Abstract
Endothelial cell injury and dysfunction are the major triggers of pathophysiological processes leading to cardiovascular disease. Endothelial dysfunction (ED) has been implicated in atherosclerosis, hypertension, coronary artery disease, vascular complications of diabetes, chronic renal failure, insulin resistance and hypercholesterolemia. Although now recognized as a class of physiological second messengers, reactive oxygen species (ROS) are important mediators in cellular injury, specifically, as a factor in endothelial cell damage. Uncontrolled ROS production and/or decreased antioxidant activity results in a deleterious state referred to as ‘oxidative stress’. A candidate factor in causing ROS production in endothelial cells is tumor necrosis factor alpha (TNF-α), a pleiotropic inflammatory cytokine. TNF-α has been shown to both be secreted by endothelial cells and to induce intracellular ROS formation. These observations provide a potential mechanism by which TNF-α may activate and injure endothelial cells resulting in ED. In this review, we focus on the relationship between intracellular ROS formation and ED in endothelial cells or blood vessels exposed to TNF-α to provide insight into the role of this important cytokine in cardiovascular disease.
Keywords: Cardiovascular disease, endothelial cell, inflammation, mitochondria, NAD(P)H oxidase, nitric oxide
INTRODUCTION
The endothelium, the largest organ in the body, has long been considered as simply being a “layer of nucleated cellophane”, a mechanical barrier between vessel wall and blood stream, or a relatively inert container for blood [1, 2]. However, following the breakthrough discovery of nitric oxide (NO) in the 1980s, this view of it being a non-reactive mechanical barrier, allowing only passive diffusion of biomolecules, began to change. The importance of the endothelial cell layer for vascular homeostasis and its underlying mechanisms has been increasingly appreciated. Now, thousands of scientific papers are published every year related to the biological, pharmacological and toxicological facets of endothelial cell biology. Endothelial cells respond to various mechanical and biological stimuli by synthesizing and releasing numerous factors that regulate angiogenesis, inflammation and hemostasis, as well as vascular tone and permeability[3]. Endothelial dysfunction (ED), a term first described in studies of human hypertension, using the forearm vasculature, has became a prominent term in endothelial research. Accumulating evidence suggests that ED is intimately involved in the pathogenesis of most cardiovascular diseases being associated with atherosclerosis, hypertension, coronary and peripheral artery dysfunction, chronic heart failure, diabetes, insulin resistance, hypercholesterolemia, hyperhomocysteinemia, inflammation [3]. However, the exact role and detailed mechanisms leading to ED remain incompletely understood.
Reactive oxygen species (ROS), sometimes referred to as free radicals, describes a number of molecules, including chemical species with one unpaired electron, derived from the metabolism of molecular oxygen. Thus, ROS includes a series of very small and highly reactive molecules such as hydroxyl radical (·OH), superoxide anion (O2·−), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and singlet oxygen (1O2) [4, 5]. Interestingly NO, which itself reacts with ROS species and is required to form ONOO− from O2·−, has also been considered as a member of the ROS family, although in the strictest sense it does not belong to ROS [6]. ROS form as natural byproducts of the normal metabolism of oxygen and are involved in a variety of cellular processes from cell proliferation to cell adaptation to hypoxia, from apoptosis to carcinogenesis, to maintain or reestablish redox homeostasis, acting as intracellular second messengers or modulating signal transduction pathways [7–9]. Under physiological conditions, the deleterious effects of ROS are minimized by antioxidant defense mechanisms that prevent their formation in excess, act as scavengers or repair the resultant damage. Such defense mechanisms involve a strong antioxidant system including superoxide dismutase (SOD), glutathione peroxidase, catalase and a variety of DNA-repairing enzymes [10].
Uncontrolled ROS production and/or decreased antioxidant activity, however, results in a deleterious state called oxidative stress. ROS are generated in multiple compartments and by multiple enzymes within the cell. Important sources include the mitochondria, NAD(P)H oxidase, cyclooxygenase (COX), lipoxidase (LOX), xanthine oxidase (XO), cytochrome P450 (CYP450) as well as a number of other physiologically relevant systems [11]. Vascular and, specifically, endothelial cells express nearly all of these enzymes and endothelial cells are, therefore, viewed as an important source of vascular ROS formation [12–14]. Further, intracellular ROS formation in endothelial cells has been shown to play a critical role in ED, which might be a common initial pathway for many cardiovascular diseases.
Tumor necrosis factor alpha (TNF-α), a 17 kDa polypeptide, was originally discovered as a factor produced by macrophages in endotoxin exposed rabbits that could cause hemorrhagic necrosis of experimental tumors [15]. Now TNF-α is considered one of the most important cytokines being recognized as a major effector of macrophage-mediated host defense and tissue injury, while also playing a crucial role in innate and adaptive immunity, cell proliferation, and apoptotic processes [16]. In pathological processes, tissue fixed macrophages, such as Langerhan’s cells, Kupffer cells, and astroglia, are believed to be major sources of TNF-α. However, other cell types, including endothelial cells, epithelial cells, monocytes, T-cells, smooth muscle cells, adipocytes and fibroblasts, secrete significant amounts of TNF-α when exposed to the appropriate stimuli [17].
As demonstrated for other cytokines, TNF-α signals target cells through binding to specific cell surface membrane receptors. Two distinct receptors mediate the biological activities of TNF-α, one of a molecular mass of 55 kDa (p55, R1) and the other of 75 kDa (p75, R2). The former is constitutively expressed and historically has been considered the main mediator for TNF-α responses while the latter expression is inducible [17]. Several comprehensive reviews have been published in recent years describing the biology and function, receptor requirements, and signaling pathways of TNF-α [17–20]. Though it had long been noticed that TNF-α could cause injury to endothelial cells resulting in ED, the mechanisms have not been fully understood. In this review, we focus on intracellular ROS formation and ED in TNF-α treated endothelial cells and vessels, in an effort to provide insight into the important role of TNF-α in cardiovascular disease.
TNF-α INDUCES INTRACELLULAR ROS FORMATION IN ENDOTHELIAL CELLS
TNF-α is a cytokine that induces ROS through endothelial mitochondria and NAD(P) H at the plasma membrane; further TNF-α can increase nitric acid which under certain circumstances produce perinitrate.
TNF-α Induction of Mitochondrial ROS
Mitochondria, the energy centers of the cell, are the principal site of oxygen metabolism, accounting for approximately 85%–90% of oxygen consumed by cells [21, 22]. During oxygen consumption and ATP synthesis, about 1% to 3% of total oxygen utilized by mitochondria is incompletely reduced and remains as ROS [23]. ROS are mainly produced in the mitochondrial respiratory chain and two sites have been identified as significant sources of their formation. One is dependent on the autooxidation of the flavin mononucleotide from the NADH-dehydrogenase (complex I), whereas the other, depends on autooxidation of the unstable ubisemiquinone (complex III), which is an intermediate of the Q-cycle reaction [24].
Various inhibitors of mitochondrial electron transport have been used to show that the cytotoxic activity of TNF-α is mediated through alterations to mitochondrial function. Furthermore, TNF-α activates radical production in mitochondria principally at the ubiquinone site and TNF-α damages the mitochondrial chain at complex III, which consequently results in the increased production of oxygen radicals inside the mitochondrion [24]. Elimination of mitochondrial oxidative metabolism not only inhibits TNF-α cytotoxicity, but also considerably reduces TNF-α-mediated activation of NF-κB and expression of IL-6 [25]. Studies of TNF-α stimulated L929 fibroblasts have provided direct evidence for TNF-α-induced mitochondrial ROS and their involvement in cytotoxicity. Levels of TNF-α-induced mitochondrial ROS were shown to be tightly correlated with cytotoxicity and, further, the ROS formation could be effectively scavenged by the mitochondrial glutathione (GSH) system [26]. Collectively, these studies strongly suggest that TNF-α induced mitochondrial ROS formation plays an important role in TNF-α induced cytotoxicity.
Recent studies related to TNF-α induced ROS formation in mitochondria have mainly focused on the contributions of GSH and ceramide. Almost all the GSH (80–85% of total cellular GSH) is found in the cytosol while only a small fraction of the total cellular pool (about 10–15%) is sequestered in mitochondria. This results from the action of a carrier that transports GSH from the cytosol to the mitochondrial matrix [27]. GSH in the mitochondrial matrix is the only defense mechanism available to cope with the potential toxic effects of H2O2 produced endogenously in the electron transport chain. In primary cultured human umbilical vein endothelial cells (HUVEC), TNF-α treatment resulted in slightly increased ROS formation. However, depleting the mitochondrial separate GSH pool with L-buthionine-[S,R]-sulphoximine (BSO) significantly increased ROS formation, thus demonstrating the critical protective role of mitochondrial GSH in TNF-α induced intracellular ROS production in endothelial cells [28]. The important role of GSH transportation in TNF-α induced oxidative stress has been systematically reviewed by Fernandez-Checa et al. [27].
Ceramide is a sphingosine-based lipid-signaling molecule involved in the regulation of cellular differentiation, proliferation, and apoptosis. It can be generated by sphingomyelin (SM) hydrolysis at various subcellular sites by the action of at least two types of sphingomyelinases (SMases): neutral (neutral-sphingomyelinase, NSMase) and acid (acid–sphingomyelinase, ASMase). NSMase located in the vicinity of the plasma membrane hydrolyzes SM located in the outer leaflet of the plasma membrane, whereas ASMase is located in the endosome and/or lysosome compartment, which generates a distinct functional pool of ceramide [29–31]. Recent studies have demonstrated that the rapid ROS production by mitochondria in endothelial cells exposed to TNF-α is, in fact, mediated by ceramide. In these studies, treatment of HUVEC with TNF-α dose-dependently induced rapid (in approximately 1h) ROS formation. Further, inhibitors of mitochondrial respiratory chain activity revealed that complex III was the primary site of the TNF-α-induced ROS generation. Interestingly, the ROS production was inhibited by the AS-Mase inhibitor, desipramine, and totally blocked by the ceramide-activated protein kinase (CAPK) inhibitor, dimethylaminopurine. In contrast, inhibition of NAD(P)H oxidase with diphenylene iodonium (DPI), xanthine oxidase (XO) with allopurinol, nitric oxide synthase (NOS) with Nω-nitro-L-arginine (L-NNA) or cyclooxygenase (COX) pathway by mefenamic acid showed no significant inhibitory effect [32]. These results, therefore, suggest that there is a ceramide-mediated mitochondrial ROS formation pathway, which is independent of NAD(P)H oxidase, NOS, XO and COX. Thus, ROS production in endothelial mitochondria by TNF-α requires ceramide.
NAD(P)H Oxidase Mediated ROS Formation
The NAD(P)H oxidase complex, another important source of ROS, was originally identified and characterized in phagocytes [33]. The classical neutrophil NAD(P)H oxidase comprises a catalytic subunit, gp91phox, which in conjunction with p22phox forms the transmembrane portion of NAD(P)H oxidase, which is also known as flavocytochrome b558. Three cytosolic regulatory subunits are required for activation of the enzyme, these being denoted p67phox, p47phox and p40phox. Additionally, the small GTPase Rac is required for activation of the NAD(P)H complex. All the classical NAD(P)H oxidase subunits have been shown to be expressed in endothelial cells, both at the mRNA and protein levels, although there are significant differences between the phagocyte oxidase and the enzyme in endothelial cells [34].
Activation of NAD(P)H oxidase occurs through a complex series of events involving both phosphorylation and translocation. Traditionally, activation of NAD(P)H oxidase is viewed to follow phosphorylation of p47phox on serine residues [35], which then initiates an intramolecular shift revealing the C-terminal proline rich domain of p47phox so that it can interact with the C-terminal SH3 domain of p67phox [36–38]. p67phox is constitutively bound to p40phox through a PB1 domain[39, 40]. Upon interaction with p47phox the complex traffics to the membrane where p47phox interacts with p22phox in the p22phox/Nox dimer [37, 38, 41] and p67phox interacts directly with the Nox subut in the p22phox/Nox dimer [42–45]. Additionally, activated Rac binds to the N-terminus of p67phox and is required for generation of O2− [46, 47]. For a complete review regarding the activation of NAD(P)H oxidase, we recommend Takeya and Sumimoto [48].
Of the 5 Nox subunits, Nox2 has been the most widely studied as it is the Nox originally described in neutrophils [49]. In endothelial cells although Nox1, 2, 4, and 5 have all been shown to be expressed [50–54], Nox 2 and 4 appear to be the most biologically significant [51, 53, 55, 56]. Further, Nox1 expression levels have been reported to be low to nonexistent in some endothelial cells [51, 56]. Endothelial cells express all of the cytosolic subunits of NAD(P)H oxidase [50, 51]; however there are low levels of p51Nox/Noxa1 suggesting that p67phox is predominant in endothelial cells [50]. The levels of p47phox and p41Nox/Noxo1 are similar. It has become clear that there are significant differences between endothelial cells and phagocytes and further, it is also becoming evident that heterogeneity may exist between endothelial cells. Conceivably, species differences may contribute to these seemingly discordant findings.
Previous studies in cultured rat aortic smooth-muscle cells have shown that TNF-α activates, in a time- and dose-dependent manner, an O2·− producing NAD(P)H oxidase and prolonged treatment with TNF-α increased p22phox mRNA expression suggesting NAD(P)H oxidase as a source for TNF-α induced free radical production [57]. Recent studies demonstrated that in endothelial cells TNF-α could not only induce NAD(P)H oxidase expression but also activate NAD(P)H oxidase directly.
In pulmonary artery endothelial cells (PAEC), TNF-α treatment elicited an increase in the formation of O2·− and induced gp91phox expression, which could be blocked by the continual presence of SOD and apocynin but not catalase, providing evidence for the role of NAD(P)H oxidase in TNF-α induced ROS production [58]. In cultured pig pulmonary artery vascular smooth muscle cells (PAVSMC) and endothelial cells (PAEC), TNF-α treatment also induces O2·− and gp91phox expression, which can be inhibited by DPI and apocynin, but not via the COX inhibitor salicylic acid [59].
In studies of coronary microvascular endothelial cells (CMEC), deletion of p47phox did not result in a reduction in NAD(P)H-dependent ROS production in the absence of agonist stimulation. However, while pre-stimulation with TNF-α significantly increased NAD(P)H-dependent O2·− production in the wild-type (p47phox+/+) cells, this response was completely lost in p47phox−/− cells. Transfection of the full-length p47phox cDNA into p47phox−/− cells caused expression of p47phox protein and restoration of the O2·− response to TNF-α. Thus these data show that p47phox is necessary for TNF-α and NAD(P)H oxidase-mediated activity in endothelial cells [60]. However, while this study utilized exogenous NAD(P)H to assess NAD(P)H oxidase activity, as has been used previously by several groups both in intact cells and intact tissues [60], this approach is subject to criticism since the effect of exogenous NAD(P)H in this setting is uncertain. For example, studies have indicated that exogenous NAD(P)H might, itself, induce endothelial cell ROS formation through both NAD(P)H oxidase-dependent and independent mechanisms [61].
Tumor necrosis factor receptor-associated factors (TRAFS) were initially discovered as adaptor proteins that couple the TNFR family to signaling pathways. Recruitment of TRAF adapter proteins to the cytoplasmic domains of receptor molecules can lead to the assembly of larger signaling complexes that consist of distinct TRAF adapter molecules and other effector proteins with enzymatic functions [62]. A yeast two-hybrid screen found evidence of an association between TRAF4 and p47phox, raising the possibility that TRAF-mediated and NAD(P)H oxidase-dependent downstream redox signaling may in fact be linked [63]. In human microvascular endothelial cells (HMEC), the acute response to TNF-α involves a rapid PKC dependent phosphorylation of p47phox, an increase in p47phox-TRAF4 association, translocation of p47phox-TRAF4 to the cell membrane, increased formation of p47phox-p22phox complexes, and activation of NAD(P)H oxidase and ROS generation. NAD(P)H oxidase activation leads to a rapid (within 5 min) activation of ERK1/2 and p38MAPK (but not JNK), which requires both p47phox and TRAF4. These data suggested that p47phoxphosphorylation and TRAF4 are required for acute TNF-α signaling [64].
A study examining the role of a Rac1-dependent oxidase in regulating TNF-induced ROS production in HUVEC showed that Rac1 is partly responsible for the TNF-α induced oxidative burst but does not regulate TNF-α induced mitochondrial ROS generation [65]. These data, therefore, suggest the possibility that there might be at least two different pathways involved in TNF-α induced ROS formation in endothelial cells [66]. Another group provided evidence that both complex III of the mitochondrial respiratory chain and the gp91phox subunit of NAD(P)H oxidase are the primary sources of ROS in TNF-α treated HUVEC [67]. This further suggests that there may be an internal relationship between mitochondria and NAD(P)H oxidase in TNF-α treated endothelial cells. ROS-mediated advanced glycation end product receptor (RAGE) is induced via the activation of NF-κB and the stimulation of HUVEC by TNF-α; this stimulates NAD(P)H oxidase, which leads to generation of ROS and activation of mitochondrial respiratory chain [67]. This in turn stimulates NF-κB activity and induction of RAGE expression [67].
TNF-α, Nitric Oxide Synthase and Nitric Oxide
NO is a reactive nitrogen species, generated by nitric oxide synthase (NOS) in a two-step, five-electron oxidation of the terminal guanidino nitrogen of L-arginine with N-hydroxy-L-arginine as the intermediate. Three distinct iso-forms of NOS have been well characterized and commonly referred as endothelial (eNOS), neuronal (nNOS), and inducible NOS (iNOS) [68]. Multiple reports demonstrate that TNF-α regulated NOS expression and/or increased activity directly affect NO production.
MacNaul et al. (1993) showed that treatment of human aortic endothelial cell (ACEC) with TNF-α for 8 h induced iNOS mRNA expression but down regulated eNOS expression [69]. While other studies subsequently supported this finding that TNF-α could significantly decrease eNOS expression in endothelial cells [70–73] another group suggested that TNF-α could activate eNOS [74]. Unlike eNOS, iNOS is regulated predominantly at the transcriptional level and not normally produced in most cells. TNF-α induced iNOS mRNA could be decreased by rooperol administration in microvascular endothelial cell (MMEC) [65]. In cultured human nasal microvascular endothelial cells (HNMEC), TNF-α induced iNOS expression, as determined by immunofluorescent staining [75]. Further, co-administration of TNF-α and LPS markedly augmented the expression of iNOS [65].
Several mechanisms have been suggested for the induction/activation of NOS by TNF-α. A recent study showed that activation of eNOS by TNF-α in HUVEC requires activation of Akt. Furthermore, eNOS is activated by TNF-α via sphingosine phosphate (S1P) receptors, activated by S1P generated through NSMase2 and sphingosine kinase type 1 (SK1) [74]. In HUVEC, the effect of TNF-α on iNOS was not affected by statin treatment while reduced eNOS protein expression was reversed by rosuvastatin and cerivastatin through inhibition of HMG-CoA reductase and subsequent blocking of isoprenoid synthesis [76].
At present there are a number of studies relating to the effects of TNF-α on endothelial cell NO production that are yet to be fully placed in context. Co-incubation of TNF-α and IFN-γ (100U/ml each) significantly induced NO formation in the rat brain microvessel-derived EC line, EC219 [77]. Scalera et al. showed that HUVECs incubated with TNF-α (0–1000 pg /ml) for 24 h had no effect on NO release as determined by a colorimetric assay based on the Griess reaction [78], whereas several other studies showed that TNF-α reduced NO formation in HUVEC [79, 80]. In bovine aortic endothelial cell (BAEC), TNF-α (100U/ml) induced NO production through activation of eNOS present in the same membrane compartment [81]. In the mouse vascular aortic endothelial cell line END-D, a low concentration of TNF-α reduced NO production, through down-regulation of cNOS [82], while in the human umbilical vein endothelial ECV304 cell line, TNF-α increased NO formation [70].
Stimulation of NO production from NOS, and eNOS specifically, is generally considered beneficial, especially in regards to endothelial function; however, NO rapidly interacts with O2·− to form ONOO- [83]. ONOO- is a stable potent oxidant; for a complete review of ONOO- biology and pathophysiology we suggest [84]. In the endothelium NO is quenched by O2·− resulting in decreased vascular relaxation, and this quenching is blocked by SOD [85]. NO does not directly interact with SOD [86]; thus, the effects of SOD on the biological function of NO appear to be due to removal of O2·− [87] However, this indicates that ONOO- would also be produced. Furthermore, since TNF-α stimulation can generate O2·− and NO, TNF-α can generate ONOO- [88], which can lead to ED [89]. Therefore, in some situations, such as excessive O2·− stress, TNF-α mediated production of NO is not necessarily beneficial and could contribute to oxidative stress by competing with SOD for O2·− and producing ONOO-.
Other Potential ROS Sources
Xanthine oxidase (XO) is another important ROS source deriving rom O2·− in endothelial cells [90]. However, the effect of TNF-α on endothelial XO is not clear. In rat pulmonary artery endothelial cells, treatment with human recombinant TNF-α induced conversion of xanthine dehydrogenase to XO with an ED50 of 12 nM suggesting that TNF-α could interact directly with endothelial cells to bring about the activation of XO [91]. Several lines of studies in cultured cells failed to inhibit TNF-α induced ROS formation in endothelial cells with the XO inhibitor allopurinol/oxypurinol which might exclude the involvement of XO [32, 60]. However, our experiments showed that TNF-α activated JNK and XO in pig coronary arterioles [75].
Neither mefenamic acid nor aspirin (COX inhibitors) showed a significant inhibitory effect on TNF-α induced ROS formation in endothelial cells suggesting a negligible for COX [32, 59]. However, in HUVEC, TNF-α treatment induced COX-2 expression both at the mRNA and protein levels together with enhanced COX-2 activity [92]. Furthermore, the induction of COX-2 involved intracellular ROS formation and GSH/GSSG alteration [92]. Another study also showed TNF-α induced both COX-1 and COX-2 expression in HUVEC [93]. In view of the role of COX in ROS formation, further studies are required to elucidate its potential role. There is also limited data on the effect of TNF-α on CYP-450 expression and activity in endothelial cells. TNF-α induced endothelial cell adhesion molecule expression has been shown to be CYP-450 dependent [94]. Further, some evidence suggests that TNF-α suppresses cytochrome P450 and UDP glucuronosyl transferase dependent enzyme activity in primary cultured pig hepatocytes [95]. Thus further studies are also warranted on the relationship between TNF-a and this enzyme system.
Although most reports indicate that TNF-α induces endothelial cell ROS formation, one study showed that net generation of cellular reactive oxygen metabolites (ROMs) in response to TNF-α was not apparent despite using four different ROM assays: cytochrome c reduction, nitroblue tetrazolium (NBT) reduction, lucigenin-enhanced chemilumines-cence, and DCFH-DA in EAhy 926 endothelial cell line. Moreover, the provision of exogenous, or stimulation of endogenous ROM, did not upregulate ICAM-1, nor enhance ICAM-1 upregulation by TNF-α [96]. This apparent inconsistency can be attributed to several factors. First, endothelial cell differences may occur between species and between primary cultured cells and cell lines. Second, differences may be due to selection of ROS probes. The detection limits of typical and modified Griess reactions for NO2− are 2 µM and 0.2 µM respectively [97]. Cytochrome c reduction is nonspecific for O2·− and reduced cytochrome c can be reoxidized by cytochrome oxidases, peroxidases, and oxidants, including H2O2 and ONOO− [12]. NBT is also nonspecific for O2·− and numerous other substances including cellular reductases, can reduce NBT [12]. While the DCFH-DA assay may best be applied as a qualitative marker of cellular oxidant stress, its oxidation by cells is complex and there is controversy regarding interpretation of results [98]. Furthermore, nearly all the probes for ROS can undergo autooxidation. Third, there are differences in the literature between the dose and the incubation time of TNF-α from picogram to nanogram and from mins to hours and even days, respectively. Prolonged incubation may conceivably increase autooxidation of the probes.
TNF-α, ROS AND ENDOTHELIAL DYSFUNCTION
The activation, injury and dysfunction of endothelial cells may be a common sequential trilogy for many cardiovascular diseases. Originally, endothelial activation referred to a state in which endothelial cells, responding to various mediators, acquire the ability to perform new functions [16], and now is defined as the increased expression or release of endothelial adhesion molecules [99]. Endothelial injury describes a state in which endothelial cell shape changes, or injury can be identified. This can be evident as defects in endothelial lining or elevation of soluble markers [99]. Generally, dysfunction of the endothelium is manifested as reduced vasodilation, increased vasoconstriction, a proinflammatory state, and prothrombic properties [100]. ED can be caused by, or is associated with, reduced bioavailability of NO, an alteration in the production of prostanoids, including prostacyclin, thromboxane A2, and/or isoprostanes, an impairment of endothelium-dependent hyperpolarization, as well as an increased release of endothelin-1 [3]. These events can be assessed by evaluating vascular functions through flow-mediated vasodilatation, coronary circulation, vascular stiffness or measurement of biochemical markers of oxidative stress such as NO and its metabolites, 8-hydroxy-2'deoxyguanosine (8-OHdG), oxidized LDL, isoprostanes, oxidatively modified tyrosine residues, adhesion molecules. TNF-α has a broad effect on endothelial cells, and the endothelial cell activation and injury by TNF-α has been reviewed in detail previously [1, 17, 20, 101]. This portion of the review therefore specifically focuses on the effects of TNF-α on endothelial function involving ROS, and particularly on vascular reactivity to TNF-α.
Direct Injury of ROS
Uncontrolled ROS production causes cell injury by DNA damage, oxidation of polyunsaturated fatty acids and amino acids, and inactivation of specific enzymes through oxidizing their co-factors [102]. Previous studies show that TNF-α induced intracellular ROS formation is a mediator of mitochondrial and nuclear DNA damage in cardiac myocytes [103], primary murine hepatocytes [104] and L929 cells fibroblasts [105]. Lipid peroxidative metabolites resulting from the interaction of free radicals with lipids are indirect indicators of oxidative stress, which include a series of small molecules such as malondialdehyde (MDA) and 4-hydroxy-alkenals (4-HNE). Lower concentrations of TNF-α (10–100pg) dose-dependently increase intracellular lipid peroxides as determined by MDA and 4-HNE in HUVECs [78]. TNF-α induced lipid peroxidation in the human umbilical vein endothelial ECV304 cell lines is also involved in the activation of NF-κB [70].
ROS AS A MEDIATOR IN TNF-α INDUCED GENE EXPRESSION
TNF-α induces profound changes in the expression of a set of 19 glycosyltransferases, which are correlated with an alteration of endothelial cell surface glycosylation in HUVEC [95]. Recent studies also found TNF-α induced stannin gene expression via a PKC-ζ dependent manner in endothelial cells [106]. MnSOD expression is induced by TNF-α via a mitochondria-to-nucleus signaling mechanism that is inhibited by NAC treatment [107], thus demonstrating a role for ROS in this signaling pathway. Several studies suggested that TNF-α upregulates ET-1 gene expression [108] and ET-1 release from endothelial cells [109, 110], while one study demonstrated lower concentrations (10–100pg/ml) of TNF-α to increase while higher concentrations (1000pg/ml) decreased ET-1 secretion [78]. TNF-α significantly induces ET-1 and IL-8 gene expression in HMECs, but possibly through different redox signaling pathways. Thus, ET-1 expression appears to be regulated by O2·−, whereas IL-8 expression appears to be mediated by H2O2 [111]. TNF-α induced RAGE expression is mediated through NF-κB and 17-beta-estradiol activation of the transcription factor Sp1,with this pathway being dependent on activation of NAD(P)H oxidase and subsequent ROS formation [112]. Additionally TNF-α, in a ROS-dependent manner, is involved in initiating cytokine signaling, caspase activation, cytochrome c release, regulation of mitochondrial membrane potential and NF-κB activation, as has been comprehensively summarized in a previous review [101].
TNF-α Induced Vascular Endothelial Dysfunction
Vasoactivity (contraction and relaxation) depends on a complex interplay between endothelial and smooth muscle cells. Endothelial cells contribute to the regulation of vasoactivity by releasing several potent vasodilators, such as NO, endothelium-derived hyperpolarizing factor, and prostacyclin, and several vasoconstrictors, including ET-1, TXA2, PGH2, and O2·−. In vitro, low concentrations (10–100 pg/ml) of TNF-α increase the intracellular content of LPO and GSH, stimulate the secretion of ET-1 and TXA2, but inhibit the secretion of PGI2 in HUVECs. In contrast, high concentrations (1000pg/ml) of TNF-α increase the secretion of PGI2 and TXA2 while decreasing ET-1 secrettion. These results suggest that TNF-α induces oxidative stress and results in altered secretion of vasodilatory substances in favor of vasoconstrictors in human endothelial cells [78].
The effect of TNF-α on vasoactivity has been widely supported by studies in several species, including humans, utilizing isolated blood vessels or in vivo studies. In rat skeletal muscle arterioles, 2 min of incubation with TNF-α has no direct vasomotor effect. However, pretreatment with endotoxin revealed that TNF-α can cause arteriolar dilation, possibly through a mechanism involving COX and NOS [113]. Infusion of TNF-α into normal SD rats does not significantly alter either mean arterial pressure or heart rate. However, isolated aorta and pulmonary artery from both TNF-α infused and normal animals treated with TNF-α for 2h showed reduced acetylcholine-induced vascular relaxation, which was sustained for at least 80 minutes. In the same system nitroglycerine-induced vascular relaxation was not altered [114]. Aortic strips from pregnant rats show a greater enhancement of phenylephrine (Phe) contraction than those from virgin rats indicating that pregnancy alters vascular function. Furthermore, TNF-α significantly inhibits acetylcholine (Ach)- and bradykinin-induced vascular relaxation and nitrite/nitrate production more prominently in pregnant than virgin rats. In TNF-α treated vessels, L-NAME and ODQ (cGMP inhibitor) inhibit Ach-mediated relaxation and enhance Phe-mediated contraction suggesting the involvement of NO-cGMP-mediated vascular relaxation in TNF-α treated vessels [115, 116]. Intraperitoneal injection of recombinant murine TNF-α (20 µg/kg) to male ACI rats caused no effect on hemodynamics or significant tissue injury, while inducing iNOS expression with a subsequent increase in NO production. Furthermore, the systemic vascular barrier was injured by TNF-α administration [117].
In anesthetized, ventilated sheep, recombinant human TNF-α (10 mg for 20 or 40 min) infused directly into the bronchial artery resulted in a significant decrease in bronchial vascular resistance after 20 minutes but this vasodilation was followed by a reversal of tone within 120 min. The observed increase in bronchial vascular resistance was suggested to result from a secondary release of ET-1 [118]. The incubation of bovine intralobar pulmonary arteries with TNF-α for 60min inhibited endothelium-dependent relaxation to acetylcholine, bradykinin and histamine. TNF-α mediated inhibition of endothelium-dependent relaxation was not reversible by L-NMMA, an inhibitor of NOS, the COX inhibitor, ibuprofen, or the platelet-activating factor antagonist, CV3908 [119]. In isolated palmar digital arteries of horses, TNF-α (5100 pg/ml) exposure significantly decreased maximal relaxation to Ach and increased maximal contraction to norepinephrine. L-arginine treatment was without effect [120]. Intravenous administration of natural human TNF-α (10 µg/kg) to anesthetized dogs induced a significant increase in mean arterial pressure and cardiac index and a significant decrease of systemic and pulmonary vascular resistance index. Furthermore, plasma levels of ET-1, ET-3, NOx, and 6-keto-PGF1 alpha significantly increased at 1 h [121]. Our own studies showed that intraluminal treatment of pig coronary arterioles with TNF-α (1ng/ml, 90 min) significantly attenuated NO release and vasodilation to adenosine which was not observed in denuded vessels or in the presence of L-NMMA. O2·− production and JNK phosphorylation was enhanced by TNF-α. Furthermore, administration of an O2·− scavenger (TEMPOL) or inhibitors of CAPK (dimethylaminopurine), JNK (SP600125 and dicumarol), and XO (allopurinol) reduced O2·− production as while restoring NO release and vasodilation to adenosine. Conversely, the effects of TNF-α were insensitive to inhibitors of p38 (SB203580), ERK (PD98059), NAD(P)H oxidase (apocynin), or mitochondrial respiratory chain (rotenone). These data, therefore, indicated that TNF-α inhibits endothelium-dependent NO-mediated dilation of coronary arterioles by ceramide-induced activation of JNK and subsequent production of O2·−via XO [91].
In humans, several lines of evidence point towards TNF-α playing an important role in eliciting ED. It has been established, through clinical studies, that there is a strong positive relationship between increased plasma TNF-α levels and reduced reactivity to Ach [122]. Though elevated concentrations of TNF-α contract the distal ends of human internal mammary arteries, it failed to relax norepinephrine-precon-tracted vessels. The observed vasoconstrictor effects were mediated by ETA receptors and were endothelium dependent [123]. In healthy volunteers, low-dose intra-arterial forearm infusion of TNF-α increased forearm vascular resistance by increasing the bioavailability of the vasoconstrictor prostaglandin while reducing the bioavailability of NO [124]. Furthermore, incubation of human temporal artery segments with TNF-α for 2 days concentration-dependently enhanced endothelin ETB receptor-mediated contraction [125]. TNF-α infusion in healthy subjects induced transient and reversible ED in vein as determined by measurements of vessel diameter [114]. In diabetic adults, TNF-α induces ED, which is reversed by the PPAR-gamma agonist pioglitazone [126]. However, other studies in human isolated resistance arteries, preconstricted with noradrenalin or a depolarizing high potassium solution, showed that TNF-α and IL-1 (alone or in combination) does not exert any relevant vasoactive effect [127]. These data suggest that TNF-α does not exert a direct effect on human resistance arteries.
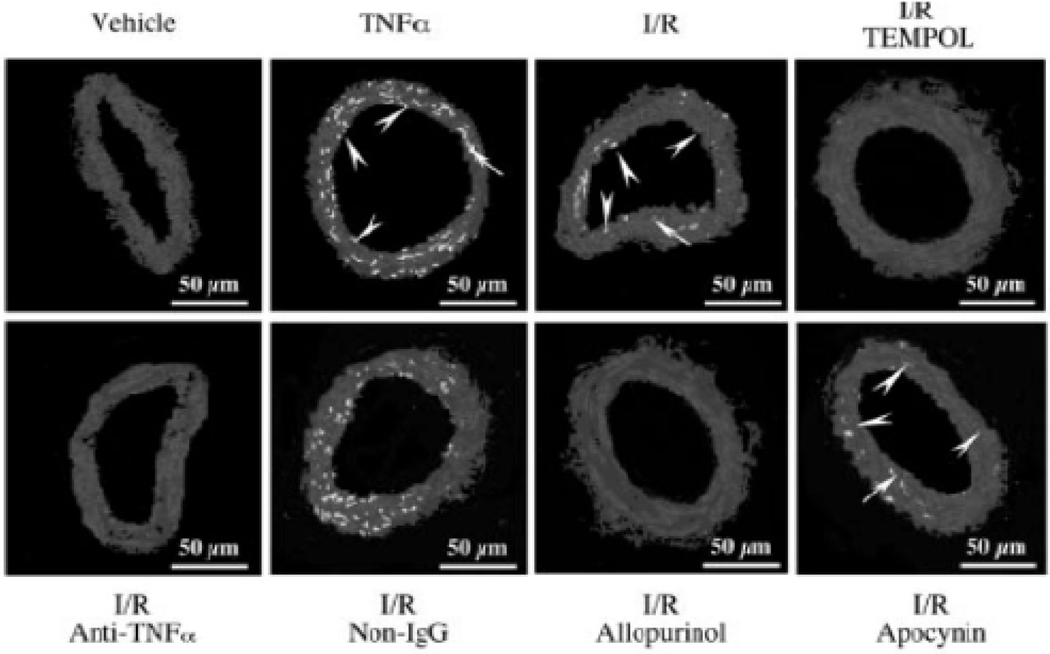
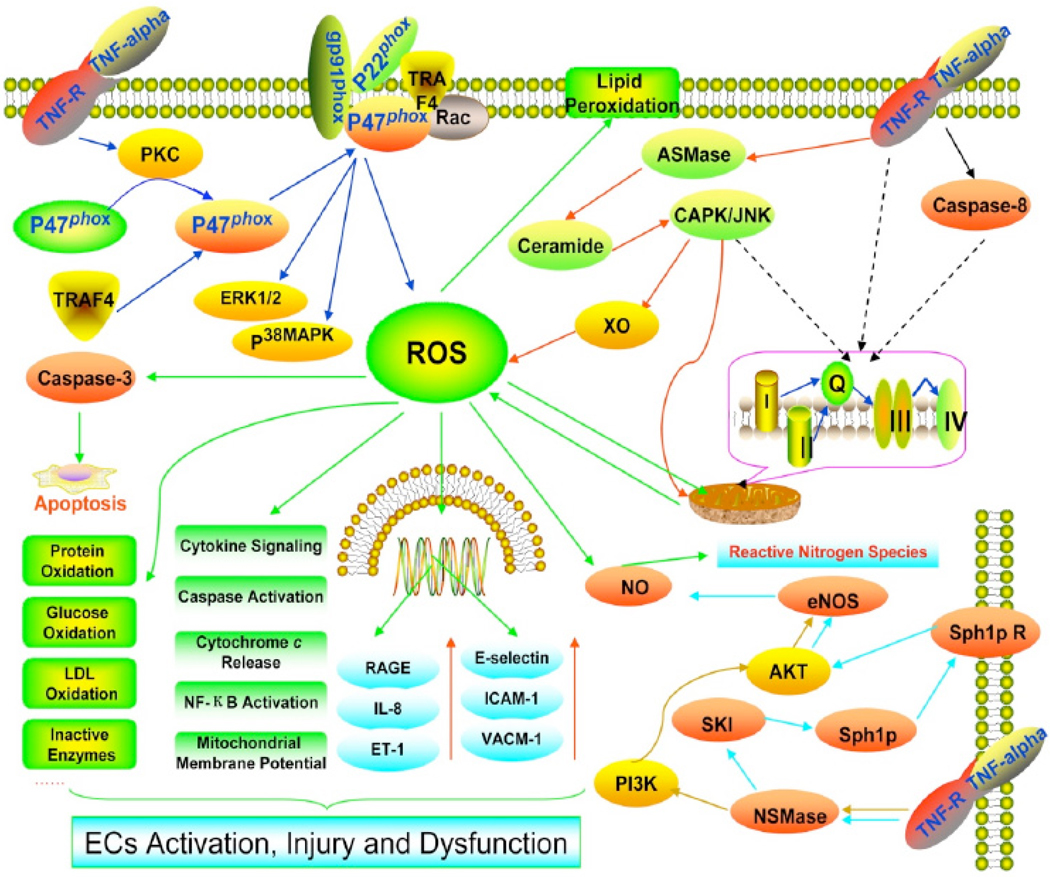
Animal models have provided more evidence for the role of ROS in TNF-α induced ED. For example, one study indicated that TNF-α contributes to ischemia-reperfusion (I/R) induced endothelial activation, in isolated rat heart, through early TNF-α cleavage and NF-κB activation [128]. Additionally, our lab has provided direct evidence for the role of ROS in TNF-α induced ED. We tested the effect of neutralizing TNF-α antibody on coronary arteriolar dilation in a murine I/R model and found that the neutralizing antibody as well as TEMPOL, allopurinol and oxypurinol restored endothelium-dependent dilation in the I/R group and reduced I/R-stimulated O2·− production (Fig. 1). Furthermore, I/R-dependent activation of XO was prevented by the antibody as well as the XO inhibitor allopurinol. These results suggest that TNF-α contributes to ED in myocardial I/R through activation of XO and production of O2·−, leading to coronary ED [129]. We also demonstrated that TNF-α upregulates expression of arginase in endothelial cells, which leads to O2·− production that then contributes to ED and I/R injury [130]. Pretreatment of SD rats with a neutralizing antisera directed against TNF-α demonstrated a significant reduction in acute lung edema in response to 4 h of ischemia and 30 min of reperfusion [131]. Furthermore, cultured endothelial cells exposed to plasma from the ischemic hind limb of a SD rat altered their shape and increased permeability in a TNF-α-dependent manner [131]. Using Zucker Obese Fatty (ZOF) mice, a model of pre-diabetic metabolic syndrome, we discovered that administration of the O2·− scavenger TEMPOL, NAD(P)H oxidase inhibitor (apocynin), or anti-TNF-α restored endothelium-dependent dilation in pressurized coronary small arteries [124]. Furthermore, administration of anti-TNF-α or apocynin inhibited O2·− formation and restored eNOS expression [132]. These results demonstrate the ED occurring in the metabolic syndrome may be the result of effects of the inflammatory cytokine TNF-α and subsequent production of O2·− [132]. Similar results were obtained with type 2 diabetic Leprdb mice [133]. In summary, among the mechanisms responsible for TNF-α induced endothelial dysfunction is most likely to play the primary role of ROS (Fig. 2).
Fig 1.
DHE-fluorescence imaging of O2 in coronary arterioles . DHE fluorescence was markedly elevated in both endothelial (arrow head) and vascular smooth muscle (arrow) cells of arteriolar sections after TNF-α treatment (1 ng/mL, 60 minutes, n=4) and I/R (n=4) compared with vehicle (sham). Apocynin and nonimmune IgG did not, but TEMPOL, allopurinol, and anti-TNF-α, markedly reduced the fluorescent signals (n=4). (Adapted from Figure 1 in Zhang, C et al. [129].
Fig 2.
Role of ROS in TNF-α induced endothelial dysfunction. At least four sources of ROS exist in TNF-α treated endothelial cells: the NADPH oxidase, the mitochondria, the XO and the eNOS. The binding of TNF-α to its receptor: 1. induces PKC phosphorylate p47phox and phosphorylated p47phox to recruit adaptor protein TRAF4. The p47phox-TRAF4 complex translocates to the membrane and activated NADPH oxidase; 2. induces mitochondrial ROS formation through ceramide-CAPK or caspase 8 ; 3. activates ASMase then through creamide-CAPK-JNK pathway to activate XO ; 4. activates NSMase then through PI3K–AKT or Sphingosine 1 phosphate (Sph1p) receptor to regulate eNOS resulting in NO production .TNF-α induced ROS may exert a variety of effects including causing direct injury to lipid membranes, proteins, glucose, LDL, enzymes, DNA. ROS also acts as a second messenger to induce RAGE, IL-8, ET-1, E-selectin, ICAM-1, VCAM-1 expression and induces cytokine signaling, caspase activation, cytochrome c release, NF-κB activation, mitochondrial membrane potential changes etc. Collectively, these events contribute to TNF-α induced endothelial activation, injury and dysfunction.
CONCLUSION
Endothelial activation, injury and dysfunction precede the development of a number of cardiovascular diseases especially atherosclerosis. Excessive production of ROS may cause oxidative damage in a molecule, a cellular organelle, a cell tissue and even an organ resulting in a steady state condition generally referred to as oxidative stress. Importantly, many of the accepted cardiovascular risk factors, including hyperlipidemia, hypertension, diabetes and smoking, are associated with overproduction of ROS or increased oxidative stress. Furthermore, increased oxidative stress is considered to be a major mechanism involved in the pathogenesis of ED and may serve as a common pathogenic mechanism manifesting the effect of risk factors on the endothelium. Recent studies show that TNF-α, one of the most important inflammatory cytokines, also plays an important role in ED. ROS appears to serve as one of the key mediators involved in TNF-α induced cellular responses thus linkings these two mechanisms. Elucidating the exact mechanism(s) underlying these actions will require further study.
Acknowledgments
FUNDING SOURCES
This study was supported by grants from American Heart Association Scientist Development Grant (110350047A), Pfizer Atorvastatin Research Award (2004-37) and NIH grants (RO1-HL077566 and RO1-HL085119) to Dr. Cuihua Zhang.
REFERENCES
- 1.Mantovani A, Sozzani S, Introna M. Endothelial activation by cytokines. Ann N Y Acad Sci. 1997;832:93–116. doi: 10.1111/j.1749-6632.1997.tb46240.x. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P. Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 4.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 6.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 7.Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol. 2000;88:1880–1889. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 8.Droge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 9.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kowaltowski AJ, Vercesi AE. Reactive oxygen generation by mitochondria. Mitochondria in Pathogenesis. 2002:281–300. [Google Scholar]
- 11.Shah AM, Channon KM. Free radicals and redox signalling in cardiovascular disease. Heart. 2004;90:486–487. doi: 10.1136/hrt.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 13.Azevedo LC, Janiszewski M, Soriano FG, et al. Redox mechanisms of vascular cell dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:159–164. doi: 10.2174/187153006777442431. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 15.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pober JS, Min W. Endothelial cell dysfunction, injury and death. Handb Exp Pharmacol. 2006:135–156. doi: 10.1007/3-540-36028-x_5. [DOI] [PubMed] [Google Scholar]
- 17.Luster MI, Simeonova PP, Gallucci R, et al. Tumor necrosis factor alpha and toxicology. Crit Rev Toxicol. 1999;29:491–511. doi: 10.1080/10408449991349258. [DOI] [PubMed] [Google Scholar]
- 18.Beyaert R, Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity What we do understand and what we do not. FEBS Lett. 1994;340:9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- 19.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. Faseb J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 20.Pober JS. Endothelial activation: intracellular signaling pathways. Arthritis Res. 2002;3(4 Suppl):S109–S116. doi: 10.1186/ar576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 22.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;9:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 25.Schulze-Osthoff K, Beyaert R, Vandevoorde V, et al. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. Embo J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goossens V, Grooten J, De Vos K, et al. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, et al. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 28.Chen KH, Reece LM, Leary JF. Mitochondrial glutathione modulates TNF-alpha-induced endothelial cell dysfunction. Free Radic Biol Med. 1999;27:100–109. doi: 10.1016/s0891-5849(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 29.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee S. Neutral sphingomyelinase: past, present and future. Chem Phys Lipids. 1999;102:79–96. doi: 10.1016/s0009-3084(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 31.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 32.Corda S, Laplace C, Vicaut E, et al. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 33.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 34.Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 35.Ago T, Nunoi H, Ito T, et al. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 36.Finan P, Shimizu Y, Gout I, et al. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J Biol Chem. 1994;269:13752–13755. [PubMed] [Google Scholar]
- 37.Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci U S A. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumimoto H, Kage Y, Nunoi H, et al. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci U S A. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito T, Matsui Y, Ago T, et al. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. Embo J. 2001;20:3938–3946. doi: 10.1093/emboj/20.15.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsunawaki S, Mizunari H, Nagata M, et al. A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox. Biochem Biophys Res Commun. 1994;199:1378–1387. doi: 10.1006/bbrc.1994.1383. [DOI] [PubMed] [Google Scholar]
- 41.Leusen JH, Bolscher BG, Hilarius PM, et al. 156Pro-->Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Exp Med. 1994;180:2329–2334. doi: 10.1084/jem.180.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang PM, Cross AR, Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc Natl Acad Sci U S A. 2001;98:3001–3005. doi: 10.1073/pnas.061029698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang PM, Cross AR, Quinn MT, et al. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc Natl Acad Sci U S A. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han CH, Freeman JL, Lee T, et al. Regulation of the neutrophil respiratory burst oxidase Identification of an activation domain in p67(phox) J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 45.Nisimoto Y, Motalebi S, Han CH, et al. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558) J Biol Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 46.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 47.Koga H, Terasawa H, Nunoi H, et al. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 48.Takeya R, Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol Cells. 2003;16:271–277. [PubMed] [Google Scholar]
- 49.Rotrosen D, Yeung CL, Leto TL, et al. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992;256:1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- 50.Ago T, Kitazono T, Kuroda J, et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 51.Ago T, Kitazono T, Ooboshi H, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 52.BelAiba RS, Djordjevic T, Petry A, et al. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 53.Petry A, Djordjevic T, Weitnauer M, et al. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 54.Van Buul JD, Fernandez-Borja M, Anthony EC, et al. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 55.Datla SR, Peshavariya H, Dusting GJ, et al. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 56.Li JM, Fan LM, George VT, et al. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Keulenaer GW, Alexander RW, Ushio-Fukai M, et al. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329(Pt 3):653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muzaffar S, Shukla N, Angelini GD, et al. Superoxide auto-augments superoxide formation and upregulates gp91(phox) expression in porcine pulmonary artery endothelial cells: inhibition by iloprost. Eur J Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 59.Muzaffar S, Shukla N, Angelini G, et al. Nitroaspirins and morpholinosydnonimine but not aspirin inhibit the formation of super-oxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation. 2004;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- 60.Li JM, Mullen AM, Yun S, et al. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 61.Park L, Anrather J, Zhou P, et al. Exogenous NADPH increases cerebral blood flow through NADPH oxidase-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:1860–1865. doi: 10.1161/01.ATV.0000142446.75898.44. [DOI] [PubMed] [Google Scholar]
- 62.Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs)--a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 63.Xu YC, Wu RF, Gu Y, et al. Involvement of TRAF4 in oxidative activation of c-Jun N-terminal kinase. J Biol Chem. 2002;277:28051–28057. doi: 10.1074/jbc.M202665200. [DOI] [PubMed] [Google Scholar]
- 64.Li JM, Fan LM, Christie MR, et al. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bereta J, Bereta M, Allison AC, et al. Inhibitory effect of dicatechol rooperol on VCAM-1 and iNOS expression in cytokine-stimulated endothelium. Life Sci. 1997;60:325–334. doi: 10.1016/s0024-3205(96)00633-9. [DOI] [PubMed] [Google Scholar]
- 66.Deshpande SS, Angkeow P, Huang J, et al. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. Faseb J. 2000;14:1705–1714. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- 67.Mukherjee TK, Mukhopadhyay S, Hoidal JR. The role of reactive oxygen species in TNFalpha-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells. Biochim Biophys Acta. 2005;1744:213–223. doi: 10.1016/j.bbamcr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 69.MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196:1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 70.Xia Z, Liu M, Wu Y, et al. N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol. 2006;550:134–142. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Patel JM, Li YD, et al. Proinflammatory cytokines down-regulate gene expression and activity of constitutive nitric oxide synthase in porcine pulmonary artery endothelial cells. Res Commun Mol Pathol Pharmacol. 1997;96:71–87. [PubMed] [Google Scholar]
- 72.Seidel M, Billert H, Kurpisz M. Regulation of eNOS expression in HCAEC cell line treated with opioids and proinflammatory cytokines. Kardiol Pol. 2006;64:153–158. discussion 9–60. [PubMed] [Google Scholar]
- 73.Goodwin BL, Pendleton LC, Levy MM, et al. Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1115–H1121. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 74.De Palma C, Meacci E, Perrotta C, et al. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 75.Zhang C, Hein TW, Wang W, et al. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Jantzen F, Konemann S, Wolff B, et al. Isoprenoid depletion by statins antagonizes cytokine-induced down-regulation of endothelial nitric oxide expression and increases NO synthase activity in human umbilical vein endothelial cells. J Physiol Pharmacol. 2007;58:503–514. [PubMed] [Google Scholar]
- 77.Murata J, Corradin SB, Janzer RC, et al. Tumor cells suppress cytokine-induced nitric-oxide (NO) production in cerebral endothelial cells. Int J Cancer. 1994;59:699–705. doi: 10.1002/ijc.2910590519. [DOI] [PubMed] [Google Scholar]
- 78.Scalera F. Intracellular glutathione and lipid peroxide availability and the secretion of vasoactive substances by human umbilical vein endothelial cells after incubation with TNF-alpha. Eur J Clin Invest. 2003;33:176–182. doi: 10.1046/j.1365-2362.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- 79.Ma ZC, Gao Y, Wang J, et al. Proteomic analysis effects of ginsenoside Rg1 on human umbilical vein endothelial cells stimulated by tumor necrosis factor-alpha. Life Sci. 2006;79:175–181. doi: 10.1016/j.lfs.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Z, Wang SQ, Liu Y, et al. Cryptotanshinone inhibits endothelin-1 expression and stimulates nitric oxide production in human vascular endothelial cells. Biochim Biophys Acta. 2006;1760:1–9. doi: 10.1016/j.bbagen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 82.Morikawa A, Koide N, Kato Y, et al. Augmentation of nitric oxide production by gamma interferon in a mouse vascular endothelial cell line and its modulation by tumor necrosis factor alpha and lipopolysaccharide. Infect Immun. 2000;68:6209–6214. doi: 10.1128/iai.68.11.6209-6214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxynitrite in alkaline aqueous solutions. Inorg Chem. 1985;24:3504–3505. [Google Scholar]
- 84.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 86.Gorren AC, de Boer E, Wever R. The reaction of nitric oxide with copper proteins and the photodissociation of copper-NO complexes. Biochim Biophys Acta. 1987;916:38–47. doi: 10.1016/0167-4838(87)90208-1. [DOI] [PubMed] [Google Scholar]
- 87.Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phelps DT, Ferro TJ, Higgins PJ, et al. TNF-alpha induces peroxynitrite-mediated depletion of lung endothelial glutathione via protein kinase C. Am J Physiol. 1995;269:L551–L559. doi: 10.1152/ajplung.1995.269.4.L551. [DOI] [PubMed] [Google Scholar]
- 89.Neumann P, Gertzberg N, Vaughan E, et al. Peroxynitrite mediates TNF-alpha-induced endothelial barrier dysfunction and nitration of actin. Am J Physiol Lung Cell Mol Physiol. 2006;290:L674–L684. doi: 10.1152/ajplung.00391.2005. [DOI] [PubMed] [Google Scholar]
- 90.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friedl HP, Till GO, Ryan US, et al. Mediator-induced activation of xanthine oxidase in endothelial cells. Faseb J. 1989;3:2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- 92.Eligini S, Barbieri SS, Cavalca V, et al. Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc Res. 2005;65:683–693. doi: 10.1016/j.cardiores.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 93.Schmeck B, Brunsch M, Seybold J, et al. Rho protein inhibition blocks cyclooxygenase-2 expression by proinflammatory mediators in endothelial cells. Inflammation. 2003;27:89–95. doi: 10.1023/a:1023278600596. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki M, Ostanin D, Elrod JW, et al. TNF-alpha -induced endothelial cell adhesion molecule expression is cytochrome P-450 monooxygenase dependent. Am J Physiol Cell Physiol. 2003;284:C422–C428. doi: 10.1152/ajpcell.00271.2002. [DOI] [PubMed] [Google Scholar]
- 95.Monshouwer M, Witkamp RF, Nujmeijer SM, et al. Suppression of cytochrome P450- and UDP glucuronosyl transferase-dependent enzyme activities by proinflammatory cytokines and possible role of nitric oxide in primary cultures of pig hepatocytes. Toxicol Appl Pharmacol. 1996;137:237–244. doi: 10.1006/taap.1996.0077. [DOI] [PubMed] [Google Scholar]
- 96.Arai T, Kelly SA, Brengman ML, Takano M, Smith EH, et al. Ambient but not incremental oxidant generation effects intercellular adhesion molecule 1 induction by tumour necrosis factor alpha in endothelium. Biochem J. 1998;331(Pt 3):853–861. doi: 10.1042/bj3310853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marzinzig M, Nussler AK, Stadler J, et al. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide. 1997;1:177–189. doi: 10.1006/niox.1997.0116. [DOI] [PubMed] [Google Scholar]
- 98.Chen XP, Du GH. Dichlorodihydrofluorescin diacetate, forty years' application and controversy. Herald of Medicine. 2007;26:343–349. [Google Scholar]
- 99.Vallet B. Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction? Crit Care. 2003;7:130–138. doi: 10.1186/cc1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 101.Shakibaei M, Schulze-Tanzil G, Takada Y, et al. Redox regulation of apoptosis by members of the TNF superfamily. Antioxid Redox Signal. 2005;7:482–496. doi: 10.1089/ars.2005.7.482. [DOI] [PubMed] [Google Scholar]
- 102.Karihtala P, Soini Y, Auvinen P, et al. Hyaluronan in breast cancer: correlations with nitric oxide synthases and tyrosine nitrosylation. J Histochem Cytochem. 2007;55:1191–1198. doi: 10.1369/jhc.7A7270.2007. [DOI] [PubMed] [Google Scholar]
- 103.Suematsu N, Tsutsui H, Wen J, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 104.Wheelhouse NM, Chan YS, Gillies SE, et al. TNF-alpha induced DNA damage in primary murine hepatocytes. Int J Mol Med. 2003;12:889–894. [PubMed] [Google Scholar]
- 105.Park YM, Han MY, Blackburn RV, et al. Overexpression of HSP25 reduces the level of TNF alpha-induced oxidative DNA damage biomarker, 8-hydroxy-2'-deoxyguanosine, in L929 cells. J Cell Physiol. 1998;174:27–34. doi: 10.1002/(SICI)1097-4652(199801)174:1<27::AID-JCP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 106.Reese BE, Davidson C, Billingsley ML, et al. Protein kinase C epsilon regulates tumor necrosis factor-alpha-induced stannin gene expression. J Pharmacol Exp Ther. 2005;314:61–69. doi: 10.1124/jpet.105.084236. [DOI] [PubMed] [Google Scholar]
- 107.Rogers RJ, Monnier JM, Nick HS. Tumor necrosis factor-alpha selectively induces MnSOD expression via mitochondria-to-nucleus signaling, whereas interleukin-1beta utilizes an alternative pathway. J Biol Chem. 2001;276:20419–20427. doi: 10.1074/jbc.M008915200. [DOI] [PubMed] [Google Scholar]
- 108.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- 109.Corder R, Carrier M, Khan N, et al. Cytokine regulation of endothelin-1 release from bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1995;26 Suppl 3:S56–S58. [PubMed] [Google Scholar]
- 110.Tang C, Wu AH, Xue HL, et al. Tanshinone IIA inhibits endothelin-1 production in TNF-alpha-induced brain microvascular endothelial cells through suppression of endothelin-converting enzyme-1 synthesis. Acta Pharmacol Sin. 2007;28:1116–1122. doi: 10.1111/j.1745-7254.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 111.Zhao RZ, Chen X, Yao Q, et al. TNF-alpha induces interleukin-8 and endothelin-1 expression in human endothelial cells with different redox pathways. Biochem Biophys Res Commun. 2005;327:985–992. doi: 10.1016/j.bbrc.2004.12.109. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka N, Yonekura H, Yamagishi S, et al. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 113.Glembot TM, Britt LD, Hill MA. Endotoxin interacts with tumor necrosis factor-alpha to induce vasodilation of isolated rat skeletal muscle arterioles. Shock. 1996;5:251–257. doi: 10.1097/00024382-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 114.Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535–H2541. doi: 10.1152/ajpheart.1994.266.6.H2535. [DOI] [PubMed] [Google Scholar]
- 115.Davis JR, Giardina JB, Green GM, et al. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R390–R399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 116.Giardina JB, Green GM, Cockrell KL, et al. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R130–R143. doi: 10.1152/ajpregu.00704.2001. [DOI] [PubMed] [Google Scholar]
- 117.Worrall NK, Chang K, LeJeune WS, et al. TNF-alpha causes reversible in vivo systemic vascular barrier dysfunction via NO-dependent and -independent mechanisms. Am J Physiol. 1997;273:H2565–H2574. doi: 10.1152/ajpheart.1997.273.6.H2565. [DOI] [PubMed] [Google Scholar]
- 118.Wagner EM. TNF-alpha induced bronchial vasoconstriction. Am J Physiol Heart Circ Physiol. 2000;279:H946–H951. doi: 10.1152/ajpheart.2000.279.3.H946. [DOI] [PubMed] [Google Scholar]
- 119.Greenberg S, Xie J, Wang Y, et al. Tumor necrosis factor-alpha inhibits endothelium-dependent relaxation. J Appl Physiol. 1993;74:2394–2403. doi: 10.1152/jappl.1993.74.5.2394. [DOI] [PubMed] [Google Scholar]
- 120.Baxter G. Effects of tumor necrosis factor on in vitro digital arterial responses in horses. Am J Vet Res. 1994;55:551–555. [PubMed] [Google Scholar]
- 121.Mitaka C, Hirata Y, Ichikawa K, et al. Effects of TNF-alpha on hemodynamic changes and circulating endothelium-derived vasoactive factors in dogs. Am J Physiol. 1994;267:H1530–H1536. doi: 10.1152/ajpheart.1994.267.4.H1530. [DOI] [PubMed] [Google Scholar]
- 122.Holm T, Aukrust P, Andreassen AK, et al. Peripheral endothelial dysfunction in heart transplant recipients: possible role of proin-flammatory cytokines. Clin Transplant. 2000;14:218–225. doi: 10.1034/j.1399-0012.2000.140307.x. [DOI] [PubMed] [Google Scholar]
- 123.Iversen PO, Nicolaysen A, Kvernebo K, et al. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflugers Arch. 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- 124.Nakamura M, Yoshida H, Arakawa N, et al. Effects of tumor necrosis factor-alpha on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J Cardiovasc Pharmacol. 2000;36:487–492. doi: 10.1097/00005344-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 125.White LR, Juul R, Skaanes KO, et al. Cytokine enhancement of endothelin ET(B) receptor-mediated contraction in human temporal artery. Eur J Pharmacol. 2000;406:117–122. doi: 10.1016/s0014-2999(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 126.Martens FM, Rabelink TJ, op 't Roodt J, et al. TNF-alpha induces endothelial dysfunction in diabetic adults, an effect reversible by the PPAR-gamma agonist pioglitazone. Eur Heart J. 2006;27:1605–1609. doi: 10.1093/eurheartj/ehl079. [DOI] [PubMed] [Google Scholar]
- 127.Pickkers P, Netea MG, van der Meer JW, et al. TNFalpha and IL-1beta exert no direct vasoactivity in human isolated resistance arteries. Cytokine. 2002;20:244–246. doi: 10.1006/cyto.2002.2004. [DOI] [PubMed] [Google Scholar]
- 128.Kupatt C, Habazettl H, Goedecke A, et al. Tumor necrosis factor-alpha contributes to ischemia- and reperfusion-induced endothelial activation in isolated hearts. Circ Res. 1999;84:392–400. doi: 10.1161/01.res.84.4.392. [DOI] [PubMed] [Google Scholar]
- 129.Zhang C, Xu X, Potter BJ, et al. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 130.Gao X, Xu X, Belmadani S, et al. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–1275. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- 131.Gilmont RR, Dardano A, Engle JS, et al. TNF-alpha potentiates oxidant and reperfusion-induced endothelial cell injury. J Surg Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- 132.Picchi A, Gao X, Belmadani S, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 133.Gao X, Belmadani S, Picchi A, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]