Abstract
Background
Opioid dependence is common in HIV clinics. Buprenorphine/naloxone (BUP) is an effective treatment for opioid dependence that may be used in routine medical settings.
Objective
To compare clinic-based treatment with BUP (clinic-based BUP) with case-management and referral to an opioid treatment program (referred-treatment).
Design
Single-center, 12-month randomized trial. Participants and investigators were aware of treatment assignments.
Setting
HIV clinic in Baltimore, Maryland.
Patients
93 HIV-infected, opioid-dependent subjects who were not receiving opioid agonist therapy and were not dependent on alcohol or benzodiazepines.
Intervention
The clinic-based BUP strategy included BUP induction and dose titration, urine drug test monitoring, and individual counseling; the referred-treatment arm included case management and referral to an opioid treatment program.
Measurements
Initiation and long-term receipt of opioid agonist therapy, urine drug test results, visit attendance with primary HIV providers, use of antiretroviral therapy, and changes in HIV RNA levels and CD4 cell counts.
Results
The average estimated participation in opioid agonist therapy was 74% (95% CI 61%–84%) in clinic-based BUP and 41% (29%–53%) in referred-treatment (p<0.001). Opioid and cocaine positive urine drug tests were significantly less frequent in clinic-based BUP than in referred-treatment, and study subjects in clinic-based BUP attended significantly more HIV primary care visits than study subjects in referred-treatment. Use of antiretroviral therapy and changes in HIV RNA levels and CD4 cell counts did not differ in the 2 arms of the study.
Limitations
This was a small single-center study, follow-up was only fair, and there was an imbalance in recent drug injection in the study arms at baseline.
Conclusions
This study suggests that management of HIV-infected, opioid-dependent patients with a clinic-based BUP strategy facilitates access to opioid agonist therapy and improves substance abuse treatment outcomes.
Primary Funding Source
Health Resources Services Administration, Special Projects of National Significance.
Keywords: HIV, opioid dependence, buprenorphine, opioid agonist treatment, methadone, opioid treatment program
INTRODUCTION
Until recently, the treatment of opioid dependence with opioid agonist treatment was available only in highly regulated opioid treatment programs. In 2002, the sublingual combination tablet buprenorphine/naloxone (BUP) was approved by the Food and Drug Administration for office-based treatment of opioid-dependent individuals (1,2). The treatment of opioid dependence in medical settings has particular resonance in HIV care. Injection drug use is a major risk factor for the acquisition of HIV and opioid dependence is highly prevalent in HIV clinics.
It is not known how treating opioid-dependent patients with BUP in an HIV clinic compares to the traditional model of referring them to specialized opioid treatment programs. To address this question, we conducted a single-center, non-blinded, 12-month, randomized trial comparing clinic-based treatment with BUP (clinic-based BUP) to case-management and referral to an opioid treatment program (referred-treatment) for opioid-dependent participants in an urban HIV clinic. We hypothesized that clinic-based BUP would lead to improved engagement and outcomes for both substance abuse and HIV treatment compared to referred-treatment.
METHODS
Setting
We conducted this study in the Johns Hopkins HIV Clinic between November 2005 and April 2009. Our study was one of 10 sites supported by the Health Resources Services Administration Special Projects of National Significance to conduct demonstration projects that included the integration of BUP treatment into HIV primary care (available at http://www.bhives.org). The Johns Hopkins Medicine and New York Academy of Medicine Institutional Review Boards approved this study.
Participant selection and randomization
Individuals were eligible for the study if they were at least 18 years of age, received care in the Johns Hopkins HIV Clinic, met Diagnostic and Statistical Manual IV criteria for opioid dependence (3), had a positive urine drug test for opioids, sought opioid agonist therapy (and were willing to receive either BUP or methadone), and provided written, informed consent. Individuals were excluded if they were currently receiving opioid agonist therapy, were pregnant or unwilling to use birth control if female (because of inadequate BUP safety data in this population), were allergic to BUP, required chronic opioid treatment for pain, or had an alanine aminotransferase level greater than 5-times the upper limit of normal. Additionally, because of safety concerns regarding use of BUP in combination with abuse of benzodiazepines or alcohol, we excluded individuals with active benzodiazepine or alcohol dependence at screening.
Using a statistical software package, we generated a random, non-stratified treatment allocation list prior to study inception, with block sizes that varied randomly between 2 and 10. An individual not involved with the study placed treatment assignment cards into sequentially-numbered, opaque envelopes. At the time a participant enrolled in the study, the study coordinator opened the next treatment allocation envelope in the sequence and recorded the treatment assignment and envelope sequence number in the study record. Neither participants nor investigators were blinded to treatment assignments.
Interventions
The clinic-based BUP arm was managed by a licensed practical nurse with training and experience as a substance abuse counselor (interventionist). The interventionist met briefly with participants after randomization to schedule a physician visit and BUP induction date (generally within 7 days) and to instruct participants how to prepare for induction. We used a 2-day BUP induction protocol in which up to 3 BUP doses were given each day according opioid withdrawal severity, measured by the Clinical Institute Narcotic Assessment (4). Following induction, participants were dosed in the clinic with BUP three-times weekly for 2 to 4 weeks until stabilized, at which point they were switched to weekly reporting. A treatment team physician met with subjects 4–6 weeks after starting BUP and at other times as indicated. Subjects who missed more than 3 consecutive days of BUP dosing had to repeat BUP induction (5).
At reporting visits, which lasted between 10 and 40 minutes, clinic-based BUP participants received unstructured individual counseling, provided urine samples for point-of-care urine drug tests, took observed BUP doses, and received take-home supplies of BUP to last until their next visit. A treatment team, comprising the interventionist and 2 to 5 BUP-prescribing physicians, met weekly to discuss participants’ progress in treatment. The treatment team set reporting frequencies, which ranged from thrice weekly to monthly, according to urine drug test results and other factors. We required at least 4 consecutive urine drug tests negative for opioids and cocaine before reporting intervals were increased beyond one week. Participants’ primary HIV providers were variably involved with BUP treatment, but their involvement was not required in our model.
Participants assigned to referred-treatment were enrolled in an intensive case management program that has operated in the clinic since 1998. We used study funds to support staff time that was devoted to this project. However, the case managers had no investment (positive or negative) in the trial or its outcome. A social worker or registered nurse in the case management program met with referred-treatment participants shortly after randomization and developed treatment plans that were primarily focused on linking participants to opioid treatment programs, but may have included other issues, like food and housing needs. Case managers used the Baltimore Substance Abuse Systems hotline to identify opioid treatment programs according to proximity, insurance requirements, treatment availability, and participant preference. Since several opioid treatment programs in the area had Ryan White grant support to treat uninsured HIV-infected individuals (including a Johns Hopkins-affiliated opioid treatment program), the lack of medical insurance was not a barrier to program entry. After referral to an opioid treatment program, case managers followed-up with participants to ascertain if they had attended scheduled appointments, and assist them with rescheduling if needed.
Participant follow-up
At each study visit - baseline, 1, 3, 6, 9, and 12 months - participants provided urine samples for urine drug tests and responded to questions administered by audio computer-assisted self interview (6). Only urine drug tests obtained at study visits, and not those obtained by opioid treatment programs or the clinic-based BUP intervention, were used for study outcomes. CD4 lymphocyte counts and HIV RNA levels were measured at baseline, 6 months, and 12 months. Participants received a modest reimbursement for study visits to compensate for travel and time. We abstracted clinic medical records at baseline and quarterly to assess visits with HIV primary care providers and use of ART.
Sample size considerations, outcomes, and statistical methods
We selected a sample size of 120 to provide 80% power to detect a relative hazard 2.0 or greater for initiation of opioid agonist therapy, assuming 50% of subjects overall would initiate opioid agonist therapy and a 2-sided type-1 error of 0.05. Outcomes included self-reported participation in opioid agonist therapy at study visits, study-visit urine drug tests positive for opioids (opiates or oxycodone) or cocaine, visits with primary HIV providers, months of antiretroviral therapy use, changes in HIV RNA levels and CD4 cell counts, and proportion with emergency department visits or hospitalizations.
We adhered to the intent-to-treat principle in all analyses. Stata version 10/11 (StataCorp, College Station, Texas) and R (http://www.r-project.org) software were used for statistical analyses. We used the Fischer’s exact test and the Wilcoxon rank sum test to compare discrete and continuous variables, respectively. We compared time to initiation of opioid agonist therapy in the study arms with the log rank test, censoring at time of death or last study visit. We used mixed effects logistic and linear models to assess longitudinal outcomes (7), including participation in opioid agonist therapy, urine drug test results for opioids and cocaine, HIV RNA levels, and CD4 cell counts. For discrete outcomes, we obtained marginal probabilities from mixed effect models using the gllamm procedure in Stata (8). To assess the influence of missing data on longitudinal outcomes, we conducted random effects pattern-mixture models, in which patterns of missing observations were incorporated as covariates into models (9–11). Additionally, because of a difference in recent injection drug use in the study arms at baseline, we conducted post-hoc sensitivity analyses that included a term for recent injection drug use in the models.
Role of the funding source
The funding source for this trial had no role in the design, implementation, or interpretation of results.
RESULTS
Participant disposition and characteristics
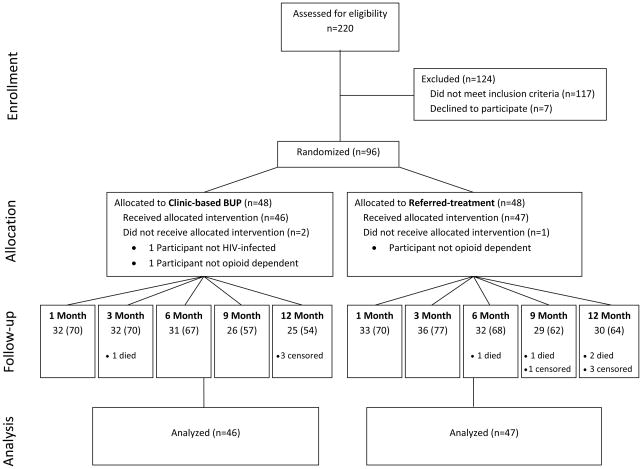
A total of 220 individuals were screened for the study, of which 96 were randomized (Figure 1). Among 117 individuals who did not meet inclusion criteria, 40 (34%) were not opioid dependent, 24 (21%) were not treated in the HIV clinic, 21 (18%) were receiving methadone, 13 (11%) met criteria for alcohol dependence, and 19 (16%) were excluded for other reasons. Two subjects allocated to clinic-based BUP and 1 subject allocated to referred-treatment were excluded from the study and analysis due to protocol violations identified shortly after enrollment (2 were not using opioids and 1 was HIV-seronegative).
Figure 1.
Disposition of HIV-infected, opioid-dependent participants enrolled in a randomized trial comparing clinic-based BUP to referred-treatment. Follow-up shows the number (%) of subjects attending follow-up study visits. Censored individuals had not completed follow-up when the study concluded.
A total of 93 participants (46 in clinic-based BUP and 47 in referred-treatment) were included in intent-to-treat analyses. Five subjects died during the study: 1 in clinic-based BUP from complications of end-stage renal disease and 4 in referred-treatment from complications of end-stage renal disease (1), pneumonia and sepsis (1), and unknown causes (2). Nine and 12-month visits were administratively censored in 1 and 6 subjects, respectively, at cessation of study follow-up on April 15, 2009. Participant follow-up visit attendance was similar in the 2 arms (Figure 1). Baseline characteristics were similar in the study arms (Table), with the exceptions that clinic-based BUP participants were less likely report injection drug use in the 30 days prior to enrollment (p=0.001) and were less likely to be hepatitis C virus co-infected (p=0.045).
Table.
Baseline characteristics of HIV-infected, opioid-dependent participants enrolled in a randomized trial comparing clinic-based BUP to referred-treatment
| Characteristic | Referred-treatment (n=47) |
Clinic-based BUP (n=46) |
|---|---|---|
| Female, n (%) | 12 (26) | 14 (30) |
| African American, n (%) | 47 (100) | 44 (96) |
| Age, years, median (IQR) | 46 (42–52) | 45 (42–50) |
| High school graduate or equivalent, n (%) | 22 (48) | 27 (59) |
| Housing status, n (%) | ||
| Own or rent home | 18 (38) | 27 (59) |
| Stay with family/friends | 22 (47) | 14 (30) |
| Group home | 2 (4) | 2 (4) |
| Homeless, shelter, or single room occupancy | 5 (11) | 3 (7) |
| Employed, n (%) | 15 (32) | 13 (28) |
| Depression scorea, median (IQR) | 9 (6–13) | 10 (6–14) |
| Incarcerated ≥ 3 days in lifetime, n (%) | 37 (79) | 35 (76) |
| Type of opioid used in prior month, n (%) | ||
| Heroin | 47 (100) | 42 (91) |
| Prescription opioid | 12 (26) | 13 (28) |
| Days opioid used in prior month, median (IQR) | 30 (20–30) | 30 (20–30) |
| Used cocaine in prior month, n (%) | 37 (79) | 30 (65) |
| Days usedb, median (IQR) | 10 (5–30) | 15 (4–25) |
| Used alcohol in prior month, n (%) | 28 (60) | 22 (48) |
| Days usedb, median (IQR) | 8 (3–15) | 9 (4–15) |
| Injected drugs in prior month, n (%) | 36 (77) | 20 (43)c |
| Years of opioid use, median (IQR) | 20 (14–26) | 18 (10–25) |
| Hospitalized in prior 3 months, n (%) | 11 (23) | 15 (33) |
| AIDS-defining opportunistic condition in prior 3 months, n (%) | 3 (7) | 6 (13) |
| Hepatitis C antibody positive,d n (%) | 38 (86) | 30 (67)e |
| Taking antiretroviral therapy, n (%) | 24 (51) | 25 (54) |
| Nadir CD4 count, cells × 109/L, median (IQR) | 206 (40–351) | 110 (12–232)f |
| Current CD4 count, cells × 109/L, median (IQR) | 304 (177–482) | 228 (68–397) |
| HIV RNA < 400 copies/mL, n (%) | 19 (40) | 19 (41) |
| HIV RNA log10 copies/mL in subjects with HIV RNA > 400 copies/mL, median (IQR) | 4.6 (3.0–5.0) | 4.4 (3.9–5.1) |
BUP, buprenorphine/naloxone; IQR, interquartile range
CES-D 10-item survey addressing symptoms in previous week, higher scores correspond to more severe depressive symptoms (12).
Days of substance use in prior month among those reporting any use.
P=0.001 compared to referred-treatment arm
Hepatitis C status unknown for 4 subjects.
P=0.045 compared to referred-treatment arm.
P=0.089 compared to referred-treatment arm.
Services provided
Of 46 subjects assigned to clinic-based BUP, 41 (89%) received at least 1 dose of BUP. The median daily BUP dose was 16 mg (interquartile range [IQR] 8–24mg). Of 41 subjects who initiated BUP, 12 (29%) were re-induced to BUP once and 6 (15%) were re-induced 2 or more times.
Of 47 subjects assigned to referred-treatment, 37 (78%) attended appointments with a case manager, which were usually performed on the day of randomization. Those seen in the case management program had a median of 3 contacts with case managers during the study period (IQR 2–5). Among 10 subjects in referred-treatment in whom case manager documentation specified an opioid treatment program referral date, the average wait for the appointment was 6.7 days (range 2–15 days). During the time of the study, the average delay between first contact at an opioid treatment program and enrollment to the program was 12 days in Baltimore City, declining from 15 days in 2005 to 9 days in 2009 (personal communication, Bill Rusinko, Director of Research, Maryland Alcohol and Drug Administration). Thirty subjects (64%) in referred-treatment started opioid agonist therapy during the study period. Of these, 16 and 14 reported receiving methadone and BUP, respectively.
Substance abuse treatment outcomes
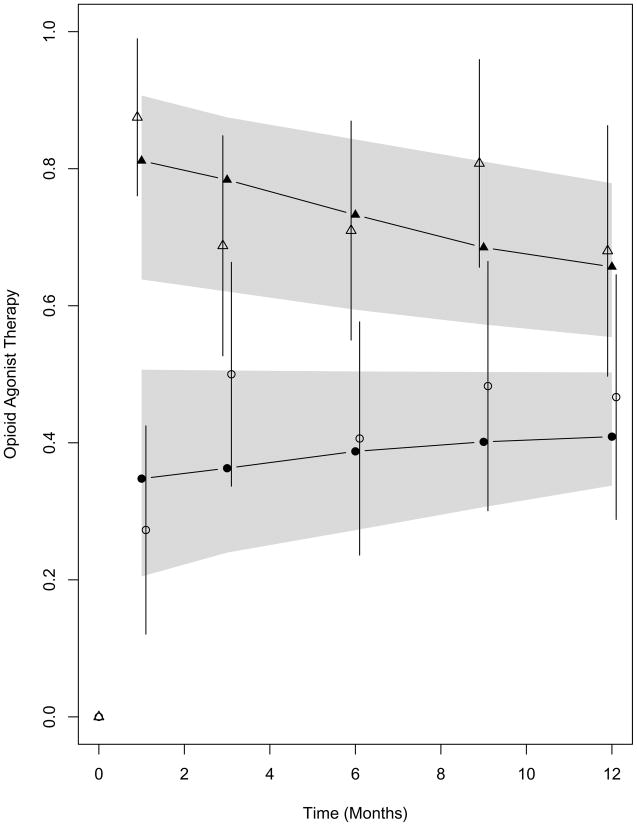
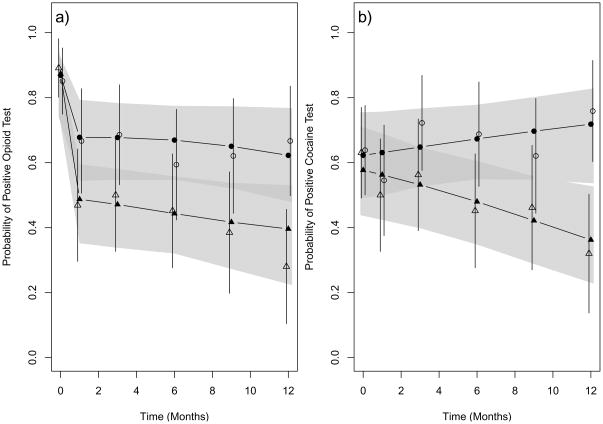
Initiation of opioid agonist therapy was substantially more rapid in the clinic-based BUP arm than in the referred-treatment arm: at 2 weeks, 84% (95% CI 72%–93%) in clinic-based BUP had initiated opioid agonist therapy compared to 11% (5%–24%) in referred-treatment (p<0.001). Clinic-based BUP subjects were significantly more likely to participate in opioid agonist therapy throughout 12-month follow-up (Figure 2). After randomization, the average estimated participation in opioid agonist therapy was 74% (95% CI 61%–84%) in clinic-based BUP and 41% (29%–53%) in referred-treatment (p<0.001, likelihood ratio test). Figure 3 shows urine drug test results for opioids and cocaine in the two study arms. After randomization, the average estimated percentages of opioid positive and cocaine positive urine drug tests were significantly lower in clinic-based BUP than referred-treatment (44% [32%–58%] versus 65% [95% CI, 52%–76%] for opioids, p=0.015, and 51% [39%–61%] versus 66% [54%–76%] for cocaine, p=0.012).
Figure 2.
Probability of receiving opioid agonist therapy at study follow-up visits. Observed estimates are shown for clinic-based BUP (open triangles) and referred-treatment (open circles) with vertical lines representing 95% confidence intervals. Mixed-effects model-based estimates for receiving opioid agonist therapy are shown for clinic-based BUP (solid triangles) and referred-treatment (solid circles) with 95% confidence bands shown as shading. The average model-based estimates for receiving opioid agonist therapy were significantly higher for clinic-based BUP than referred-treatment (p<0.001)
Figure 3.
Probability of positive opioid (a) and cocaine (b) urine drug tests at study visits. Observed estimates are shown for clinic-based BUP (open triangles) and referred-treatment (open circles) with vertical lines representing 95% confidence intervals. Mixed-effects model-based estimates for positive urine drug tests are shown for clinic-based BUP (solid triangles) and referred-treatment (solid circles) with 95% confidence bands shown as shading. The average model-based estimates for opioid positive and cocaine positive urine drug test were significantly lower in clinic-based BUP than in referred-treatment (p=0.015 and p=0.012, respectively).
HIV treatment outcomes
Subjects in clinic-based BUP had significantly more visits with their primary HIV providers during the study than subjects in referred-treatment (median 3.5 [IQR 2–4] versus 3.0 [IQR 1–3] visits, respectively, p=0.047). There was no significant difference between the study arms in the number of months subjects received antiretroviral therapy (11 months [IQR 0–12] for clinic-based BUP versus 12 months [IQR 0–12 months] for referred-treatment, p=0.85). Changes from baseline in HIV RNA levels and CD4 cell counts were not significantly different in the study arms, p=0.31 and p=0.161, respectively. Thirty five percent in clinic-based BUP and 36% in referred-treatment had ≥1 emergency department visit or hospitalization during the study (p = 1.00).
Sensitivity analyses
We used random effects pattern-mixture models to assess the influence of missing data on repeated measure outcomes. The inclusion of missing pattern groups (main effects and interactions with covariates of interest) significantly improved the fit of the model for opioid agonist therapy participation (p=0.023). However, the inferred study arm treatment difference in this outcome was not altered (electronic-only appendix). Pattern mixture modeling did not improve the overall fit of the models for opioid-positive urine drug tests or cocaine-positive urine drug tests (likelihood ratio tests, p=0.31 and p=0.80, respectively).
In a post-hoc analysis we examined the sensitivity of key study outcomes to adjustment for an imbalance in recent drug injection at baseline in the study arms. Inclusion of a term for recent drug injection at baseline did not significantly improve model fit for opioid agonist therapy participation, opioid-positive urine drug tests, or cocaine-positive drug tests (likelihood ratio test range, p=0.157 to p=0.65) and did not alter the statistical inferences from the models.
DISCUSSION
We conducted a randomized controlled trial comparing clinic-based BUP to referred-treatment for opioid-dependent patients in an HIV clinic. Compared to referred-treatment, the clinic-based BUP strategy was associated with higher participation in opioid agonist therapy over 12 months of follow-up and significantly reduced rates of positive urine drug tests. Additionally, clinic-based BUP subjects attended more visits with their primary care providers during follow-up than referred-treatment subjects. However, we found no evidence that treatment arm was associated with use of antiretroviral therapy or with changes in HIV RNA, CD4 cell counts, or emergency department use and hospitalizations.
It seems likely that streamlined access to opioid agonist therapy was a major mechanism underlying the results we observed. Two weeks after randomization, 84% in clinic-based BUP had initiated opioid agonist therapy compared to just 11% in referred-treatment. Even when expedited by a case-management program, referred-treatment entailed following-up for an intake appointment at a new clinic, completing intake evaluation, and usually waiting until a treatment slot became available. The average wait time for an opioid treatment program assessment visit was 7 days in our study and the average delay between first contact at an opioid treatment program and intake was 12 days in our city during the period the study was conducted. It is notable that rapid treatment intake in the clinic-based BUP arm appeared to yield benefits that persisted out to 12 months.
A number of studies have compared the efficacy of BUP and methadone in head-to-head clinical trials (13), which was not the goal of our study. Consistent with the tenets of comparative effectiveness research (14), our objective was to compare the effectiveness of two treatment strategies, clinic-based BUP and referral to an opioid treatment program, that may be available to opioid-dependent HIV-infected patients. Two prior studies randomized individuals recruited from substance abuse treatment settings to treatment in an opioid treatment program or in an office-based setting (15,16). One study, which randomized subjects who were receiving methadone at an opioid treatment program to continue in the program or to receive methadone in an office setting, found similar outcomes in the 2 arms, but higher participant satisfaction with the office-based strategy (15). The second study, which randomized subjects from an opioid treatment program waiting list to either program-based or office-based treatment with BUP, found office-based BUP to be associated with higher retention at 12-weeks and lower rates of opiate-positive urine drug tests compared to program-based BUP (16).
Our study differed from these studies in two ways. First, subjects in our study were recruited from a medical setting (HIV clinic) rather than a substance abuse setting. Second, the comparison arm in our study included the step of referring participants to opioid treatment programs, reflecting the process that occurs in clinical practice. Successful referral of substance-dependent individuals from medical care settings to substance abuse treatment is challenging. For example, one observational study that followed 40 patients with substance-related medical conditions after discharge from the hospital, found that only 1 (3%) followed-up with outpatient substance abuse treatment to which he had been referred (17).
In addition to lower rates of opioid use, we found that rates of cocaine use were also lower in clinic-based BUP than in referred-treatment. We hypothesize that this difference was due to higher treatment engagement in the former than the latter group, rather than to a pharmacodynamic effect of BUP. In a randomized trial comparing BUP and methadone in opioid-dependent cocaine abusers, BUP was no more effective than methadone in reducing cocaine use (18).
Our study has limitations. First, as a single-center study, our results may not generalize to other settings. The treatment model we used was relatively intensive and may not be practical in smaller treatment centers. Additionally, the performance of our control condition, referred-treatment, was inherently tethered to the availability of opioid treatment services in our area. The relative effectiveness of clinic-based BUP may differ in settings in which opioid treatment services are substantially more or less available than in our community. To ensure that referred-treatment was consistently implemented in our study, all subjects allocated to this arm were enrolled in an established case management program in the HIV clinic. Second, the manufacturer provided BUP to sites participating in this demonstration project for use in participants who had no source of payment, eliminating medication insurance coverage as a barrier to treatment in the clinic-based BUP arm. This highlights public health importance of facilitating access to BUP when treatment with this drug is indicated.
Third, owing to the constraints of enrolling from a single clinic, our sample size was small and we enrolled only 78% of our target. This limited our ability to detect smaller, but potentially clinically-significant, differences between the study arms in HIV treatment outcomes. Larger, multicenter, clinical trials of BUP delivery in HIV care settings are warranted to confirm and extend our findings. Fourth, follow-up rates in the study were only fair. However, while sensitivity analyses with pattern-mixture models did provide evidence that differences in opioid agonist therapy participation were sensitive to missing data, the inferences remained the same (i.e., higher participation in clinic-based BUP). Finally, the study arms were imbalanced with regard to recent drug injection, with statistically significantly more subjects in referred-treatment reporting recent injection than in clinic-based BUP. Notably, the study arms were well-matched with regard to demographic and socioeconomic factors, and other indicators of drug use severity including, years of use, history of incarceration, and co-occurring cocaine or alcohol use. Drug injection may be an indicator of more severe drug dependency and be associated with poorer response to treatment (19), which could bias study outcomes in favor of clinic-based BUP. In post-hoc analyses, we found that outcome differences in the study arms were not appreciably altered by adjustment for baseline injection drug use in multivariate models.
Our study has several implications. Provision of clinic-based BUP should be considered in all HIV treatment settings where opioid dependence is common, and HIV care policy makers should consider including clinic-based BUP as a core quality-of-care measure. Treatment models that integrate substance abuse and medical services have been proposed as a means to improve outcomes for both types of conditions and to improve patient satisfaction (20–23). HIV clinics are arguably the medical venue in which the availability of effective clinic-based treatment for opioid dependence can have the greatest impact (24). Opioid dependence is highly prevalent in many HIV treatment settings and is detrimental to retention in care and adherence to life-saving antiretroviral therapy (25–27). Moreover, ongoing drug use in HIV-infected individuals sustains behaviors that risk transmission to others.
In summary, we conducted a randomized trial comparing two treatment strategies for opioid dependent individuals attending an urban HIV clinic. Compared to referred-treatment, we found that clinic-based BUP led to more rapid initiation of opioid agonist therapy, greater use of opioid agonist therapy over 12-month follow-up, lower urine drug test positivity rates for opioids and cocaine, and a higher number of visits with HIV primary care providers during follow-up.
Acknowledgments
Grant support: This study was supported by the Health Resources Services Administration Special Projects of National Significance (H97HA03794). The project was also supported by Grant Number UL1 RR 025005 from the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research. Drs. Lucas, Lau, Fiellin, and Moore were also supported by the National Institutes of Health (K23DA015616, R01DA018577, K01AI071754, R01DA019511, R01DA025991, R01DA020576, K24DA000432, R01DA011602, and R01AA016893). Reckitt Benckiser Pharmaceuticals, Inc. provided buprenorphine/naloxone for uninsured subjects in the study.
Footnotes
ClinicalTrials.gov registry number: NCT00130819
Protocol and statistical code: available to interested readers by contacting Dr. Lucas at glucas@jhmi.edu.
Data: not available.
Disclaimer: The views here express the authors’ personal views and not those of the Substance Abuse and Mental Health Services Administration, the National Center for Research Resources, the National Institutes of Health, the US Department of Health and Human Services, or the US government.
Reference List
- 1.Fiellin DA, O’Connor PG. New federal initiatives to enhance the medical treatment of opioid dependence. Ann Intern Med. 2002;137(8):688–692. doi: 10.7326/0003-4819-137-8-200210150-00014. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med. 2008;148(9):662–670. doi: 10.7326/0003-4819-148-9-200805060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 4.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83(2):193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. [PubMed] [Google Scholar]
- 6.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280(5365):867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 7.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2. Oxford: Oxford University Press; 2002. [Google Scholar]
- 8.Rabe-Hesketh S, Skrondal A, Pickles A. Generalized multilevel structural equation modeling. Psychometrika. 2004;69(2):167–190. [Google Scholar]
- 9.Hogan JW, Laird NM. Mixture models for the joint distribution of repeated measures and event times. Stat Med. 1997;16(1–3):239–257. doi: 10.1002/(sici)1097-0258(19970215)16:3<239::aid-sim483>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2(1):64–78. [Google Scholar]
- 11.Little RJA. Pattern-mixture models for multivariate incomplete data. Journal of the American Statistical Association. 1993;88(421):125–134. [Google Scholar]
- 12.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 13.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Volpp KG, Das A. Comparative effectiveness--thinking beyond medication A versus medication B. N Engl J Med. 2009;361(4):331–333. doi: 10.1056/NEJMp0903496. [DOI] [PubMed] [Google Scholar]
- 15.Fiellin DA, O’Connor PG, Chawarski M, Pakes JP, Pantalon MV, Schottenfeld RS. Methadone Maintenance in Primary Care: A Randomized Controlled Trial. JAMA. 2001;286(14):1724–1731. doi: 10.1001/jama.286.14.1724. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med. 1998;105(2):100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- 17.O’Toole TP, Strain EC, Wand G, McCaul ME, Barnhart M. Outpatient treatment entry and health care utilization after a combined medical/substance abuse intervention for hospitalized medical patients. J Gen Intern Med. 2002;17(5):334–340. doi: 10.1046/j.1525-1497.2002.10638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 19.Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willenbring ML, Olson DH. A randomized trial of integrated outpatient treatment for medically ill alcoholic men. Arch Intern Med. 1999;159(16):1946–1952. doi: 10.1001/archinte.159.16.1946. [DOI] [PubMed] [Google Scholar]
- 21.Umbricht-Schneiter A, Ginn DH, Pabst KM, Bigelow GE. Providing medical care to methadone clinic patients: referral vs on-site care. Am J Public Health. 1994;84(2):207–210. doi: 10.2105/ajph.84.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269(15):1953–1959. [PubMed] [Google Scholar]
- 23.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286(14):1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan LE, Fiellin DA. Buprenorphine: its role in preventing HIV transmission and improving the care of HIV-infected patients with opioid dependence. Clin Infect Dis. 2005;41(6):891–896. doi: 10.1086/432888. [DOI] [PubMed] [Google Scholar]
- 25.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 27.Kavasery R, Galai N, Astemborski J, Lucas GM, Celentano DD, Kirk GD, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50(4):360–366. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]