Abstract
This article examines age differences in individual’s ability to produce the durations of learned auditory and visual target events either in isolation (focused attention) or concurrently (divided attention). Young adults produced learned target durations equally well in focused and divided attention conditions. Older adults in contrast showed an age-related increase in timing variability in divided attention conditions that tended to be more pronounced for visual targets than for auditory targets. Age-related impairments were associated with a decrease in working memory span; moreover, the relationship between working memory and timing performance was largest for visual targets in divided attention conditions.
Many everyday tasks that humans perform involve assessments of temporal extent. For example, an individual’s ability to judge (in the absence of an explicit clock) whether a stoplight that is visited on a regular commute is shorter or longer than usual requires some internal mechanism that measures time, as well as a mechanism that permits comparison of moment-by-moment time estimates with an individual’s temporal memory of the stoplight’s duration. From an information processing perspective, behavioral paradigms that assess timing have become an increasingly popular tool used by psychologists and neuroscientists to probe age-related changes in cognition (Craik & Hay, 1999; Lustig & Meck, 2001; McAuley, Jones, Holub, Johnston & Miller, 2006; McCormack, Brown, Maylor, Darby, & Green, 1999; Vanneste & Pouthas, 1999; Wearden, Wearden, & Rabbitt, 1997). This is in part because of (1) evidence that the component attention, memory, and decision processes involved in timing may be shared by human and non-human animals (Allan & Gibbon, 1991; Wearden & Ferrara, 1993; Wearden & McShane, 1988) and (2) the systematic sensitivity of timing paradigms to behavioral, pharmacological, and neurobiological manipulations in humans and animal models (Lustig & Meck, 2001; Macar, Grondin, & Casini, 1994; Meck, 1996; Meck & Williams, 1997; Olton, Wenk, Church, & Meck, 1988)
The focus of this article is on age-related changes in the ability to time two events concurrently (a divided attention task) compared with timing an event in isolation (a focused attention task). The general importance of attention in the perception of time is evident in commonplace intuitions about awareness of the passage of time. Indeed, situations that increase attention to time, such as boredom and anticipation, produce an apparent lengthening of external time, whereas situations that reduce attention to time, such as participating in an engaging activity, produce an apparent shortening of external time. These attentional distortions in the perception of time are aptly captured by the expressions “a watched pot never boils” and “time flies when you’re having fun”, respectively; see Brown (1997) for an excellent review.
Dual-task studies of attention and timing in young adults have supported the view that attention has the potential to modulate the rate of accumulation of pulses generated by an internal pacemaker, where the number of pulses is assumed to provide a representation of temporal extent. Thus, when asked to report the duration of an event, young participants often make shorter verbal estimates and longer productions during dual-task (divided attention) conditions than in single-task (focused attention) conditions (Brown, 1985; Hicks, Miller, Gaes & Bierman, 1977; Macar, Grondin & Casini, 1994; Zakay, 1993).. As one example, Brown (1985) asked participants to provide verbal estimates of the duration of a figure-drawing task that varied in difficulty, but was always the same duration. Brown found that as the drawing task became more difficult (i.e., more attention was allocated to drawing), verbal estimates became shorter and more variable. Effects of dual-task conditions on interval timing performance tend to be robust and have been found using different methods and a range of concurrent non-temporal tasks, including card sorting, mental rotation, visual search, and anagram solving (Brown, 1997; Fortin & Breton, 1995; Hicks et al., 1977; Macar et al., 1994; Zakay, 1993).
With respect to aging, one common intuition about the perception of time is that time passes more quickly as we get older. Although there is some evidence to support this observation, previous studies of aging and timing are mixed (Block, Zakay, & Hancock, 1998; McCormack et al., 1999; Salthouse, Wright, & Ellis, 1979; Vanneste & Pouthas, 1999; Wearden et al., 1997). Some studies found shorter verbal estimates and longer productions of duration with increased age, findings consistent with the view that time passes more quickly as we get older (Craik & Hay, 1999; McCormack et al., 1999). On the other hand, the opposite pattern has also been found (Block et al., 1998). Finally, others have reported similar timing performance for younger and older adults (Salthouse et al., 1979; Wearden et al., 1997). Reasons for the varied findings may be due to differences in task complexity, age ranges compared, level of training, and amount of feedback provided to participants on performance. In general, studies involving simpler tasks, more training, and greater feedback on performance were less likely to find age differences in timing performance than studies where the opposite was true.
The present study considered effects of aging on attention and timing in the context of a divided attention task in which individuals were asked to concurrently time an auditory and a visual event; thus, both tasks in the dual-task required temporal judgments. Previous evidence for age-related impairments during concurrent performance of two temporal tasks has been found using a simultaneous version of a temporal-bisection task (Lustig & Meck, 2001). In this study, younger and older adults were exposed to both short and long auditory and visual anchor durations and then asked to judge whether a series of simultaneously presented test durations were either short or long in reference to the learned anchors. Older adults were more variable in their timing than were younger adults during concurrent timing (divided attention) conditions, especially for visual stimuli. Young adults, in contrast, were able to time multiple stimuli as well as a single stimulus, independent of modality.
The present study extends the work of Lustig & Meck (2001) using a divided attention version of the peak-interval (PI) procedure (Roberts, 1981), referred to as a simultaneous temporal processing (STP) task (Meck, 1987; Meck & Williams, 1997; Olton et al., 1988; Pang, Yoder, & Olton, 2001). Like the temporal bisection task used by Lustig & Meck (2001), the PI procedure and variants have been particularly useful for cross-species comparisons of attention and timing (Wearden & McShane, 1988). However, as far as the authors are aware, no previous study has used the STP paradigm with human participants.
Previous animal studies of attention and timing using the STP procedure have shown little decrement in timing performance when young rats divide attention across multiple intervals compared with a single interval (Meck, 1987; Meck & Williams, 1997). Response distributions in both focused and divided attention conditions are typically centered on the target duration (reflecting accurate timing) and scalar in variability, consistent with Weber’s law (Gibbon, Church & Meck, 1984; Meck & Williams, 1997; Olton et al., 1988; Pang & McAuley, 2003; Rakitin et al., 1998). Perhaps most impressive is that young rats have been shown to simultaneously time up to three durations as well as they can time a single duration, as evidenced by the consistency of peak times on divided attention and focused attention trials (Meck & Church, 1984). With respect to aging, however, Meck and colleagues reported that for isolated-interval timing, aged rats produced over-estimates of duration that were proportional to the signal being timed (Meck, 1987; Meck & Williams, 1997; Olton et al., 1988).
For the present study, we were interested in the concurrent timing performance of older adults with the STP procedure. We attempted to address several main questions. First, would older adults tested on the STP procedure show impairments in concurrent timing performance relative to younger adults? Second, would any age difference interact with modality as previously observed by Lustig and Meck (2001)? One reason why age-related impairments might be expected to be greater for visual stimuli than for auditory stimuli is that auditory stimuli capture and hold attention relatively easily, whereas visual stimuli appear to require greater attentional control (Lustig & Meck, 2001; Meck, 1984). If aging produces a larger decrement of controlled rather than automatic aspects of attention, age-related impairments in divided attention would be expected to exact a greater toll for visual stimuli than for auditory stimuli. So the last question addressed was whether working memory span and Stroop interference, two measures that have been proposed to index controlled aspects of attention (Engle, Tuholski, Laughlin & Conway, 1999; West, 2004), would predict divided attention performance, especially in the visual domain.
Methods
Participants
Forty young adults (18–39 years; n = 26, female; n = 14, male), twenty two young-old adults (60–74 years; n = 13, female; n = 9, male) and seventeen old-old adults (75+ years; n = 10, female; n = 7, male) participated in the study. Young adult participants were undergraduates at Bowling Green State University who participated in return for extra credit in an introductory psychology course. Young-old and old-old adult participants were active members of the local community with normal hearing. All older adults had at least a high school level of education (51.3% completed high school, 33.3% completed college, and 15.4% had at least some graduate education); they received $25 for their participation. Average self-reported health ratings for three groups were 3.96 (0.16), 3.73 (0.49) and 3.59 (0.50), respectively, on a 4-point scale ranging from 1–bad to 4–good.
Design
The study implemented a 3 (age) × 2 (attention condition) × 2 (duration) × 2 (modality) mixed factorial design. Participants in the three age groups (18–39 years, 60–74 years, 75+ years) were tested in focused and divided attention timing conditions using 6 second and 12 second target durations that were paired with an auditory stimulus (tone) or a visual stimulus (white square). Pairing of modality with target duration was counterbalanced across participants. For approximately half of the participants in each age group (18–39 years, n = 22; 60–74 years, n = 12; 75+ years, n = 7), the 6 second (short) target was marked by a tone and the 12 second (long) target was marked by a white square, while for the remaining participants (18–39 years, n = 18; 60–74 years, n = 10; 75+ years, n = 10) the short target was marked by the white square and the long target was marked by a tone.
Materials
Participants sat facing a computer monitor and responded using the computer keyboard. The auditory stimulus was a 440 Hz tone presented through headphones at a comfortable listening level (≈ 70 dB); the visual stimulus was a 5 × 5 cm white-square that appeared on a black background in the center of the computer screen. Stimulus presentation and response collection were conducted using the E-prime software (Psychological Software Tools, 2002).
Procedure
Fixed-interval training
During an initial fixed-interval (FI) training block, participants were presented with either a tone or white square stimulus on each trial and made a single response as close in time to when they thought the stimulus would end. On all trials, the offset of the stimulus always occurred at the target time (6 or 12 sec following stimulus onset); the response period continued following stimulus offset in order to permit both ‘early’ and ‘late’ responses. Participants were told to use the stimulus offset as feedback to improve performance. There were 20 randomly mixed FI trials (10 auditory targets, 10 visual targets).
Focused attention testing
Following initial FI training, random probes were introduced where the stimulus did not end at the target duration, but rather stayed on until the end of the trial. Participants were informed about the inclusion of probe trials and were instructed that for all trials they should respond when they thought that the stimulus should end. Inclusion of probe trials encourages stimulus timing, rather than simple reactions to stimulus offset. Focused attention testing consisted of 40 trials (20 FI trials, 20 probe trials), with an equal number of auditory and visual targets.
Divided attention testing
Participants completed three divided attention blocks that tested concurrent timing. During the first test block, participants were presented with the long stimulus followed by the onset of the short stimulus after a random 2 or 4 second delay. Both stimuli were presented simultaneously for 2 or 4 seconds before one stimulus (the non-target) was terminated, signaling that the remaining stimulus (target) was to be timed. On each trial, the target stimulus was randomly selected to be either the short or long stimulus. Participants’ task was to press the response button when they thought that the target stimulus would end. The target FI (6 sec or 12 sec) was always measured from the onset of the target stimulus, not from the offset of the non-target stimulus. The period of time that the two stimuli overlapped was the divided attention interval; during this time the participant need to be able to time both stimuli concurrently in order to perform the task accurately. The configuration was the same for the second and third blocks, except for the inclusion of random probe trials. As before, participants were informed about the probe trials and were instructed that for all trials they should respond when they thought that the target stimulus should end. The first block consisted of 48 trials (24 auditory targets, 24 visual targets). The second and third blocks comprised 96 trials (48 FI trials, 48 probe trials), with an equal number of auditory and visual targets.
Non-timing measures
Participants returned the next day to complete a standard Stroop color-naming task (Stroop, 1935) and a reading span working memory task (Salthouse & Meinz, 1995; Ybarra & Park, 2002). For the Stroop task, participants were presented with neutral trials, which contained the stimulus @@@@@ in different colors, and incongruent trials, which contained color words in a different color (e.g., the word green written in red ink). For both kinds of trials, participants were instructed to name the color that they saw as quickly as possible. The computer recorded the response time (RT) of each trial. Each participant completed 20 trials of each trial type. Stroop interference was measured as the mean RT difference between the neutral trials and the incongruent trials for each participant. The working memory task involved presentation of simple sentences with the participant instructed to select an answer to a question about the sentence from a set of three alternatives, while also remembering the last word in each sentence. After a designated number of sentences (starting with one), the word RECALL appeared on the screen and participants typed the last word of each sentence in order. If participants made correct responses to the questions and correctly recalled the last word of each sentence for at least two of three trials, then the designated number of sentences increased by one; otherwise the program stopped. Participant’s working memory span was defined by the final series length.
Results
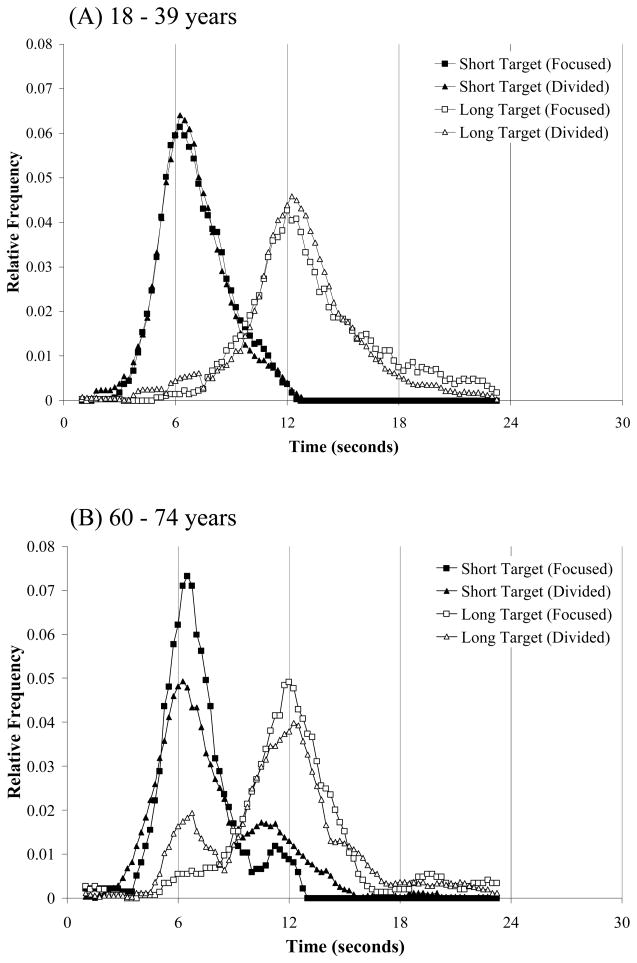
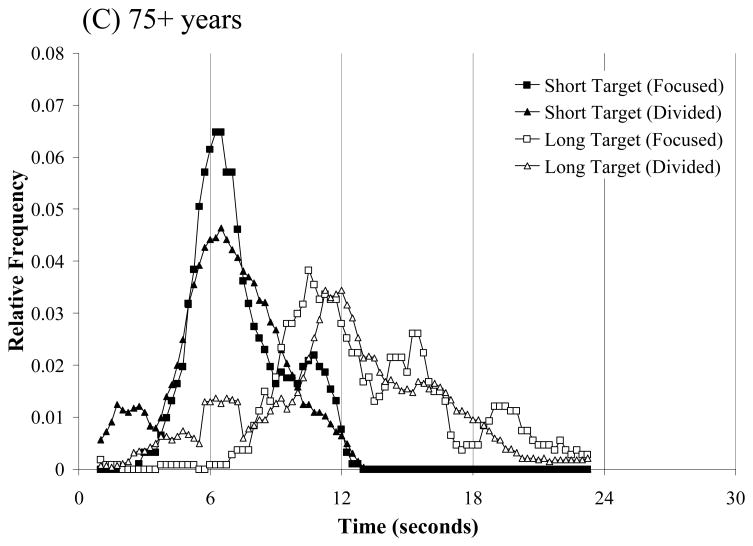
Temporal productions on probe trials were binned in 0.25 second intervals and combined across participants within each age group in order to create composite temporal response functions. Figure 1 shows the composite temporal response curves for the three age groups (18–39 years, Panels A; 60–74 years, Panel B; 75+ years, Panel C). Each panel shows the relative frequency distribution of produced time intervals for the 6- and 12-sec targets timed in isolation (focused attention testing) and concurrently (divided attention testing). Estimates of peak time, a directional measure of timing accuracy corresponding to the peak of the response curve, were obtained using the best-fitting Gaussian function (Buhusi, Sasaki & Meck, 2002); the peak-time data are shown in Table 1 with separate estimates for auditory and visual stimuli. Several aspects of the composite temporal response curves and peak-time estimates stand out. First, temporal response functions for young adults were strikingly similar when target intervals were timed in isolation (focused attention) compared to when they were timed concurrently (divided attention). Overall, temporal response curves were approximately centered on the short (6 sec) and long (12 sec) target intervals in both focused and divided attention conditions (see Figure 1). Peak-time estimates in Table 1 show that temporal productions tended to be slightly longer that target durations; moreover, when separated by modality, there was some support for peak-time estimates for auditory target durations to be longer than peak-time estimates for visual target durations. This modality effect did not turn out to be statistically reliable, however. ANOVA on mean produced durations for young adults revealed no effect of attention condition, no effect of modality, and no interaction between attention condition and modality (all p’s > 0.1).
Figure 1.
Temporal response functions for short and long target intervals for participants aged 18–39 years (Panel A), 60–74 years (Panel B), and 75+ years (Panel C) during focused and divided attention timing conditions.
Table 1.
Peak-time estimates from composite temporal response functions for three age groups separated by modality (A = Auditory Target Duration; V = Visual Target Duration).
| Age Group | Focused Attention | Divided Attention | ||||||
|---|---|---|---|---|---|---|---|---|
| Short | Short | Long | Long | Short | Short | Long | Long | |
| A | V | A | V | A | V | A | V | |
| 18–39 yrs | 6.89 | 6.28 | 12.45 | 12.09 | 7.00 | 6.30 | 12.73 | 12.20 |
| 60–74 yrs | 6.69 | 6.24 | 12.27 | 11.77 | 6.76 | 6.10 | 11.99 | 11.80 |
| 75+ yrs | 6.35 | 6.48 | 11.82 | 11.41 | 6.45 | 6.96 | 12.83 | 11.47 |
Also evident in Figure 1 is that both older adult age groups (Panels B and C) had more difficulty timing the target stimuli in divided attention conditions than in focused attention conditions; see also corresponding peak-time estimates separated by modality in Table 1. During focused attention conditions, both older adult age groups produced durations that, on average, were relatively close approximates to the short and long targets and were not reliably different from the produced durations of young adults. ANOVA on mean produced durations during focused attention with age and modality as factors revealed no effect of age, no effect of modality, and no interaction between the two factors for both short and long targets (all p’s > 0.1). Similarly, during divided attention testing, ANOVA on mean produced durations revealed no effects of age or modality, or interaction between the two factors (all p’s > 0.1).
However, as suggested by Figure 1, the ANOVAs on mean produced durations mask several interesting aspects of the data; temporal response functions in divided attention conditions were often multimodal for older adults, with a primary peak at/or near the cued target time (reported in Table 1) and at least one additional, secondary, peak at a non-target time, For the participants in the 60–74 year age group, secondary peaks at non-target times were apparent at approximately 10.75 sec for the short (6-s) target duration and at approximately 6.75 sec for the long (12-s) target duration; for the 75+ year age group, a secondary peak was apparent at approximately 6.5 sec for the long target duration. The 75+ year age group additionally produced what appear to be three peaks in timing the long target duration during focused attention conditions.
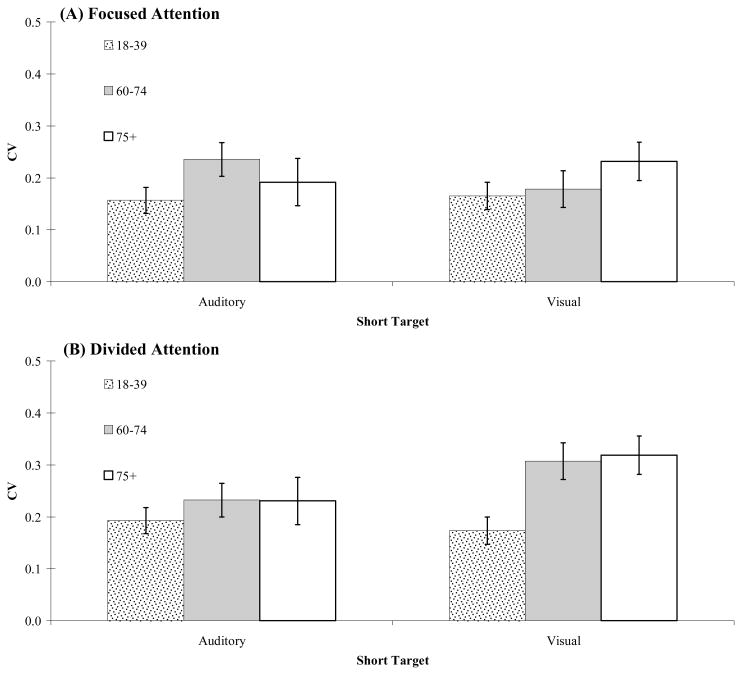
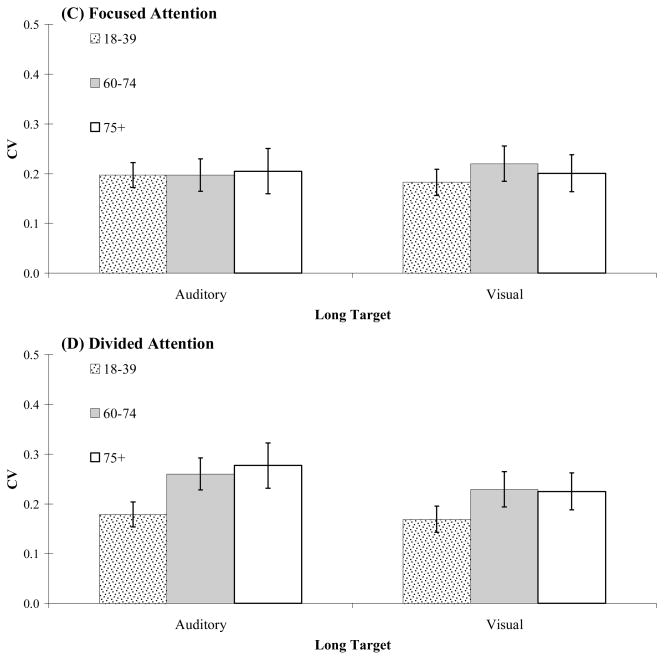
Estimates of timing variability for each participant were obtained by dividing the standard deviation of produced time intervals by the mean, providing a normalized variability score (or coefficient of variation, CV). An overall comparison of CV scores for focused and divided attention testing is shown in Figure 2 (Panels A–D). A 3 (age group) × 2 (attention condition) × 2 (target duration) mixed measures ANOVA on CV scores revealed a main effect of age group, F(2,76) = 4.13, MSE = 0.026, p = 0.02, a main effect of attention condition, F(1,76) = 9.66, MSE = 0.011, p = 0.003, and a marginally significant interaction between age group and attention condition, F(2,76) = 2.75, MSE = 0.011, p = 0.07. During focused attention, CV scores increased only slightly across the tested age range (18–39 years, M = 0.18, SE = 0.014; 60–74 years, M = 0.20, SE = 0.018; 75+ years, M = 0.21, SE = 0.022). However, during divided attention, both groups of older adults were substantially more variable in their timing (60–74 years, M = 0.26, SE = 0.023; 75+ years, M = 0.27, SE = 0.028) than their 18–39 year old counterparts (M = 0.18, SE = 0.017). There was no main effect of duration; moreover, duration did not interact with either age or attention condition (all p’s > 0.1).
Figure 2.
Timing variability (coefficient of variation) for the three age groups for auditory and visual target durations. Panels A and B show short targets in focused and divided attention conditions, respectively. Panels C and D show long targets in focused and divided attention conditions, respectively.
To examine effects of modality, separate 3 (age group) × 2 (attention condition) x 2 (modality) ANOVA were conducted for short and long target durations.1 For short targets, the ANOVA revealed a main effect of age, F(2,73) = 5.31, MSE = 0.015, p = 0.007, a main effect of attention, F(1,73) = 8.15, MSE = 0.007, p = 0.006, but no main effect of modality, or interaction between age group and modality. There was, however, a marginal three-way interaction between age, attention and modality, F(2,73) = 2.82, MSE = 0.007, p = 0.06. This interaction highlights the finding of generally larger age-related increases in timing variability during concurrent timing of short visual targets than for short auditory targets. The same modality interaction, however, did not emerge for long targets. For long targets, the ANOVA revealed a main effect of attention, F(1,73) = 7.72, MSE = 0.007, p = 0.007, an interaction between age and attention, F(2,73) = 4.45, MSE = 0.007, p = 0.015, but no main effects of age or modality, or other interactions (all p’s > 0.1). Simple effects analyses reveal that the interaction between age and attention condition was driven by an age-related increase in variability during concurrent timing (divided attention), F(2,76) = 4.41, p = 0.01; no significant effect of age on variability was found for focused attention testing, F(2, 76) = 0.23, p = 0.79.
Bivariate correlations between age (in years), working memory span (WM), Stoop interference (SI) and measures of timing variability were examined. As expected, WM decreased with increasing age, r(77) = −0.70, p < 0.001 and SI increased with increasing age, r(77) = 0.55, p < 0.001; WM and SI were also negatively correlated, r(77) = −0.42, p < 0.001. With respect to timing performance, age, WM, and SI were not correlated with either signed or absolute constant error (two measures of timing accuracy). However, both age and WM were correlated with timing variability in divided attention conditions (concurrent timing), but not focused attention conditions (see Table 1). In general, WM was a better predictor of variability in concurrent visual timing, r(77) = −0.38, p < 0.001, than variability in concurrent auditory timing, r(77) = −0.25, p = 0.028. When corrections are made for multiple comparisons, WM only reliably predicts variability in concurrent visual timing. SI did not predict any of the timing measures, including CV (all p’s > 0.2) and did not help to account for the observed greater age-related impairments in concurrent visual timing compared with concurrent auditory timing.
Discussion
A common requirement of juggling more than one task simultaneously is that both tasks are accomplished at the right time. Depending on task constraints, this may require that the two tasks are timed relatively independently of one another. The present study addressed effects of age on concurrent timing of auditory and visual events using a simultaneous temporal processing task (Meck, Church, & Wenk, 1986; Meck & Williams, 1997). Participants were first trained to produce the duration of a tone and a white square that were 6 sec or 12 sec, respectively; they were then tested on their ability to produce the learned event durations in isolation (focused attention condition) or concurrently (divided attention condition). Results from this work reveal that young adults were able to produce the duration of the auditory and visual events equally well in focused and divided attention conditions.
The lack of divided attention decrement during concurrent timing is consistent with previous studies of simultaneous temporal processing in young adult rats (Meck, 1987; Meck & Williams, 1997). However, these findings notably differ from the work of Brown and colleagues who have reported decreases in temporal accuracy and increases in variability when young adult humans are asked to time multiple intervals compared to when they are asked to time a single interval (Brown, Stubbs & West, 1992; Brown & West, 1990). Although the reasons for these differences across studies and species are unclear, it is possible that the differences partially reflect methodological differences and potentially the use of slightly different dependent measures. With respect to temporal accuracy, Brown and West (1990) emphasized absolute temporal error independent of direction, whereas the current study primarily considered directional differences in timing (i.e., degree of over- and under-estimation). Some neurobiological support for separating magnitude and direction of temporal distortions comes from Meck (2002) who has suggested that sodium-dependent high affinity choline uptake serves as indicator of the magnitude of timing errors, while theta rhythm activity in the frontal cortex serves as an indicator of direction of errors (over- or under-estimation).
In contrast to the young adult data, both groups of older adults in the present study showed a large divided attention decrement in timing performance that was generally more pronounced for visual stimuli than for auditory stimuli. Age-related impairments in timing appeared as increased variability of produced durations during concurrent timing performance (divided attention condition) relative to when the auditory and visual stimuli were timed in isolation (focused attention condition). A close inspection of the aggregate temporal response curves for the two older age groups reveals that age-related increases in timing variability may have been partly driven by a difficulty of older participants in forming distinct temporal memories for the 6-sec and 12-sec target intervals. For concurrent timing (and in some cases for isolated-interval timing), temporal response curves were multimodal for both older age groups with peaks occurring at approximately 6-sec and 12-sec. Although this finding of multiple peaks in the aggregate response curves is intriguing, there is not enough data for each participant to permit a more comprehensive by-subject analysis; it does suggest, however, that this may be a promising avenue of future research.
The divided attention decrement observed with both groups of older adults is consistent with Vanneste & Pouthas (1999) and Lustig & Meck (2001) using different methods. Vanneste & Pouthas (1999) examined a temporal reproduction task that required up to three concurrent estimates of duration and found that increasing the number of concurrent to-be-timed intervals led to less accurate and more variable timing performance in older adults than in young adults. Using a concurrent timing version of the temporal bisection task, Lustig & Meck reported no differences in timing accuracy, but did find an age-related increase in timing variability. Similar to the present study, effects of age interacted with modality during concurrent temporal bisection, with visual timing conditions showing greater age-related impairments than auditory timing conditions. In more general terms, overall larger age impairments in visual timing compared with auditory timing are consistent studies reporting evidence of auditory dominance in temporal processing (Guttman, Gilroy, & Blake, 2005; Lustig, 2003; Penney, Gibbon, & Meck, 2000, Repp & Penel, 2002).
Lustig & Meck (2001) hypothesized that differences in the amount of controlled attention devoted to the different modalities may be responsible for the observed interactions between age and modality, with visual events requiring more controlled attention than auditory events. A controlled attention hypothesis receives some support in the present study. Working memory span, which has been previously proposed to index controlled attention (Engle et al., 1999; Kane, Bleckley, Conway & Engle, 2001), was found to be associated with increased timing variability in divided attention conditions; shorter WM span was linked to increased timing variability. Moreover, this relationship was strongest for visual timing in divided attention conditions; see also the work of Vanneste & Pouthas (1999) who found that shorter WM span was associated with increased timing variability in the most attention demanding conditions.
Finally, an additional aspect of Lustig & Meck’s study deserves some discussion. In their study, an intriguing interaction was observed between age and the time of day that testing occurred. Participants were tested either before 9 am or after 4 pm, with older adults predominantly characterized as “Moderately Morning” individuals and younger adults predominantly characterized as “Moderately Evening” individuals based on the MEQ (Horne & Ostberg, 1976). Older adults tested in the morning (their optimal time of day) were found to show greater sensitivity to visual stimuli than to auditory stimuli, while the reverse was true for those tested late in the afternoon (their non-optimal time of day). Lustig and Meck argue that allocation of attention to different sensory channels is modulated by circadian rhythm phase. Although time of day was not a variable in the present design (all testing took place between 9 am and 5pm) these previous results do suggest that the time-of-testing should be looked at more closely in the context of interactions between age, attention, and temporal processing.
In summary, the current study examined concurrent timing of auditory and visual events by young and elderly adults using a modified simultaneous temporal processing procedure that has been widely used in non-human animal studies, but rarely used in human studies. Consistent with animal studies of concurrent timing involving rats (Meck, 1987; Meck & Williams, 1997), young adults were found to be able to produce the duration of the two target events equally well in focused and divided attention conditions. Older adults in contrast showed a large divided attention decrement in timing performance that in some cases was more pronounced for visual targets than for auditory targets. Age-related impairments were found to be associated with a decrease in working memory span and this relationship was found to be largest for visual targets in divided attention conditions. Finally, these findings converge with those of Vanneste & Pouthas (1999) and Lustig & Meck (2001) using a different task and provide some support for a controlled attention explanation of age and modality differences in concurrent timing performance. More broadly, this research contributes to the growing body of work showing age-related interactions between attention and timing processes (Anderson, Craik, & Naveh-Benjamin, 1998; Craik, 1977; Hartley & Little, 1999; McDowd & Craik, 1988; McDowd & Shaw, 2000; Salthouse, Rogan, & Prill, 1984)
Table 2.
Correlations between age, working memory span (WM) and magnitude of Stroop interference (SI) with timing variability (as measured by CV) in focused and divided attention conditions for auditory and visual target events.
| Predictor | Focused Attention | Divided Attention | ||
|---|---|---|---|---|
| Auditory | Visual | Auditory | Visual | |
| Age | 0.10 | 0.17 | 0.27* | 0.39** |
| WM | −0.20 | −0.21 | −0.25* | −0.38** |
| SI | −0.01 | 0.09 | 0.05 | 0.14 |
Correlation is significant at the .05 level (2-tailed)
Correlation is significant at the .01 level (2-tailed)
Acknowledgments
The authors are grateful to Scott Brown and James Cutting for their comments on an earlier version of this manuscript. The authors thank Steve Borawski and Nathaniel Miller for their assistance in data collection and the other members of the Rhythm, Attention, and Perception Lab at Bowling Green State University for their assistance in the completion of this project. Portions of these data were previously presented at the 2003 meeting of the Society for Neuroscience. This research was supported by PHS grant AG20560.
Footnotes
To examine effects of modality, separate 3 (age group) × 2 (attention condition) × 2 (modality) ANOVA were conducted for short and long target durations rather than the omnibus 4-way ANOVA with duration as the four factor due to the fact that the two (reversed) pairings of modality and target duration prevents a simple interpretation of the 4-way analysis. To understand why this produces a problem of interpretation, it is important to first note that the counterbalancing means that for approximately half of the participants in each age group, the 6 second (short) target was marked by a tone and the 12 second (long) target was marked by a white square (short-auditory/long-visual pairing), while for the remaining participants, the short target was marked by the white square and the long target was marked by a tone (short-visual/long-auditory pairing). Thus, as a result of this aspect of the design, any effects of modality (or interactions with modality) in the 4-way ANOVA would need to be qualified by the duration-modality pairing.
References
- Allan LG, Gibbon J. Human bisection at the geometric mean. Learning & Motivation. 1991;22(1–2):39–58. [Google Scholar]
- Anderson ND, Craik FIM, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: I. Evidence from divided attention costs. Psychology & Aging. 1998;13(3):405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Block RA, Zakay D, Hancock PA. Human aging and duration judgments: A meta-analytic review. Psychology & Aging. 1998;13(4):584–596. doi: 10.1037//0882-7974.13.4.584. [DOI] [PubMed] [Google Scholar]
- Brown SW. Time perception and attention: The effects of prospective versus retrospective paradigms and task demands on perceived duration. Perception & Psychophysics. 1985;38(2):115–124. doi: 10.3758/bf03198848. [DOI] [PubMed] [Google Scholar]
- Brown SW. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Perception & Psychophysics. 1997;59(7):1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Brown SW, West AN. Multiple timing and the allocation of attention. Acta Psychologica. 1990;75(2):103–121. doi: 10.1016/0001-6918(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Brown SW, Stubbs DA, West AN. Attention, multiple timing, and psychophysical scaling of temporal judgments. In: Macar F, Pouthas V, Friedman WJ, editors. Time, action and cognition: Towards bridging the gap. Vol. 66. Dordrecht ; Boston: Kluwer Academic; 1992. pp. 129–140. [Google Scholar]
- Craik FIM. Age differences in human memory. In: Birren JE, Shaie KW, editors. Handbook of the psychology of aging. New York: Van Nostrand Reinhold; 1977. pp. 384–420. [Google Scholar]
- Craik FIM, Hay JF. Aging and judgments of duration: Effects of task complexity and method of estimation. Perception & Psychophysics. 1999;61:549–560. doi: 10.3758/bf03211972. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fortin C, Breton R. Temporal interval production and processing in working memory. Perception & Psychophysics. 1995;57:203–215. doi: 10.3758/bf03206507. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84(3):279–325. [Google Scholar]
- Gibbon J, Church R, Meck W. Annals of the New York Academy of Sciences. 1984. Scalar timing in memory; pp. 52–77. [DOI] [PubMed] [Google Scholar]
- Guttman SE, Gilroy LE, Blake R. Hearing what the eyes see: Auditory encoding of visual temporal sequences. Psychological Science. 16:228–225. doi: 10.1111/j.0956-7976.2005.00808.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AA, Little DM. Age-related differences and similarities in dual-task interference. Journal of Experimental Psychology: General. 1999;128(4):416–449. doi: 10.1037//0096-3445.128.4.416. [DOI] [PubMed] [Google Scholar]
- Hicks RE, Miller GW, Gaes G, Bierman K. Concurrent processing demands and the experience of time-in-passing. American Journal of Psychology. 1977;90(3):431–446. [Google Scholar]
- Horne JA, Ostberg O. A self assessment questionaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychological Science. 2001;12(6):478–484. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- Lustig . Grandfather’s clock: Attention and interval timing in older adults. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 351–370. [Google Scholar]
- Macar F, Grondin S, Casini L. Controlled attention sharing influences time estimation. Memory & Cognition. 1994;22(6):673–686. doi: 10.3758/bf03209252. [DOI] [PubMed] [Google Scholar]
- McAuley JD, Jones MR, Holub S, Johnston HM, Miller NS. The time of our lives: Lifespan development of timing and event tracking. Journal of Experimental Psychology: General. 2006;135:348–367. doi: 10.1037/0096-3445.135.3.348. [DOI] [PubMed] [Google Scholar]
- McCormack T, Brown GDA, Maylor EA, Darby RJ, Green D. Developmental changes in time estimation: Comparing childhood and old age. Developmental Psychology. 1999;35(4):1143–1155. doi: 10.1037//0012-1649.35.4.1143. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Craik FI. Effects of aging and task difficulty on divided attention performance. Journal of Experimental Psychology: Human Perception & Performance. 1988;14(2):267–280. doi: 10.1037/0096-1523.14.2.267. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Shaw RJ. Attention and aging: A functional perspective. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Meck WH. Attentional bias between modalities: Effect on the internal clock, memory, and decision stages used in animal time discrimination. Annals of the New York Academy of Sciences. 1984;423:528–541. doi: 10.1111/j.1749-6632.1984.tb23457.x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Vasopressin metabolite neuropeptide facilitates simultaneous temporal processing. Behavioural Brain Research. 1987;23(2):147–157. doi: 10.1016/0166-4328(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Research: Cognitive Brain Research. 1996;3(3–4):227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Simultaneous temporal processing. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(1):1–29. [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL. Arginine vasopressin inoculates against age-related increases in sodium-dependent high-affinity choline uptake and discrepancies in the content of temporal memory. European Journal of Pharmacology. 1986;130(3):327–331. doi: 10.1016/0014-2999(86)90287-6. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8(14):3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wenk GL, Church RM, Meck WH. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia. 1988;26(2):307–318. doi: 10.1016/0028-3932(88)90083-8. [DOI] [PubMed] [Google Scholar]
- Pang KC, Yoder RM, Olton DS. Neurons in the lateral agranular frontal cortex have divided attention correlates in a simultaneous temporal processing task. Neuroscience. 2001;103(3):615–628. doi: 10.1016/s0306-4522(01)00018-5. [DOI] [PubMed] [Google Scholar]
- Pang KCH, McAuley JD. Importance of frontal motor cortex in divided attention and simultaneous temporal processing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 351–370. [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Differential effects of auditory and visual signals on clock speed and temporal memory. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1770–1787. doi: 10.1037//0096-1523.26.6.1770. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SH, Meck WH. Peak-interval timing in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:19–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Repp BH, Penel A. Auditory dominance in temporal processing: New evidence from synchronization with simultaneous visual and auditory sequences. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1085–1099. [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working-memory, and speed. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 1995;50(6):P297–P306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Rogan JD, Prill KA. Division of attention: Age differences on a visually presented memory task. Memory & Cognition. 1984;12(6):613–620. doi: 10.3758/bf03213350. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Wright R, Ellis CL. Adult age and the rate of an internal clock. Journal of Gerontology. 1979;34(1):53–57. doi: 10.1093/geronj/34.1.53. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Pouthas V. Timing in aging: The role of attention. Experimental Aging Research. 1999;25(1):49–67. doi: 10.1080/036107399244138. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Ferrara A. Subjective shortening in humans’ memory for stimulus duration. Quarterly Journal of Experimental Psychology: Comparative & Physiological Psychology. 1993;46(2):163–186. [PubMed] [Google Scholar]
- Wearden JH, McShane B. Interval production as an analogue of the peak procedure: Evidence for similarity of human and animal timing processes. Quarterly Journal of Experimental Psychology: Comparative & Physiological Psychology. 1988;40(4-B):363–375. [Google Scholar]
- Wearden JH, Wearden AJ, Rabbitt PMA. Age and IQ effects on stimulus and response timing. Journal of Experimental Psychology: Human Perception & Performance. 1997;23(4):962–979. [Google Scholar]
- West R. The effects of aging on controlled attention and conflict processing in the Stroop task. Journal of Cognitive Neuroscience. 2004;16:103–113. doi: 10.1162/089892904322755593. [DOI] [PubMed] [Google Scholar]
- Ybarra O, Park DC. Disconfirmation of person expectations by older and younger adults: Implications for social vigilance. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2002;57(5):P435–P443. doi: 10.1093/geronb/57.5.p435. [DOI] [PubMed] [Google Scholar]
- Zakay D. The roles of non-temporal information processing load and temporal expectations in children’s prospective time estimation. Acta Psychologica. 1993;84(3):271–280. doi: 10.1016/0001-6918(93)90064-x. [DOI] [PubMed] [Google Scholar]