abstr. 2008;34 (Abstract 368.6) [Google Scholar]
- 181.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]