Abstract
Pancreatic adenocarcinoma is an aggressive cancer with a greater than 95% mortality rate and short survival after diagnosis. Chemotherapeutic resistance hinders successful treatment. This resistance is often associated with mutations in codon 12 of the K-Ras gene (K-Ras 12), which is present in over 90% of all pancreatic adenocarcinomas. Codon 12 mutations maintain Ras in a constitutively active state leading to continuous cellular proliferation. Our study determined if TRAIL resistance in pancreatic adenocarcinomas with K-Ras 12 mutations could be overcome by first sensitizing the cells with Benzyl isothiocyanate (BITC). BITC is a component of cruciferous vegetables and a cell cycle inhibitor. BxPC3, MiaPaCa2 and Panc-1 human pancreatic adenocarcinoma cell lines were examined for TRAIL resistance. Our studies show BITC induced TRAIL sensitization by dual activation of both the extrinsic and intrinsic apoptotic pathways.
Keywords: TRAIL resistance, K-RAS, BITC, pancreatic adenocarcinoma, chemotherapeutic resistance
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States.1,2 Pancreatic cancer includes exocrine, neuroendocrine, and adenocarcinoma forms, with adenocarcinoma being the most common and having the least favorable prognosis. There are three predominant treatment options; surgery, radiation, and chemotherapy. Pancreatic adenocarcinomas are largely resistant to radiation and chemotherapy and often inoperable. The combination of late detection and chemotherapeutic resistance in pancreatic cancers is responsible for a greater than 95% mortality rate.1,2
TRAIL (TNF-related apoptosis-inducing ligand) is a potential chemotherapeutic agent. TRAIL death receptors are highly expressed on the surface of transformed cancer cells but generally absent on most normal cells.3 The cytotoxic effects of TRAIL are pronounced on cancerous cells and trigger apoptosis, but leave most noncancerous tissues unaffected.4–6 Chemotherapeutic resistance to TRAIL-induced apoptosis has been associated with mutations in codon 12 of the K-Ras gene (K-Ras12).4,5
Over ninety percent of pancreatic adenocarcinomas harbor a mutation within codon 12 of the K-Ras gene.7,8 Although other mutations, such as p53, p16, and SMAD 4 have been reported in pancreatic adenocarcinomas, mutations of K-Ras are shown to be an initiating factor in the formation of pancreatic cancers.8 Ras is a G protein that regulates migration, cytoskeletal formation, apoptosis, and cellular proliferation, predominantly through the MAP kinase signal transduction pathways.9–11 Ras cycles between an inactive GDP-bound state and an active GTP- bound state.12–16 Endogenous GTPase activity is responsible for the inactivation of Ras, thus inhibiting Ras-mediated signaling.17,18 Mutations within codon 12 of K-Ras inhibit this endogenous GTPase activity, thereby maintaining Ras in its GTP-bound active state.13 Previous studies have shown that constitutive activation of Ras leads to continuous cellular proliferation.
Benzyl isothiocyanate (BITC) has been shown to inhibit cell cycle progression.19 BITC is present in cruciferous plants and is a member of the isothiocyanate family, which have been found to be protective against carcinogenesis.20–22 BITC has recently been shown to induce G2/M cell cycle arrest by decreasing Cdk1, CyclinB1 and Cdc25B protein levels.19,23,24 In high doses, BITC produces the formation of reactive oxygen species and can induce cell death.19,24,25
Two major pathways, the extrinsic (death receptor) pathway and the intrinsic (mitochondrial) pathway mediate apoptosis.3 The extrinsic cell death pathway begins with external death receptors on the cell surface. When ligands such as TNF alpha, Fas, or TRAIL bind to their specific receptor, intercellular signaling results in the cleavage and activation of caspase 8.26,27 Caspase 8 can then cleave effector caspase 3, inducing apoptosis directly.28 In addition, in several cell types, caspase 8 can also trigger the activation of the intrinsic cell death pathway via cleavage of Bid, a proapoptotic protein.29 Truncated Bid (t-Bid) is capable of interacting with other pro-apoptotic proteins leading to loss of mitochondrial membrane integrity, which has previously been shown to trigger the release of cytochrome C.29,30 Cytochrome C release is associated with the activation of caspase 9 and subsequently leads to the activation of effector caspase 3.31–34
BxPC3, MiaPaCa2 and Panc-1 cell lines were chosen for the current study because these cell lines represent the most common mutations found in human pancreatic adenocarcinomas (Table 1).35–37 BxPC3 cells, isolated from human pancreatic adenocarcinoma, are tumorigenic but are wildtype at codon 12 of the K-Ras gene. Panc-1 cells, harbor a glycine to aspartate amino acid change within codon 12, and MiaPaCa2 cells, a glycine to cysteine amino acid change. Our results indicate that BITC sensitizes chemotherapeutically-resistant human pancreatic adenocarcinoma cells to TRAIL-induced apoptosis via the activation of dual apoptotic pathways.
Table 1. K-Ras 12 mutations in BxPC3, MiaPaCa2, and Panc-1 human pancreatic cancer cell Lines.
Observed nucleotide and amino acid changes within codon 12 of K-Ras in BxPC3, MiaPaCa2, and Panc-1 cell lines.
Materials and Methods
Materials
Panc-1 and BxPC3 cells were purchased from ATCC. Dr. B. Weissman of the University of North Carolina, Chapel Hill School of Medicine kindly provided MiaPaCa2 cells. Goat-anti-rabbit (W401B) and goat-anti-mouse (W402B) HRP-conjugated secondary antibodies were purchased from Promega. The primary antibodies used were: rabbit polyclonal anti-XIAP (Cell Signaling Cat # 2042), rabbit polyclonal PARP (Cell Signaling Cat # 9541S), mouse monoclonal caspase 8 (Cell Signaling Cat # 9746), mouse monoclonal active caspase 9 (Upstate Biotech Cat # 05-572), and rabbit polyclonal Bid (Cell Signaling Cat # 2002). Mouse monoclonal pan actin was a kind gift from Dr. J. Lessard at Cincinnati Children’s Hospital. Rabbit polyclonal active caspase 3 antibody was a kind gift from Dr. K. Tomaselli at Idun Pharmaceuticals. For apoptotic death quantitation, the Roche Cell Death ELISA Assay was used (Roche Cat # 1774425). Human recombinant TRAIL was purchased from R&D Systems (Cat # 375-TEC). Apoptosis-inducing, anti-Fas antibody (clone CH-11) was purchased from Millipore. BITC was purchased from Sigma-Aldrich (Cat # 252492). Nonmethylated Q-VD-OPh (Cat # A0004) was a kind gift from Apoptrol, LLC.
Cell culture
BxPC3, MiaPaCa2, and Panc-1 cells were maintained in RPMI 1640 with L-glutamine, supplemented with 5% heat-inactivated fetal bovine serum and 1% antibiotic/antimycotic. Cells were maintained at 5% CO2 and 37 °C. All cell lines were maintained at 1 × 105 cells/ml.
Treatment protocol
For collection of whole cell lysates used in Western blot analysis, cells were plated at 2 × 105 cells/ml and allowed to attach overnight. TRAIL was solubilized in 0.1% BSA/water. A stock solution of BITC (100 mM) was prepared in DMSO and subsequently diluted in fresh medium so that the final concentration of DMSO was less than 0.1%. An equal volume of DMSO was added for the vehicle controls. Both BITC alone and BITC with TRAIL groups were treated with a final concentration of 5 µM BITC for 18 hrs. The following morning, vehicle and BITC alone groups were treated with 5 µl 0.1% BSA, whereas in the TRAIL and BITC combined group, TRAIL was added to a final concentration of 10 ng/ml TRAIL for 6 hrs. Total treatment time with BITC was 24 hrs. Cells were then collected for whole cell lysates. In cells treated with Q-VD-OPh, cells were preincubated for 30 minutes with the pan-caspase inhibitor, prior to beginning the experimental treatments. Jurkat human T cells (1 × 106 cells/ml) were treated with 100 ng/ml of apoptosis-inducing anti-Fas or 1 µg/ml Actinomycin D for 4 hrs as positive controls.
Western blots
Whole cell lysates were collected in CHAPS buffer (50 mM PIPES pH 6.5, 2 mM EDTA pH 8.0, 0.1% CHAPS) containing protease inhibitor, sonicated, and centrifuged at 12,000 rpm for 10 minutes at 4 °C. Protein concentrations were determined using the Bradford assay.38 Cell lysates were treated with reducing sample buffer, and boiled for 5 minutes at 100 °C. Proteins were resolved on an SDS polyacrylamide gel via electrophoresis.31–34 Separated proteins were subsequently transferred onto a PVDF membrane in Western transfer buffer overnight at 0.1 A. Proteins were visualized using Ponceau S staining and the membrane was then blocked at room temperature for one hour in PBS blocking buffer (1X PBS, 5% (w/v) non-fat dry milk, and 0.05% Tween-20) for caspase 3 and 9 and Bid. Blots were blocked in TBS blocking buffer (1X TBS, 5% (w/v) non-fat dry milk, and 0.10% Tween-20) for XIAP, PARP and caspase 8. Blots were probed with antibodies specific for XIAP, cleaved PARP, active caspase 8 and 9 at (1:1,000) overnight at 4 °C. Actin was incubated at (1:16,000) for 40 minutes at room temperature (RT) and active caspase 3 at (1:4,000) for 2 hours at RT. Blots were then incubated with Pierce SuperSignal® West Pico Chemiluminescent Substrate following the manufacturer’s recommendations and exposed to X-ray film.
Cell death ELISA
For cell death ELISA analysis, cells were plated at 1 × 105 cells/ml. Vehicle and TRAIL alone groups were treated with a final concentration of 0.2% DMSO. BITC and BITC with TRAIL groups were treated for 18 hrs at a final concentration of 5 µM BITC. The following morning, the vehicle and BITC groups were treated with 2 µl 0.1% BSA. Cells treated with TRAIL were incubated with a final concentration of 10 ng/ml TRAIL for 6 hrs. Total treatment time with BITC was 24 hrs. Cells were then collected for analysis with the cell death ELISA assay following the manufacturer’s protocol. Roche Cell Death ELISA Assay was used to detect the fold increase in apoptotic cell death.29,30
Statistical analysis
Statistical analysis was performed using a one-way ANOVA. Results were considered to be statistically significant at p ≤ 0.05. All experiments were performed independently, a minimum of 3 times.
Results
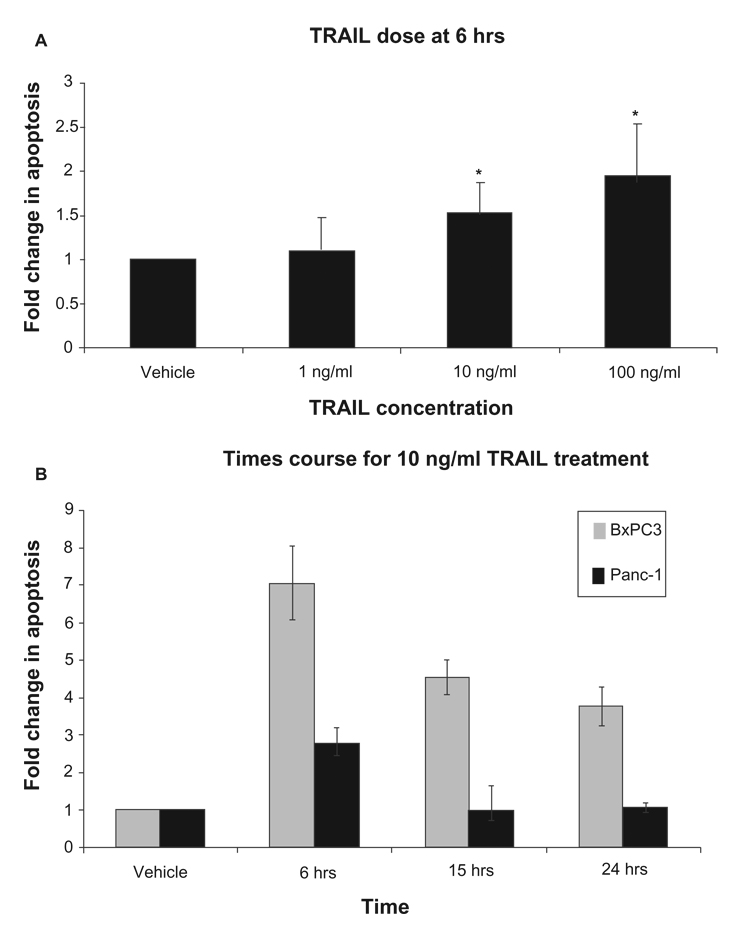
Resistance to TRAIL-induced apoptosis has previously been reported in pancreatic cancer cells.32 To determine the optimal dosage of TRAIL needed to induce apoptosis, we first treated Panc-1 cells with increasing doses of 1, 10, and 100 ng/ml TRAIL for 6 hours (Fig. 1A).6,24 TRAIL-induced apoptosis was evaluated using the cell death ELISA assay. A dose responsive increase in apoptosis was observed with increasing amounts of TRAIL. There was no significant increase in apoptosis with 1 ng/ml TRAIL compared to the vehicle treated cells. A significant 1.5 fold increase in apoptotic death was observed with 10 ng/ml TRAIL when compared to the vehicle alone and the 100 ng/ml TRAIL showed a 1.95 fold increase in cell death (P < 0.05). Since a significant increase in apoptosis was observed at 10 ng/ml TRAIL and as there was no significant difference in apoptotic death between 10 ng/ml TRAIL or 100 ng/ml TRAIL; 10 ng/ml TRAIL was chosen for all further studies.
Figure 1.
Time and dose response of Panc-1 and BxPC3 cells to TRAIL. A) Panc-1 cells were plated 1 × 105 cells/ml. Cells were treated with vehicle, 1, 10 and 100 ng/ml TRAIL for 24 hours and the dose response was analyzed by cell death ELISA assay, B) BxPC3 and Panc-1 cells were plated at 1 × 105 cells/ml. Cells were treated with 10 ng/ml TRAIL for 6, 15 or 24 hours and the optimal time course was analyzed using the cell death ELISA. The results are represented by fold increase in apoptosis compared to vehicle. Error bars represent standard deviation. *Denotes significance to p ≤ 0.05 when compared with control.
We determined the optimal duration for TRAIL treatment. Panc-1 and BxPC3 cells were treated with 10 ng/ml TRAIL for 6, 15 or 24 hours and apoptosis was analyzed using the cell death ELISA assay (Fig. 1B). Compared to the vehicle, there was a 7.04-fold increase in cell death at 6 hrs in BxPC3 cells, at 15 hrs there was a 4.53 fold increase in cell death, and at 24 hrs there was a 3.76 fold increase in cell death. In Panc-1 cells, at 6 hrs there was a 2.76 fold increase, at 15 hrs there was a 0.96 fold increase in cell death, and at 24 hrs there was a 1.05 fold increase in cell death. Higher levels of TRAIL-induced apoptosis were observed in wildtype K-Ras 12 BxPC3 cells, than in the K-Ras 12 mutant Panc-1 cell line. The greatest increase in apoptotic death in both cell lines was observed at 6 hours and this time point was chosen for all further experiments.
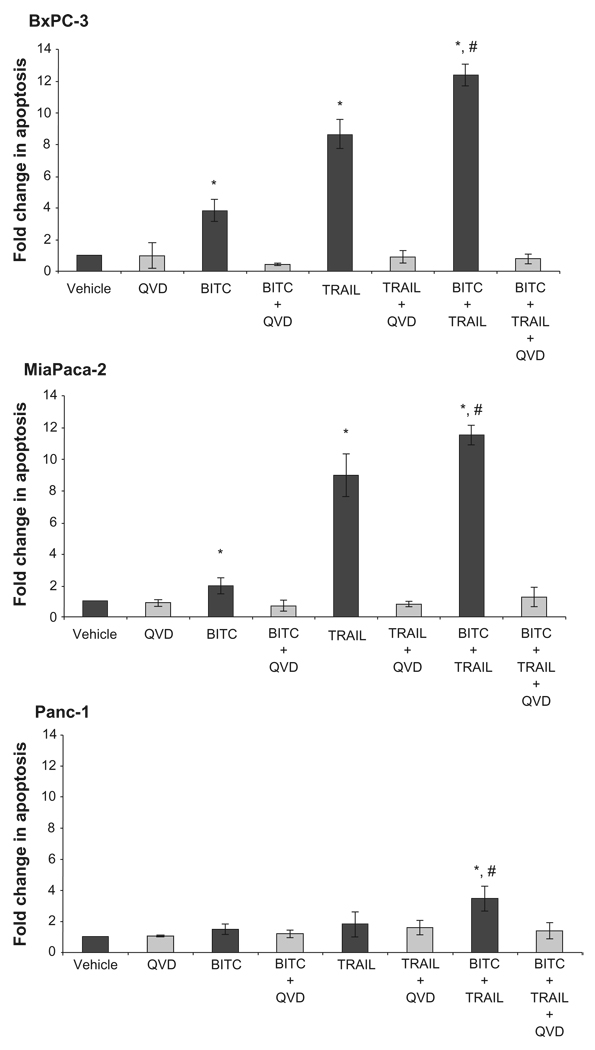
BITC is known to induce cell cycle arrest in human pancreatic cancer cells.18,22,24 We analyzed the effect of BITC on TRAIL-induced apoptosis in BxPC3, MiaPaCa2 and Panc-1 cells by the cell death ELISA assay. Cells were treated with vehicle, BITC alone, TRAIL alone, or BITC in combination with TRAIL. Because BITC is a known cell cycle inhibitor, the fold change in apoptosis was normalized to cell number to accurately reflect the amount of apoptosis. The normalized cell death ELISA results indicate that BITC alone caused a modest increase in apoptosis in BxPC3, MiaPaCa2 and Panc-1 cell lines (Fig. 2). BxPC3 and MiaPaCa2 cells were the most sensitive to TRAIL-induced apoptosis with a greater than 8-fold increase in apoptosis compared to vehicle. Panc-1 cells were the most resistant to TRAIL with only a 1.24 fold increase in apoptosis.
Figure 2.
BITC and TRAIL-induced apoptosis in BxPC3, MiaPaCa2, and Panc-1 cells. Analysis of apoptotic induction in BxPC3, MiaPaCa2, and Panc-1 cells using the cell death ELISA assay. BxPC3, MiaPaCa2, and Panc-1 cells were plated at 1 × 105 cells/ml. Cells were treated with vehicle, 5 µM BITC 24 hrs, and 10 ng/ml TRAIL for 6 hrs or both BITC and TRAIL. The pan-caspase inhibitor, Q-VD-OPh (QVD) was added at 5 µM for 30 minutes prior to treatment where indicated. Cell death ELISA results are represented by fold change in apoptosis compared to the vehicle. Error bars represent standard deviation. *Denotes significance to p ≤ 0.05 when compared with control. #Denotes significance to p ≤ 0.05 when comparison was made between TRAIL alone and BITC + TRAIL.
BxPC3 cells had a 3.84 fold increase in apoptosis upon treatment with BITC alone, an 8.65 fold increase was observed with TRAIL alone, and a 12.39 fold increase was seen when cells were treated with BITC combined with TRAIL. MiaPaCa2 cells had a 2.08 fold increase in apoptosis upon treatment with BITC, a 9.12 fold increase with TRAIL alone, and an 11.72 fold increase with BITC combined with TRAIL. Panc-1 cells underwent a 1.49 fold increase in apoptosis upon treatment with BITC, a 1.82 fold increase with TRAIL alone, and a 3.45 fold increase with BITC combined with TRAIL compared to vehicle. Compared to TRAIL treatment alone, BITC combined with TRAIL produced a significant increase in apoptotic cell death in all three cell lines examined. In addition, treatment of BxPC3, MiaPaCa2, and Panc-1 cells with non-methylated Q-VD-OPh, a pan caspase inhibitor, prevented apoptosis in all three cell lines examined (Fig. 2).31
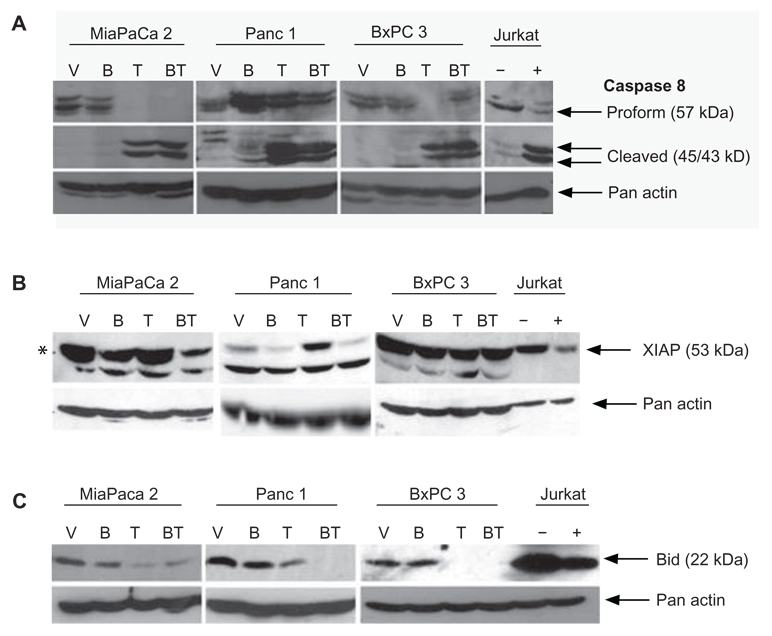
Western blot analysis was performed to identify the apoptotic pathways involved in the BITC and TRAIL-induced apoptosis of pancreatic adenocarcinoma cells. BxPC3, MiaPaCa2 and Panc-1 cells were treated with vehicle, BITC, TRAIL, or BITC combined with TRAIL and examined for activation of the extrinsic apoptotic cell death pathway (Fig. 3). In BxPC3 cells, MiaPaCa2, and Panc-1 cells, vehicle or BITC alone did not activate initiator caspase 8. Treatment with TRAIL alone induced the cleavage of the inactive proform of caspase 8, indicating the activation of caspase 8, although no further increase in caspase 8 cleavage was observed with the combination of BITC and TRAIL in all three cell lines (Fig. 3A).
Figure 3.
Analysis of the extrinsic apoptotic Pathway in human pancreatic cancer cells. Activation of caspase 8 was evaluated in BxPC3, MiaPaCa2 and Panc-1 cell lines upon treatment with vehicle (V), 5 µM BITC for 24 hrs (B), 10 ng/ml TRAIL (T) for 6 hrs or both 5 µM BITC and 10 ng/ml TRAIL (BT). Total treatment time was 24 hours. Whole cell lysates were collected, separated, and analyzed by Western blotting described in the Methods and Materials. The blots were probed with antibodies to initiator caspase 8 A), XIAP B), or Bid C) and reprobed with actin to ensure equal loading. Jurkat human T cells treated with vehicle (−), anti-Fas antibody (A, B) (+), or hrTRAIL (C) (+) were used as negative and positive controls, respectively.
X-linked inhibitor of apoptosis protein (XIAP) is a prosurvival protein and a direct target of caspase 8.42 Thus if caspase 8 becomes cleaved (and thus activated), caspase 8 will cleave its targets. We examined the XIAP protein to infer the extent of caspase 8 activation by visualizing the loss of the intact 53 kDa XIAP protein in BxPC3, MiaPaCa2, and Panc-1 cells (Fig. 3B). No detectable XIAP cleavage was observed in BxPC3 cells treated with BITC alone, TRAIL alone, or BITC combined with TRAIL. In Panc-1 cells, addition of BITC resulted in the loss of intact XIAP. TRAIL alone, however, was unable to induce XIAP cleavage. BITC combined with TRAIL led to a reduction in intact XIAP compared to the vehicle. In MiaPaCa2 cells, there was no major cleavage of XIAP with vehicle, BITC, and TRAIL alone. Treatment of TRAIL, combined with BITC in MiaPaCa2 cells, did trigger the loss of mature XIAP protein, indicative of caspase 8 cleavage. The combined treatment of BITC with TRAIL led to a reduction in intact XIAP protein in MiaPaCa2 and Panc-1 cell lines compared to TRAIL alone.
In many cell types, the extrinsic (death receptor) pathway is known to activate caspase 8 and simultaneously trigger the activation of the intrinsic (mitochondrial) pathway via cleavage of the protein, Bid, resulting in truncated Bid (t-Bid).29,30 BxPC3, MiaPaCa2 and Panc-1 were cells treated with vehicle, BITC alone, TRAIL alone, or BITC combined with TRAIL and examined for Bid cleavage (Fig. 3C). The loss of intact 22 kDa Bid is indicative of t-Bid formation and the activation of the intrinsic pathway. Vehicle or BITC alone did not induce Bid cleavage in BxPC3 cells. TRAIL alone produced a complete loss of the inactive proform of Bid as did TRAIL combined with BITC. Similar to BxPC3 cells, MiaPaCa2 cells were absent of Bid cleavage upon treatment with vehicle or BITC alone, however, a substantial loss of intact Bid was observed when cells were treated with TRAIL alone or BITC combined with TRAIL. In Panc-1 cells, treatment with BITC alone did not reduce intact Bid protein; however, a progressive increase in cleavage was seen upon treatment with TRAIL alone and a complete loss of intact Bid was observed with BITC combined with TRAIL.
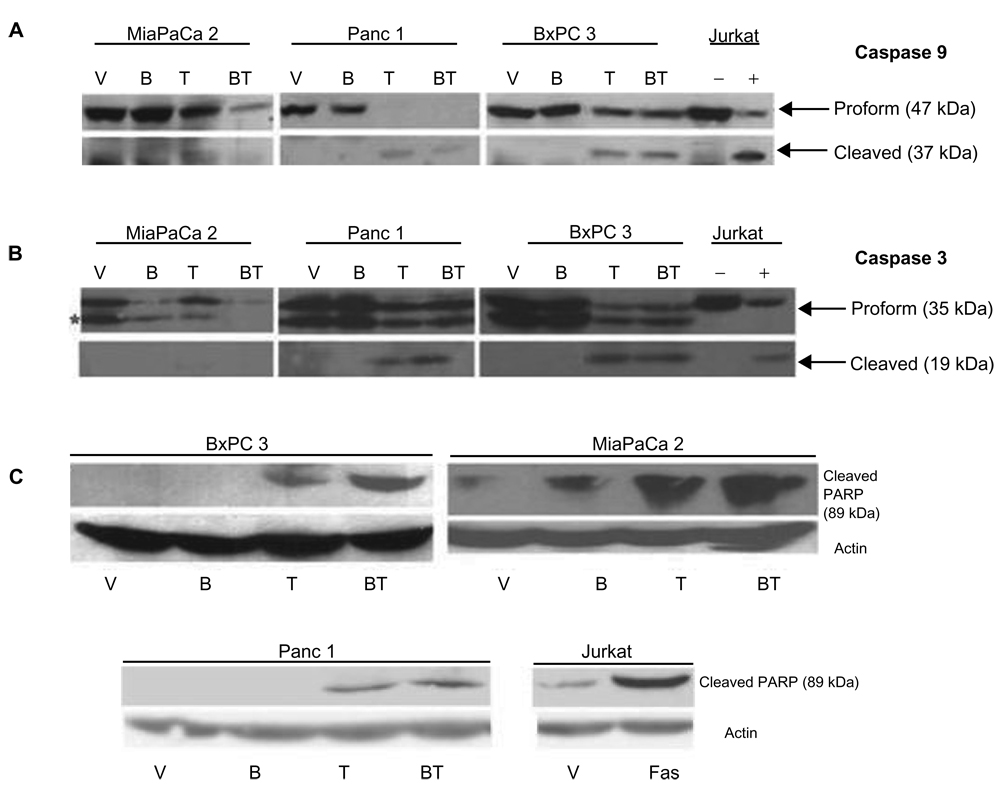
Truncation, and therefore activation, of Bid has been shown to induce the intrinsic cell death pathway.33,35 We sought to determine whether TRAIL combined with BITC activated the intrinsic, mitochondrial cell death pathway (Fig. 4). BxPC3, MiaPaCa2 and Panc-1 cells were examined for the activation of caspase 9, the initiator caspase for the intrinsic apoptotic death pathway (Fig. 4A). In BxPC3 cells treated with BITC alone, no cleavage of caspase 9 was observed. Upon treatment with TRAIL alone, there was a decrease of the proform and detection of an activated caspase 9 cleavage product. BITC in combination with TRAIL slightly increased the amounts of cleaved active caspase 9. In MiaPaCa2 cells, there was no appearance of the cleaved, active caspase 9 fragment upon treatment of the vehicle, BITC alone, or TRAIL alone. TRAIL combined with BITC resulted a dramatic decrease in inactive procaspase 9, however, little cleaved caspase 9 could be detected. In Panc-1 cells, there was no cleavage of caspase 9 with BITC alone. TRAIL-induced a decrease in the proform and an increase in the active cleaved fragment. BITC combined with TRAIL produced no further increase in cleaved caspase 9.
Figure 4.
Analysis of the intrinsic apoptotic pathway in human pancreatic cancer cells. Activation of caspase 9 was evaluated in BxPC3, MiaPaCa2 and Panc-1 cell lines upon treatment with vehicle (V), 5 µM BITC for 24 hrs (B), 10 ng/ml TRAIL (T) for 6 hrs or both 5 µM BITC and 10 ng/ml TRAIL (BT). Total treatment time was 24 hours. Whole cell lysates were collected, separated, and analyzed by Western blotting described in the Methods and Materials. The blots were probed with antibodies to initiator caspase 9 A), caspase 3 B), or PARP C). Arrows indicated procaspase 9 and the cleaved/active fragment. Asterisk (*) indicates an unrelated, crossreactive band. Jurkat human T cells treated with vehicle (−), actinomycin (+) (A, B), or anti-fas (+) (C) were used as negative and positive controls, respectively.
Because both the extrinsic and intrinsic cell death pathways trigger effector caspase cleavage, we evaluated the effect of BITC and TRAIL on the major effector caspase, caspase 3 (Fig. 4B). In BxPC3 cells, vehicle and BITC alone show no significant loss of inactive procaspase 3. Treatment with TRAIL produced the appearance of the cleavage product suggesting activation. TRAIL in combination with BITC also produced cleaved caspase 3, similar to that of TRAIL alone. MiaPaCa2 cells showed no cleavage of procaspase 3 when treated with vehicle or BITC alone. TRAIL treatment led to a decrease in the proform, however the cleaved form was only weakly detected. The combined treatment of BITC and TRAIL in MiaPaCa2 cells resulted in the loss of procaspase 3, but the cleaved form of caspase 3 was not detected. In Panc-1 cells, there was little difference between vehicle and BITC alone with no procaspase 3 cleavage. TRAIL alone triggered the cleavage of caspase 3 and this cleavage was further increased when Panc-1 cells were treated with BITC in combination with TRAIL.
To confirm the activation of caspase 3, we examined PARP, a direct substrate of activated caspase 3 (Fig. 4C). In BxPC3 cells, there is no appearance of cleaved PARP upon treatment with vehicle or BITC. TRAIL treatment alone induced a minor amount of PARP cleavage of PARP. A large increase in PARP cleavage was detected upon treatment with BITC combined with TRAIL. In MiaPaCa2 cells, PARP remained intact with vehicle alone and a small amount of the cleaved product was observed in cells treated with BITC alone. TRAIL increased the appearance of cleaved PARP and TRAIL with BITC led to even further PARP cleavage in MiaPaCa2 cells. In Panc-1 cells, there was no appearance of cleaved PARP upon treatment of vehicle or BITC alone. However, upon treatment of TRAIL, the appearance of the cleaved PARP was observed. TRAIL combined with BITC further increased PARP cleavage in Panc-1 cells compared to TRAIL alone.
Discussion
Pancreatic cancer is a one of the leading causes of death in the United States and chemotherapeutic resistance and high mortality in pancreatic cancer are associated with point mutations within codon 12 of K-Ras.39,40 K-Ras regulates cellular proliferation in most cell types and mutations within codon 12 disrupt the normal on-off cycling leading to constitutive signaling and proliferation.14 This study determined if the cell cycle inhibitor, BITC, could sensitize pancreatic adenocarcinoma cell lines with mutated K-Ras to TRAIL-induced apoptosis.
The cell death ELISA assay was used to quantitate apoptotic death in the pancreatic adenocarcinoma cell lines. We determined that 10 ng/ml TRAIL for 6 hrs was the optimal treatment to induce apoptosis in Panc-1 cells. The time course ELISA showed a gradual decline in apoptosis from 6 hrs to 24 hours (Fig. 1B). Previous studies in our lab have shown that cells in culture can undergo secondary necrosis with extended treatment periods with cell death inducers.30 The cell death ELISA assay can exclude necrotic death and specifically measure apoptotic death and this is a likely explanation for the decrease in cell death that was observed at later time points. BxPC3 cells were very sensitive to TRAIL-induced apoptosis compared to resistant Panc-1 cells. This could be attributed to their K-Ras12 status, as BxPC3 cells are K-Ras 12 WT and Panc-1 cells are K-Ras 12 mutant.
When the cell death ELISA results were normalized, the data indicated that BITC significantly sensitizes all three human pancreatic cancer cell lines to TRAIL-induced apoptosis. K-Ras 12 wild type, BxPC3 cells undergo the greatest degree of apoptosis, as expected. The K-Ras 12 mutant MiaPaCa2 cells were moderately sensitive to TRAIL. This suggests that the K-Ras 12 (gly→cys) mutation seen in MiaPaCa2 cells may not be dominant for chemotherapeutic resistance. Alternatively, there are likely to be other potential mutations in these cell lines that may also contribute to determining overall resistance. Panc-1 cells were resistant to TRAIL-induced apoptosis, but could be sensitized to TRAIL when treated in combination with BITC. Our results demonstrate that BITC combined with TRAIL significantly increases apoptosis in these human pancreatic cancer cell lines compared to TRAIL alone.
Activation of caspase 8 was observed, as TRAIL is reported to signal through the death receptor pathway. 41 Initiator caspase 8 cleavage and activation can signal directly and induce apoptosis by cleaving effector caspase 3. In all three cell lines, caspase 8 cleavage did occur upon exposure to TRAIL, but no further increase was observed when combined with BITC. This may occur as TRAIL may have activated the maximal amount of caspase 8 activity and therefore BITC was unable to further sensitize the cells.
As a target of caspase 8, XIAP cleavage was examined. 41,42 In all three cell lines examined, BITC alone or TRAIL alone moderately reduced intact XIAP. The combination of BITC and TRAIL produced moderate cleavage of XIAP in BxPC3, but substantial XIAP cleavage in Panc-1 and MiaPaCa2 cells. The intact XIAP seen in BxPC3 cells, with the combined treatment of BITC with TRAIL, may be due to these cells being TRAIL sensitive and therefore full effects were exhibited upon treatment of TRAIL alone. Alternatively, the reduction in XIAP may be related to reduced transcription due to cell cycle inhibition via BITC.
Bid cleavage was studied as it is a direct target of caspase 8 and its truncated form can initiate the intrinsic cell death pathway. A near complete loss of intact Bid was observed in MiaPaCa2 and BxPC3 treated with TRAIL. Intact Bid still remained upon TRAIL treatment alone in Panc-1 cells. This suggests that Panc-1 cells are more resistant to TRAIL-induced apoptosis than BxPC3 cells or MiaPaCa2, however, the combined treatment of BITC with TRAIL led to a complete loss of intact Bid. In Panc-1 cells, BITC sensitization to TRAIL-induced apoptosis via BID cleavage may be a mechanism to overcome the resistance to TRAIL, and a potential target for further analysis. The resistance seen in Panc-1 cells compared to the other cell lines evaluated may suggest that particular K-Ras 12 mutations may be associated with different levels of chemotherapeutic resistance and this is currently under investigation.
Truncated Bid can lead to the activation of the intrinsic cell death pathway and caspase 9.29,30 In BxPC3 cells, caspase 9 cleavage was increased upon treatment with combined BITC and TRAIL relative to TRAIL alone. MiaPaCa2 cells had no increase in activated caspase 9 with combined treatment but do show a decrease in the caspase 9 proform, suggesting some sensitization by BITC. Despite no substantial increase in cleaved caspase 9 with combined treatment, the results suggest that MiaPaCa2 cells underwent increased apoptosis as indicated by the loss of procaspase 9 protein and the cell death ELISA assay. Panc-1 cells are more resistant to TRAIL-induced apoptosis, as suggested by lower levels of activated caspase 9 compared to BxPC3 cells.
Both the extrinsic and intrinsic cell death pathway lead to the activation of the main effector caspase, caspase 3.5,29–31 BxPC3, MiaPaCa2 and Panc-1 cell lines all show some activation of caspase 3 with TRAIL treatment. MiaPaCa2 cells show the least amount of active caspase 3. However, a substantial decrease in the caspase 3 proform suggests limited resistance to TRAIL. All three cell lines show increased activation of caspase 3 when treated with BITC in combination with TRAIL, compared to TRAIL alone. BxPC3 cells show the least amount of change between TRAIL alone and the combination of BITC and TRAIL. This may be because these cell lines are very TRAIL sensitive and its full effect is seen with TRAIL alone. Cleavage of PARP supports the levels of caspase 3 activation.
In conclusion, our results indicate that BITC sensitizes BxPC3, MiaPaCa2, and Panc-1 human pancreatic cancer cell lines to TRAIL-induced apoptosis via dual apoptotic pathways and the induction of several key apoptotic proteins, namely caspases 8, 9 and 3 as well as there respective substrates XIAP, Bid and PARP. BITC has also recently been shown to sensitize human pancreatic cancer cells to gamma irradiation therapy.43 Furthermore, the inhibition of STAT-3 and its signaling pathway in human pancreatic cancer cells has been suggested as contributing to the inhibition of cell growth and induction of apoptosis. 44 A recent report indicates that STAT-3 is in the mitochondria and plays an important role in maintaining the electron transport chain.45 Therefore, BITC’s ability to sensitize cells to apoptosis may lie in the ability to significantly dampen mitochondrial electron transport and alter the prosurvial:proapoptotic homeostatic balance and these studies are currently under investigation. Our results also indicate that Panc-1 cells are more resistant to TRAIL than BxPC3 and MiaPaCa2 cells, possibly suggesting that certain K-Ras 12 mutations lead to increased TRAIL resistance. Our data suggest that BITC may be of value for the treatment of chemotherapeutically-resistant mutant K-Ras 12 pancreatic adenocarcinomas, as BITC sensitizes human pancreatic cancer cells to TRAIL-induced apoptosis.
Acknowledgements
Special thanks to Chanel Keoni, Mary K. Leonard, and Amanda Vince for excellent technical assistance. Special thanks to Dr. J. Lessard for the gift of pan actin antibody, Dr. B. Weissman for MiaPaCa2 cells, Dr. K. Tomasselli for the caspase 3 antibody, and Apoptrol, LLC for the Q-VD-OPh NM. This research was funded in part by NIH R01 grant CA 106953, awarded to S.K.S. by the National Cancer Institute.
Footnotes
Disclosures
The authors report no conflicts of interest.
Contributor Information
Sanjay K. Srivastava, Email: sanjay.srivastava@ttuhsc.edu.
Thomas L. Brown, Email: thomas.l.brown@wright.edu.
References
- 1.American Cancer Society. Cancer facts and figures. 2008 http://www.cancer.org/docroot/STT/content/STT_1x_Cancer_Facts__Figures_2009.asp?from=fast.
- 2.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 3.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 4.Koschny R, Walczak H, Ganten TM. The promise of TRAIL-potential and risks of a novel anticancer therapy. J Mol Med. 2007;85:923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 5.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: Mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Vogler M, Durr K, Jovanovic M, Debatin KM, Fulda S. Regulation of TRAIL-induced apoptosis by XIAP in pancreatic carcinoma cells. Oncogene. 2007;26:248–257. doi: 10.1038/sj.onc.1209776. [DOI] [PubMed] [Google Scholar]
- 7.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 8.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 9.Boguski MS, McCormick F. Proteins regulating ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino R, Chatani Y, Yamori T, et al. Constitutive activation of the 41–43-kDa mitogen-activated protein kinase\signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 12.Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 13.Bos JL. Ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 14.Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 15.Wolfman A. Ras isoform-specific signaling: Location, location, location. Sci STKE. 2001 doi: 10.1126/stke.2001.96.pe2. PE2. 2001. [DOI] [PubMed] [Google Scholar]
- 16.Omerovic J, Hammond DE, Clague MJ, Prior IA. Ras isoform abundance and signaling in human cancer cell lines. Oncogene. 2008;27:2754–2762. doi: 10.1038/sj.onc.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark GJ, Quilliam LA, Hisaka MM, Der CJ. Differential antagonism of ras biological activity by catalytic and src homology domains of ras GTPase activation protein. Proc Natl Acad Sci U S A. 1993;90:4887–4891. doi: 10.1073/pnas.90.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Loganathan S, Humphreys I, Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr. 2006;136:2728–2734. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava SK, Xiao D, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Miyoshi N. Cell death induction by isothiocyanates and their underlying molecular mechanisms. Biofactors. 2006;26:123–134. doi: 10.1002/biof.5520260203. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi N, Uchida K, Osawa T, Nakamura Y. Benzyl isothiocyanate modifies expression of the G2/M arrest-related genes. Biofactors. 2004;21:23–26. doi: 10.1002/biof.552210106. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Kawakami M, Yoshihiro A, et al. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J Biol Chem. 2002;277:8492–8499. doi: 10.1074/jbc.M109760200. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 25.Singh SV, Srivastava SK, Choi S, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 26.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 27.Kamachi M, Le TM, Kim SJ, Geiger ME, Anderson P, Utz PJ. Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J Exp Med. 2002;196:1213–1245. doi: 10.1084/jem.20021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 29.Tang D, Lahti JM, Kidd VJ. Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem. 2000;275:9303–9307. doi: 10.1074/jbc.275.13.9303. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni K, Selesniemi K, Brown TL. Interferon-gamma sensitizes the human salivary gland cell line, HSG, to tumor necrosis factor-alpha induced activation of dual apoptotic pathways. Apoptosis. 2006;11:2205–2215. doi: 10.1007/s10495-006-0281-8. [DOI] [PubMed] [Google Scholar]
- 31.Caserta T, Smith A, Gultice A, Reedy M, Brown T. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 32.Khanbolooki S, Nawrocki ST, Arumugam T, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 33.Brown TL, Patil S, Cianci CD, Morrow JS, Howe PH. Transforming growth factor beta induces caspase 3-independent cleavage of alphaII-spectrin (alpha-fodrin) coincident with apoptosis. J Biol Chem. 1999;274:23256–23262. doi: 10.1074/jbc.274.33.23256. [DOI] [PubMed] [Google Scholar]
- 34.Brown TL, Patil S, Basnett RK, Howe PH. Caspase inhibitor BD-fmk distinguishes transforming growth factor beta-induced apoptosis from growth inhibition. Cell Growth Differ. 1998;9:869–875. [PubMed] [Google Scholar]
- 35.Moore PS, Sipos B, Orlandini S, et al. Genetic profile of 22 pancreatic carcinoma cell lines. analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 36.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata Y, Abe M, Motoshima K, Nakayama E, Shiku H. Frequent glycine-to-aspartic acid mutations at codon 12 of c-ki-ras gene in human pancreatic cancer in japanese. Jpn J Cancer Res. 1990;81:135–140. doi: 10.1111/j.1349-7006.1990.tb02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Reber HA, Dry SM, et al. Unfavourable prognosis associated with K-ras gene mutation in pancreatic cancer surgical margins. Gut. 2006;55:1598–1605. doi: 10.1136/gut.2005.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 41.Rudner J, Jendrossek V, Lauber K, Daniel PT, Wesselborg S, Belka C. Type I and type II reactions in TRAIL-induced apoptosis—results from dose-response studies. Oncogene. 2005;24:130–140. doi: 10.1038/sj.onc.1208191. [DOI] [PubMed] [Google Scholar]
- 42.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 43.Sahu RP, Epperly MW, Srivastava SK. Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation therapy. Front Biosci (Elite Ed) 2009 Jun 1;1:568–576. doi: 10.2741/e55. [DOI] [PubMed] [Google Scholar]
- 44.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101(3):176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegrzyn J, Potla R, Chwae YJ, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323(5915):793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]