Abstract
IFN-γ has long been recognized as a cytokine with potent and varied effects in the immune response. While its effects on specific cell types have been well studied in vitro, its in vivo effects are less clearly understood because of its diverse actions on many different cell types. While control of multiple protozoan parasites is thought to depend critically on the direct action of IFN-γ on macrophages, this premise has never been directly proven in vivo. In order to more directly examine the effects of IFN-γ on cells of the macrophage lineage in vivo, we generated mice called the ‘Macrophages Insensitive to Interferon Gamma’ (MIIG) mice, which express a dominant negative mutant IFN-γ receptor in CD68+ cells: monocytes, macrophages, dendritic cells, and mast cells. Macrophage lineage cells and mast cells from these mice are unable to respond to IFN-γ while other cells are able to produce and respond to this cytokine normally. When challenged in vitro, macrophages from MIIG mice were unable produce NO or kill Trypanosoma cruzi or Leishmania major after priming with IFN-γ. Furthermore, MIIG mice demonstrated impaired parasite control and heightened mortality after T. cruzi, L. major, and Toxoplasma gondii infection, despite an appropriate IFN-γ response. In contrast, MIIG mice displayed normal control of lymphocytic choriomeningitis virus, despite persistent insensitivity of macrophages to IFN-γ. Thus, the MIIG mouse formally demonstrates for the first time in vivo, the specific importance of direct, IFN-γ mediated activation of macrophages for controlling infection with multiple protozoan parasites.
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Keywords: Monocytes/Macrophages, Parasitic-Protozoan, Cytokines, Transgenic/Knockout Mice
Introduction
Interferon gamma (IFN-γ) is well-described as a cytokine with pleotropic effects on the immune response (1). While it was originally described as one of the substances produced by the host in order to ‘interfere’ with viral infection (2), it has since been characterized as having a multitude of effects in vivo, including upregulation of MHC molecules, induction of the immunoproteosome complex, and induction of a variety of anti-microbial effector molecules in macrophages and other cells (3). In addition to these pro-inflammatory effects, it also upregulates molecules with anti-inflammatory or immune dampening effects, such as 2,3 indoleamine dioxygenase and suppressor of cytokine signaling 1 (4). Additionally, it has significant effects on T cell differentiation and proliferation, though some of these effects are still being clarified (5-7). Perhaps the most striking effect it exerts, however, is the enhancement of microbicidal activity by macrophages and other phagocytes in vitro (8). Accordingly, in vivo, it is critical for the clearance of certain viruses and diverse intracellular organisms, including bacteria and protozoan parasites (9, 10).
Protozoan parasites affecting man and mice are a diverse group of organisms with a highly varied ecology. Despite this diversity, IFN-γ plays a pivotal role in controlling infection with many of these organisms, including Toxoplasma gondii (11), Leishmania species (12), Plasmodium species (13-15), and Trypanasoma cruzi (16). Additionally, IFN-γ appears to provide important protection from many other less well studied protozoan parasites including Cryptosporidia (17), Microsporidia (18), Entamoeba histolytica (19), and Babesia microti (20). Finally, while control of Giardia lamblia and Pneumocystis carinii are not strictly dependant on IFN-γ, this cytokine is part of the host response, and may provide partial protection from infection or reactivation (21) (22). Because many (though not all) of these pathogens have an intracellular phase of their life cycle, the consistent importance of IFN-γ for host defense is not entirely surprising. Accordingly, strong circumstantial evidence indicates that IFN-γ mediated activation of macrophages is crucial for control of many of these parasites.
Notably, the well-established role of IFN-γ as an activator or primer of macrophage anti-microbial responses is largely based on in vitro studies (8, 23), or in vivo studies where IFN-γ or its signaling is globally neutralized or genetically deleted (24). It is been inferred from such studies that IFN-γ must act directly on macrophages to initiate control of numerous intracellular pathogens. However, the direct effects of IFN-γ on macrophages have generally not been demonstrated in vivo. In order to more clearly understand the direct, macrophage-dependent effects of IFN-γ in vivo, we have developed transgenic mice, which have a macrophage-specific insensitivity to IFN-γ, called the ‘Macrophages Insensitive to Interferon Gamma’ (MIIG) mice. Macrophages from these mice are unable to respond to IFN-γ while other cell types produce and respond to this cytokine normally. In this report we have utilized the MIIG mice to formally demonstrate for the first time that IFN-γ must act directly on macrophage lineage cells in order to kill or control a variety of protozoan parasites, both in vitro and in vivo.
Materials and Methods
Construction of MIIG mice
The MIIG transgene construct was created by combining a human CD68 promoter fragment with a dominant negative truncation mutant of the IFN-γ receptor 1 chain. The CD68 promoter, originally cloned and characterized by Greaves, et al. (25) and used to produce macrophage-specific transgene expression (9, 26) (27), was kindly provided by P. Murray (St Jude Children’s Research Hospital, Memphis, TN). The promoter construct consisted of approximately 0.9 kbp of 5′ sequence upstream from the human CD68 start codon, combined with the 83 bp intron 1 from the CD68 gene, in a PCDNA3.1+ derived cloning vector, in which the CMV promoter was deleted and the CD68 promoter was cloned in at the EcoRV and NotI sites. The sequence for a myc-tagged mutant murine IFNGR1 molecule (kindly provided by R. Schreiber (Washington University, St Louis, MO) (28) (29, 30)) lacking the cytoplasmic signaling domain and functioning as a dominant negative mutant was then cloned into the CD68 promoter vector at the NotI and XbaI sites (destroying the XbaI site). The thirteen amino acid myc tag (recognized by the monoclonal antibody 9E10) was incorporated within the extracellular portion of the molecule, after the signal sequence and the first seven N-terminal amino acids, allowing detection of the myc epitope on the cell surface, without interfering with IFN-γ binding. The final construct was tested by transfection into L929 cells (via calcium:phosphate mediated transfection). Transfected and non-transfected cells were incubated with various concentrations of IFN-γ overnight, then MHC class I levels were assessed by flow cytometry. For generation of transgenic mice, the linearized transgene was injected into C57BL/6 embryos, by the University of Cincinnati transgenic core facility. Southern blot analysis of founder mice was performed by standard techniques. The probe for the southern blot was generated by a PCR reaction incorporating one primer with homology to the myc tag (CAGCGAGGAGGACTTGAAT) and a second primer binding to the IFN-γ receptor 1 gene (CCCTTTAGGCACATAAGGAA). One founder lineage, which carried 2 copies of the MIIG transgene (as assessed by southern blot, data not shown), was characterized as expressing functional transgene and was propagated by breeding transgenic animals with C57BL/6 mice (Jackson laboratories) in successive generations. Transgenic mice were screened utilizing PCR (described above). All experiment conducted with mice were approved by the Cincinnati Children’s Hospital Medical Center IACUC.
Flow cytometry
All antibodies were obtained from either Ebioscience or BD Biosciences, with the exception of anti-CD68, which was obtained from Serotec. For analysis of tissue derived macrophages or dendritic cells, tissues were first disassociated in collagenase (Liberase CI, Roche), incubated at 37C for 20 minutes with agitation, then incubated with 20mM EDTA at 4C, and forced through a 100 micron filter. Alveolar and resident peritoneal macrophages were obtained by lavage. Because collagenase decreased detection of the extracellular myc epitope, digestion was curtailed or eliminated when staining for this epitope. For phospho-STAT1 staining, cells were incubated with IFN-γ (100ng/ml, R+D Systems) for 30 minutes or 2 hours (when staining lymphocyte subsets), followed by fixation with 2% paraformaldehyde and permeabilization with 90% methanol, as per standard protocols (BD biosciences). IFN-α stimulation (1000U/ml, R+D systems) was continued for 2 hours prior to fixation and staining. Brain microglia were enriched prior to flow cytometry by centrifuging brain cell suspensions in percoll gradients (cells resuspended in 70% percoll, overlaid with 30%) (GE healthcare). Intracellular cytokine staining was performed by incubating 4 hours at 37C in the presence of brefeldin-a (1mcg/ml, Sigma-Aldrich) under the following stimulating conditions: LCMV GP33-41-synthetic peptide was added to spleen preparations at 1mcg/ml; LCMV GP61-80- bone marrow derived dendritic cells were loaded with synthetic peptide (10mcg/ml) over night then added (1:5 ratio) to spleen cell suspensions; L. major-bone marrow derived dendritic cells were infected with live L. major, 24 hours prior to plating with spleen cell suspensions.
Bone marrow culture
Bone marrow was flushed from long bones and cultured at approximately 1e6/ml in either M-CSF, GM-CSF, or Flt-3L for 6-9 days. Each of these cytokines were derived from tissue culture supernatants (cells: L929, B78HI/GMCSF, and B16Flt3, respectively) which were tittered to give optimal growth of bone marrow derived macrophages or dendritic cells.
RT PCR
Tissues from MIIG mice were disassociated in collagenase and depleted of infiltrating macrophages and hematopoietic cells by flow sorting of viable, CD45− cells. Endothelial cells were sorted from collagenase treated lungs as CD45−/ CD31+ cells. RNA was purified from sorted cell populations using Trizol and cDNA was generated by standard techniques. PCR amplification of the MIIG transgene was performed using one primer recognizing the extracellular portion of IFNGR1 and second primer which bound to the sequence of the myc tag. MIIG transgene PCR products were compared to the product of a PCR reaction amplifying a sequence from β-actin.
Trypanosoma cruzi and Toxoplasma gondii production and infection
In vitro parasite killing assay: Peritoneal thyoglycollate-elicited adherent cells were plated (1×10E5 cells/well) in 0.5mL 8-well TissueTek slides. Cells were incubated overnight and exposed to several concentrations of IFN-g, followed by infection with culture-derived trypomastigote forms of T. cruzi (Y strain) (10 to 1 parasite to host cell ratio). After 4 or 48 hrs cells were washed, fixed and Giemsa stained. Infection rates were evaluated by counting infected cells as observed by microscopy. NO measurements made using Griess reagent as described previously (31).
For T. cruzi infections, animals received blood-derived trypomastigotes (Y strain) via i.p. injection. Parasitemia was monitored throughout acute phase by counting moving parasites in blood smears, as described previously (32). In vivo Toxoplasma gondii (ME49 strain) infections were produced by injecting 50 cysts i.p., which were purified from the brains of chronically infected mice. Infection of peritoneal cells was assessed by microscopic examination of Giemsa stained cytospin preparations.
Leishmania major growth, purification and infection
L. major clone V1 (MHOM/IL/80/Friedlin) promastigotes were grown at 28°C in medium 199 (M199/S [Celgro]), supplemented with 20% FCS (Hyclone), 100 U/ml penicillin, 100 μg/ml streptomycin, 2mM L-glutamine, 25 mM HEPES, 0.1 mM adenine (in 50 mM Hepes), 5 μg/ml hemin (in 50% triethanolamine), and 2 μg/ml d-biotin and passaged at least 3 times but not more than 5 times prior to infection. Ficoll gradient purification, described previously (33), was used to purify infectious phase metacyclic promastigotes of L. major from 5 d old stationary culture. Briefly, stationary phase culture of promastigotes was initially enriched through centrifugation at 3500 rpm for 15 min at 4°C. Supernatant was removed and enriched promastigotes were resuspended in 2 ml of RPMI containing 100 U/ml penicillin, 100 μg/ml streptomycin and added slowly onto a Ficoll-Paque (Amersham Biosciences) layer consisting of 2 ml of 20% Ficoll-Paque (bottom) and 2 ml 10% Ficoll-Paque (top) and centrifuged at 2000 rpm for 15 min at 15°C without braking. Top portion containing metacyclic promastigotes in RPMI and 10% Ficoll-Paque was carefully harvested, transferred into a 15 ml centrifuge tube, washed by adding 14 ml of RPMI containing 100 U/ml penicillin, 100 μg/ml streptomycin and centrifuged at 3500 rpm for 15 min at 4°C.
In vitro infection with L. major was performed as previously described (34) with few modifications. Briefly, resident peritoneal macrophages were harvested using standard techniques, and resuspended at 1 × 106 cells/ml in complete RPMI medium (RPMI-1640 medium supplemented with 10% FCS, 2 mM L-glutamine and 50 μg/ml gentamicin). The cells were then plated in 0.2 ml on 16-chamber Lab-Tek Permanox tissue culture slides (Miles Laboratories) and allowed to adhere at 37°C in 5% CO2. Metacyclic promastigotes were opsonized with 5% normal mouse serum for 30 min at 37°C and were used to infect adherent cells at a ratio of 5:1. After overnight infection at 37°C, free parasites were removed by washing with complete RPMI medium. The cultures were incubated for an additional 48 h at 37°C, after which the slides were fixed in absolute methanol and stained with Diff-Quick (Dade Behring). The numbers of infected and non-infected macrophages, along with the numbers of amastigotes/infected macrophage were counted. Photographs depicting in vitro infection of macrophages with L. major were obtained under immersion oil at 60X (600X, final) resolution using inverted Zeiss microscope according to the manufacturer’s instructions.
LCMV production and infection
LCMV was propagated in BHK21 cells and tittered using a standard plaque assay (35) (36). The limit of detection for assessing viral load in infected tissues using this assay is approximately 100 plaque forming units/ gram of tissue. For LCMV infection, mice were injected with 200 pfu of LCMV-WE intraperitoneally.
Cytokine production assays
Bone marrow derived macrophages (cultured 7 days with M-CSF) from either WT or MIIG mice were plated (106/ml) with titrations of either CpG’s (ODN 1826, Invivogen) or LPS (salmonella derived, Sigma). The highest concentrations being 1 mcg/ml or 10 mcg/ml, respectively. Supernatants were gathered after 24 hours and IL-12p40 and IL-10 were measured by ELISA.
ELISA
Serum IFN-γ concentrations and IL-12p40 and IL-10 concentrations from culture supernatants were determined by standard ELISA techniques (R+D systems, Ebioscience). Measurement of serum IFN-γ concentrations after L. major infection was performed by an in vivo cytokine capture assay in which biotinylated anti-IFN-γ antibody (R46A2, 10 mcg) is injected one day prior to bleeding animals. The preformed antibody:cytokine complex is then detected via an ELISA technique, as described (37).
Data analysis/ Statistics
All studies were repeated at least twice with consistent results and with a minimum of 3 mice per group, though typically more (as indicated). P values were calculated using a student’s T test. Error bars displayed in figures represent standard error of the mean.
Results
Creation of ‘Macrophages Insensitive to Interferon Gamma’ (MIIG) mice
Dominantly acting non-signaling mutations of the IFN-γ receptor have been well characterized experimentally (29) and have been identified in human kindreds sufferring from excessive infections with intracellular bacteria (particularly mycobacteria) (38). Affected individuals have similar (though less severe) immunodeficiency as those with autosomal recessive deficiencies of IFN-γ or IL-12, but display autosomal dominant inheritance of this rare trait. This autosomal dominant trait was found to be due to truncation mutants of either IFN-γ receptor 1 or IFN-γ receptor 2, in which the cytoplasmic portion of the molecule is lost. Additionally, transgenic mice expressing such a dominant negative IFN-γ receptor 1 mutation in either T cells or macrophages have previously been described (28). These mice displayed the appropriate cell type-specific insensitivity to IFN-γ, though the functionality of the macrophage-specific mice (utilizing a different promoter than the current construct) was deemed to be incomplete (R. Schrieber, personal communication).
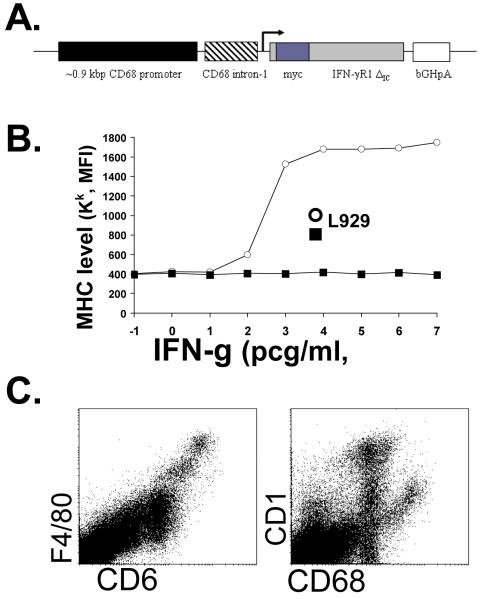
In order to better understand the in vivo effects of IFN-γ on macrophage lineage cells, we developed the construct illustrated in Figure 1A, in which the expression of a myc-tagged, cytoplasmic domain truncated IFN-γ receptor 1 molecule (IFNGR1ΔIC) is driven by the CD68 promoter. The CD68 promoter has been extensively characterized in cell lines to efficiently drive gene expression in macrophage lineage cells (25). Furthermore, this promoter has been utilized by others to produce transgenic mice with macrophage specific gene expression in vivo (26, 39). We tested our CD68/IFNGR1ΔIC construct by transfecting it into L929 cells. We were able to detect gene expression by staining for the myc tag on the cell surface. When we challenged L929 cells with a wide range of doses of IFN-γ and assessed upregulation of MHC class I on the cell surface, we found that non-transfected L929’s displayed a clear sigmoidal dose:response relationship to this cytokine (Fig. 1B). However, CD68/IFNGR1ΔIC transfected cells failed to respond to IFN-γ, even at doses which were >1000 times higher than a saturating dose in non-transfected cells.
Figure 1. Development of MIIG transgenic mice.
A. Diagram of the MIIG transgenic construct; a CD68 promoter/intron fragment was combined with a myc-tagged, dominant negative, mutant IFN-γ receptor 1 chain, which has a truncation of the cytoplasmic signaling portion. B. This promoter/gene construct was tested by transfection into L929 cells. Upregulation of MHC class I (Kk) in response to increasing amounts of IFN-γ was measured in transduced and non-transduced cells. C. Flow cytometric analysis of murine spleen cells reveals that CD68 protein is highly expressed in macrophages and to a lesser degree in CD11chigh (dendritic) cells.
While CD68 has long been considered to be a macrophage specific protein, a recent study analyzing human tissues with CD68-specific antibodies has questioned this belief (40). The authors of this study concluded that while CD68 is enriched in macrophages, it may not be truly specific for this cell type. However, similar results have not been reported in murine tissues and as illustrated in Fig. 1C, CD68 is specifically expressed at high levels in murine macrophages and at somewhat lower levels in murine dendritic cells. This expression pattern, combined with the reports from other groups utilizing this promoter, suggests that transgenic expression driven by the CD68 promoter is likely to lead to high level, specific expression in murine macrophages and to lower or perhaps variable expression in dendritic cells. After C57BL/6 embryos were injected with the CD68/IFNGR1ΔIC construct illustrated in Fig. 1A, the progeny of one transgenic founder were characterized and named the ‘Macrophages Insensitive to Interferon Gamma’ (MIIG) mice. MIIG transgenic mice were born in the expected frequency and appeared to have normal health at baseline (data not shown). Tissue morphology and cellularity was normal, with normal marrow, spleen, and lymph node cell numbers, including macrophages and lymphocyte subsets (data not shown).
Expression of the mutant IFN-γ receptor transgene and responsiveness to IFN-γ in macrophage lineage cells from MIIG mice
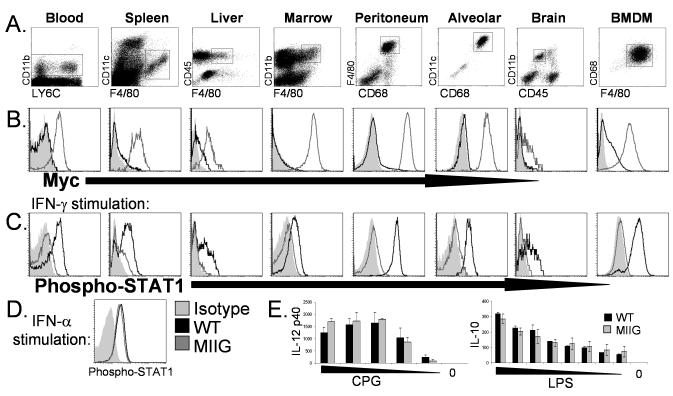
We examined macrophage lineage cells from multiple tissues in MIIG and WT mice. Dot plots in Fig. 2A illustrate how macrophage populations from each of these tissues were defined flow cytometrically. First, we examined cell surface expression of the transgenic construct by myc staining. As Fig. 2B illustrates, high levels of this transgene are expressed in monocytes and differentiated tissue macrophages from the bone marrow, spleen, liver, peritoneum, and alveolar space. Lower, but still readily detectable levels were detected on brain microglia. Next, we examined the responsiveness of macrophage lineage cells to IFN-γ by exposing freshly isolated cells to saturating amounts (100ng/ml) of this cytokine in vitro followed by staining for phospho-STAT1. Additionally, we tested bone marrow derived macrophages (BMDM’s), generated after culturing bone marrow with M-CSF for 1 week. As illustrated in Fig. 2C, monocytes, macrophages, and microglia from WT mice clearly upregulate phospho-STAT1 in response to IFN-γ. However, macrophage lineage cells from all tissues tested in MIIG mice failed to respond in this assay, indicating a profound proximal blockade of IFN-γ signaling in these cells. Of note, Ly6C+ and Ly6C- blood monocyte subsets from MIIG mice were indistinguishable by both myc and phospho-STAT1 staining (data not shown). As an additional control, phospho-STAT1 levels were measured in WT and MIIG peritoneal macrophages after exposure to IFN-α (1000U/ML) (Fig. 2D). As expected, macrophages from MIIG mice upregulated phospho-STAT1 in a normal fashion in response to IFN-α. Finally, we stimulated bone marrow derived macrophages from WT and MIIG mice with LPS or CpG’s and measured IL-10 or IL-12p40 production and found that response to these TLR ligands was unaltered in MIIG macrophages (Fig. 2E). Thus, macrophage populations from MIIG mice ubiquitously express the MIIG transgene and have a selective and severe impairment (or complete absence) of IFN-γ responsiveness.
Figure 2. Expression of the MIIG transgene and responsiveness to IFN-γ in macrophage/monocyte populations from WT and MIIG mice.
A. Dots plots illustrating how each population is defined by flow cytometry. BMDM indicates bone marrow derived macrophages, after culture with M-CSF. B. Cell surface myc staining of indicated populations. C. Phospho-STAT1 staining of the indicated population after in vitro stimulation with IFN-γ. D. Phospho-STAT1 staining of peritoneal macrophages after stimulation with IFN-α. E. IL-12p40 and IL-10 production by BMDM’s from WT and MIIG mice after stimulation with titrated amounts of either CpG (ODN 1826) or LPS. Shaded area represents isotype staining (in B) or fluorescence intensity of phospho-STAT1 in unstimulated cells (in C and D). Data are representative of three or more experiments examining greater than 6 mice.
Expression of mutant IFN-γ receptor transgene and responsiveness to IFN-γ in dendritic cells from MIIG mice
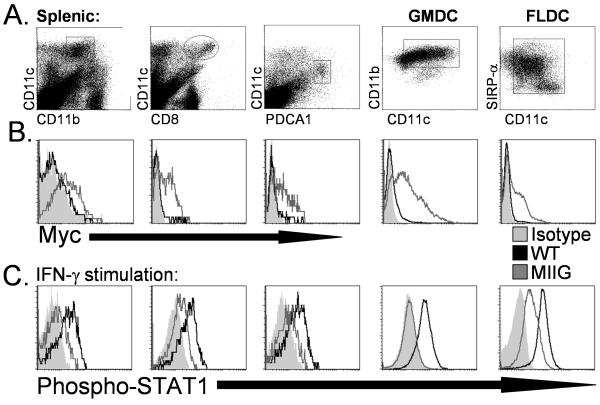
Because the CD68 promoter is expected to also drive some level of transgene expression in dendritic cells, we assessed dendritic cell subsets from MIIG mice carefully. We examined splenic dendritic cells which were either CD11b+, CD8+, or PDCA1+ from WT and MIIG mice (Fig. 3A). In addition to freshly isolated cells, we also examined dendritic cells from GM-CSF or Flt3-L cultured bone marrow. We could detect surface myc staining on each of these dendritic cell populations, though the levels were generally lower than that seen with most MIIG macrophage populations (Fig 3B). Despite this fact, all dendritic cell populations displayed significantly impaired responsiveness to IFN-γ, as measured by phospho-STAT1 accumulation after in vitro IFN-γ exposure (Fig. 3C). This defect was not quite as complete as seen in macrophages from MIIG mice, with Flt3-L culture-derived dendritic cells displaying the most clearly intermediate responsiveness to IFN-γ. Of note, dendritic cell populations from the lymph nodes of MIIG mice responded essentially identically to IFN-γ stimulation, as compared to analogous splenic dendritic cells (data not shown). Thus, dendritic cells from MIIG mice display impaired responsiveness to IFN-γ which is similar to that observed in macrophages from these mice.
Figure 3. Expression of the MIIG transgene and responsiveness to IFN-γ in dendritic cell populations from WT and MIIG mice.
A. Dots plots illustrating how each population is defined by flow cytometry. GMDC and FLDC indicate bone marrow derived dendritic cells after culture with GM-CSF or FLT3 ligand, respectively. B. Cell surface myc staining of indicated populations. C. Phospho-STAT1 staining of the indicated population after in vitro stimulation with IFN-γ. Shaded area represents isotype staining (in B) or fluorescence intensity of phospho-STAT1 in unstimulated cells (in C). Data are representative of three or more experiments examining greater than 6 mice.
Most non-macrophage lineage cells from MIIG mice do not express the mutant IFN-γ receptor transgene and respond normally to IFN-γ
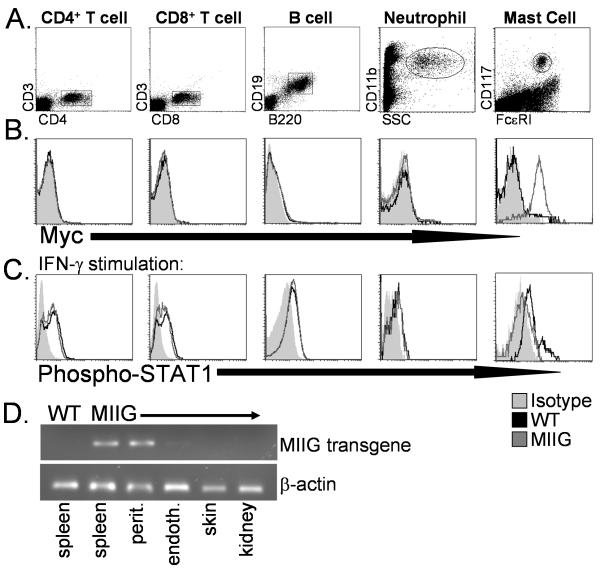
We examined transgene expression and function in non-macrophage lineage cells from MIIG mice three ways. First, we performed myc staining and found no detectable cell surface myc expression on neutrophils or lymphocytes (Fig. 4B). Due to reports that CD68 may be expressed in mast cells, we also examined peritoneal mast cells.(41) We found that these cells expressed high levels of both CD68 and the MIIG transgene (data not shown and Fig. 4B). Second, when stimulated with IFN-γ, neutrophils, CD4+/CD8+ T cells, and B cells from MIIG mice all upregulated phospho-STAT1 in a normal fashion, while mast cell displayed impaired responsiveness to this cytokine (Fig. 4C). Third, we isolated RNA from hematopoietic and non-hematopoietic tissues from MIIG and WT mice and screened for transgene expression by RT PCR. Because macrophages infiltrate virtually all tissue of the body, we depleted CD45+ cells from non-hematopoietic tissue cell suspensions prior to extracting RNA. As illustrated in Fig. 4D, spleen cells and peritoneal macrophages from MIIG mice display robust expression of MIIG transgenic RNA (as does bone marrow, not shown), while spleens from WT mice do not. We also examined endothelial cells (sorted from lung), skin cells, and kidney cells and found that they do not express detectable MIIG transgene RNA. Thus, most non-macrophage lineage cells in MIIG mice do not express the MIIG transgene or display the blockade of IFN-γ signaling which is evident in macrophages and dendritic cell from these mice. The one clear exception is mast cells, which are not considered to be macrophage lineage cells, but do express the MIIG transgene, in accordance with their status as CD68+ cells.
Figure 4. Cell type specific expression of the MIIG transgene and responsiveness to IFN-γ in non-macrophage lineage cells from WT and MIIG mice.
A. Dots plots illustrating how each population is defined by flow cytometry. B. Surface myc staining of indicated populations. C. Phospho-STAT1 staining of the indicated population after in vitro stimulation with IFN-γ. Shaded area represents isotype staining (in B) or fluorescence intensity of phospho-STAT1 in unstimulated cells (in C). D. Expression of the MIIG transgene and β-actin in indicated tissues, as measured by RT PCR of cDNA. Non-hematopoietic tissues were first depleted of CD45+ cells before extraction of RNA, in order to avoid contamination by macrophages. Data are representative of three or more experiments examining greater than 6 mice.
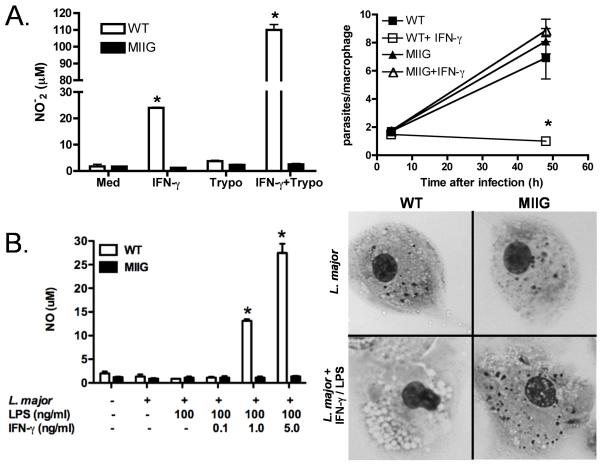
Macrophages from MIIG mice fail to produce NO or kill parasites in vitro after priming with IFN-γ
Based on our initial characterization of the MIIG mice and the known importance of IFN-γ for control of multiple protozoan parasites, we predicted that macrophages from these mice would not upregulate NO production or kill parasites after priming with IFN-γ. In order to test this prediction, we infected WT and MIIG peritoneal macrophages with either T. cruzi or L. major, in vitro (Fig. 5A and B). Production of NO was measured after parasitic infection and after stimulation with IFN-γ or IFN-γ and LPS. As expected, WT macrophages produced NO in response to IFN-γ, while those from MIIG mice failed to produce NO to any measurable degree. Furthermore, WT macrophages which had been exposed to IFN-γ (or IFN-γ + LPS) largely cleared T. cruzi or L. major infection within 48 or 72 hours, respectively. Persistent infection with L. major was readily visible in MIIG macrophages, even at 96 hours (Fig. 5B). Thus, macrophages from MIIG mice have a profound insensitivity to IFN-γ, which can be directly demonstrated (via phospho-STAT1 staining) and which clearly affects classic physiologic readouts such as NO production and microbicidal activity against protozoan parasites.
Figure 5. Macrophages from MIIG mice are unable to kill intracellular parasites or produce nitric oxide in response to IFN-γ.
A. Bone marrow derived macrophages were infected with T. cruzi (Trypo) and treated as indicated. NO production and clearance of T. cruzi infection was measured at 48 hours. B. Peritoneal macrophages were infected with L. major and cultured as indicated. NO production was measured at 72 hours. Images (600X) of L. major infected macrophages +/− IFN-γ/LPS treatment at 96 hours, with numerous visible parasites. *=p<0.01 comparing MIIG and WT macrophages treated in the same fashion. n=3 animals, with all studies repeated at least twice.
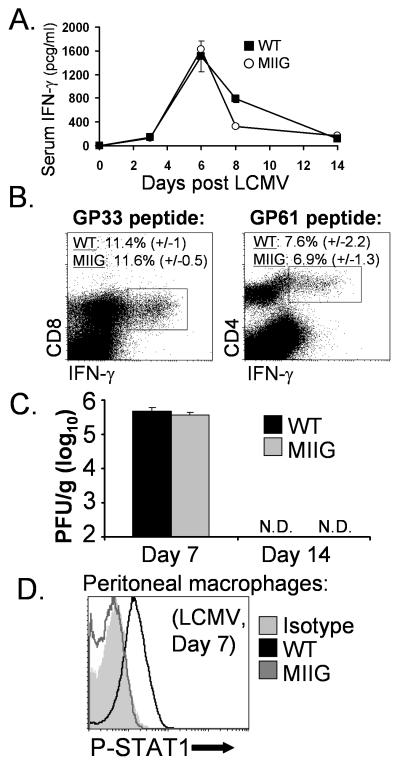
MIIG mice mount a normal anti-LCMV response and clear infection despite persistent unresponsiveness of macrophages to IFN-γ
Before characterizing the in vivo response of MIIG mice to infection with protozoan parasite species, we wanted to clearly determine whether these mice mounted a normal T cell and IFN-γ response to other types infectious agents. We chose to examine LCMV because this agent has been extensively characterized by many investigators and the course of LCMV infection appears to be only modestly influenced by IFN-γ (42, 43). First, we infected WT and MIIG animals with LCMV and measured serum IFN-γ levels over a time course which has been well characterized by us and others (44). We found that serum IFN-γ levels were comparable in MIIG and WT mice, after LCMV infection (Fig. 6A). As an additional endpoint, we assessed type I (αβ) IFN levels in the serum 1 day after LCMV infection and found no difference between WT and MIIG mice (data not shown). Next, we directly assessed the anti-LCMV T cell responses of these animals by performing intracellular cytokine staining for IFN-γ after stimulation of spleen cells with either a dominant CD8 T cell (GP33) epitope or a dominant CD4 T cell (GP61) epitope. We found that CD4 and CD8 T cell responses were comparable between WT and MIIG mice (Fig. 6B). Accordingly, when we assessed the viral burden in WT and MIIG mice using a standard viral plaque assay, we found that MIIG mice cleared the virus in a normal fashion (Fig. 6D). Finally, in order to determine whether macrophages in MIIG mice maintained their unresponsiveness to IFN-γ under inflammatory conditions, we examined peritoneal macrophages 7 days after LCMV infection. This time point is during the peak of the post-LCMV IFN-γ response (Fig. 6A), but long after the type I IFN responses has subsided (45). We found that macrophages from MIIG mice had much lower levels of phospho-STAT1 (directly ex vivo), compared to those from WT mice (Fig. 6C). This finding is particularly notable because it demonstrates that macrophages from MIIG mice fail to accumulate phospho-STAT1 in vivo, in a physiologic context associated with high IFN-γ levels. Thus, despite a selective and persistent defect of IFN-γ responsiveness in macrophages, MIIG mice respond to LCMV normally and clear the infection in a normal fashion.
Figure 6. MIIG mice mount a normal response to LCMV and clear the infection despite persistent unresponsiveness of macrophages to IFN-γ.
A. MIIG and WT mice were infected with LCMV and bled serially at the indicated time points in order to assess serum IFN-γ levels by ELISA n=6/group B. CD8 and CD4 T cell responses were quantitated at day 7 by intracellular cytokine staining. Percentages shown represent the percent of splenic CD8 or CD4 T cells, respectively, responding to the indicated peptide epitope. C. Quantitation of LCMV viral burden by standard plaque assay at the indicated days after infection. N.D. indicates none detected; pfu indicates plaque forming units. D. Phospho-STAT1 staining of peritoneal macrophages directly ex vivo (without further in vitro stimulation), 7 days after LCMV infection. n=4 animals (except where indicated), with all studies repeated at least twice.
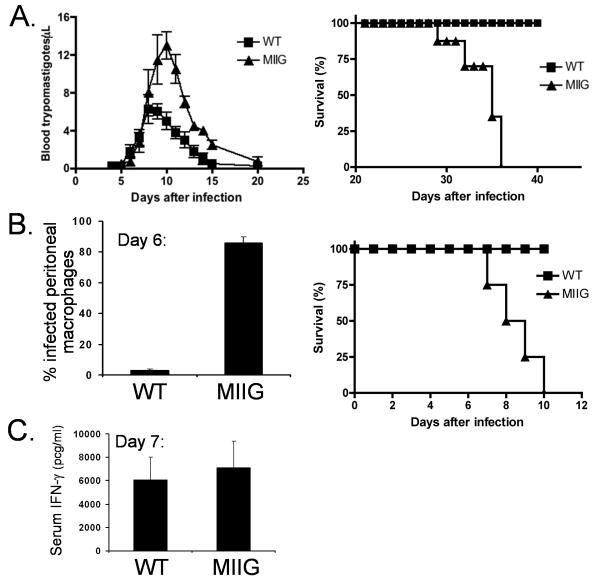
MIIG mice display impaired control of T. cruzi and T. gondii, in vivo
IFN-γ is known to be important for the clearance of both T. cruzi and T. gondii, in vivo, with a variety of data suggesting that it must act directly on macrophages (16) (11). While T. cruzi is cleared by both IFN-γ and non-IFN-γ dependant mechanisms, clearance of T. gondii infection is absolutely dependant on this cytokine (46). When we infected MIIG mice with T. cruzi, we found that these mice developed a more intense parasitemia, and succumbed to infection, unlike WT mice (Fig. 7A). Examination of infected mice revealed that heightened cardiac inflammation associated with more intensive parasite burden in cardiomyocytes appeared to underlie this mortality (data not shown). Even more strikingly, infection with T. gondii led to massive parasite burden in peritoneal cells, with very rapid death in all MIIG mice, similar to what has been reported for IFN-γ or IFN-γ receptor deficient mice (Fig. 7B). Six days after intraperitoneal T. gondii infection, nearly all peritoneal macrophages contained visible parasites, with free (extracellular) parasites evident on cytospin preparations (Fig. 7B and data not shown). This failure to control T. gondii infection occurred despite normal, robust serum IFN-γ levels in MIIG mice (Fig. 7C). Thus, these data directly demonstrate, in vivo, the critical role of a direct effect of IFN-γ on macrophages for the control of these parasites.
Figure 7. MIIG mice display impaired control of T. cruzi and T. gondii infection despite an appropriate IFN-γ response to T. gondii.
A. Parasite burden and survival were measured after infection of WT and MIIG mice with T. cruzi. n=6/group B. Parasite burden and survival was assessed after T. gondii infection of WT and MIIG mice. Parasitic infection of peritoneal macrophages was quantitated by microscopic examination of cytospin preparations. C. Serum IFN-γ was measured by ELISA, 7 days after infection with T. gondii. n=4/group
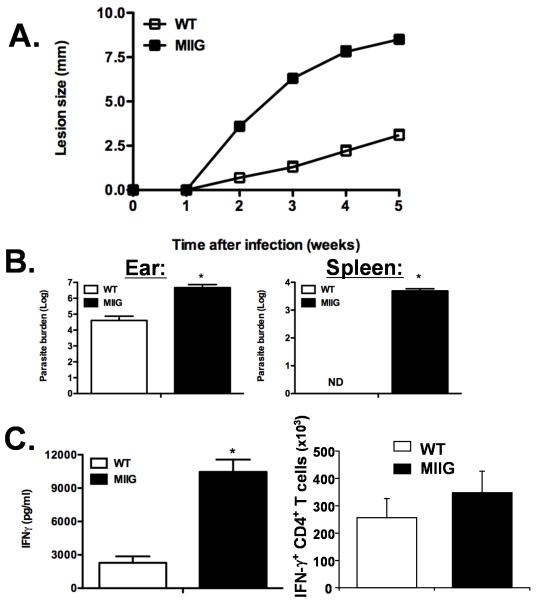
MIIG mice fail to control L. major, despite normal induction of IFN-γ producing anti-leishmanial CD4+ T cells
Similar to infection with T. cruzi and T. gondii, IFN-γ is known to be critical for controlling Leishmania infection and is thought to act principally on infected macrophages (12). In order to test this premise directly, WT and MIIG mice were infected with L. major intradermally. The size of the resulting lesions were measured serially and MIIG mice were found to have severely impaired control of the infection (Fig. 8A). We quantitated the parasite burden in these lesions and in the draining lymph nodes and found that MIIG mice had approximately 100 fold more parasites in these sites (Fig. 8B and data not shown). Notably, MIIG mice also developed disseminated spread of the infection to the spleen and liver, which was not observed in WT mice under these conditions (Fig. 8B and data not shown). We also assessed production of IFN-γ in vivo utilizing a cytokine capture assay and found that MIIG mice were producing significantly more IFN-γ than WT mice, five weeks after inoculation (Fig. 8C). This finding was not entirely surprising considering that the infection was much more widespread in the transgenic animals. Finally, we assessed the development of IFN-γ producing anti-leishmanial CD4+ T cells and found that MIIG and WT mice had a similar number of these cells in the draining lymph nodes, as determined by intracellular cytokine staining (Fig. 8C). Thus, MIIG mice fail to control L. major, despite an appropriate T cell and IFN-γ response. These data demonstrate that IFN-γ must act directly on macrophages in vivo in order to control L. major infection.
Figure 8. MIIG mice fail to control L. major infection despite an appropriate IFN-γ response.
A. WT and MIIG mice were infected intradermally with L. major and lesion size was measured serially. B. Parasite burden was quantitated in the indicated tissues 5 weeks after infection. C. IFN-γ production was measured in vivo by a cytokine capture assay (5 weeks after infection) and by intracellular cytokine staining of CD4+ T cells from the draining lymph nodes (3 weeks after infection). *=p<0.01, ND= not detected, n=6/group
Discussion
The biology of IFN-γ is relevant to numerous aspects of immune function. Our understanding of its role in host defense, tumor immunology, inflammation, and immune-mediated pathology is still evolving. While IFN-γ is largely produced by lymphocytes, it acts on many cell types due to the fact that most cells express the IFN-γ receptor and are capable of responding (1). Thus, dissecting its site of action in vivo is exceedingly difficult. In vitro and indirect in vivo data form the basis for concluding that macrophages are an important target for many of this cytokines effects. We generated the MIIG mice in order to better understand how macrophages participate in vivo in IFN-γ mediated host defense and IFN-γ driven immunopathology. In the current report, these mice have been characterized as having a specific and profound insensitivity to IFN-γ in macrophage lineage cells and mast cells. In these studies, the MIIG mice have formally demonstrated the importance of the direct effects of IFN-γ on macrophages for the control of multiple protozoan parasitic infections in vivo.
MIIG mice display faithful expression of the MIIG transgene in CD68+ cells, which include monocytes, macrophages, dendritic cells, and mast cells. Though mast cells are not considered to be part of the macrophage lineage, their expression of the MIIG transgene is consistent with their expression of CD68, a scavenger receptor also known as macrosialin, which is largely found in late endosomes and is known to bind oxidized LDL. (47) (48) The significance of mast cell expression of the MIIG transgene for the current work is probably minimal. Though mast cell degranulation may be relevant for susceptibility or resistance to cutaneous L. major infection (49), the dramatic inability of macrophages to kill or clear L. major and other parasites in vitro and in vivo is not likely to be an indirect result of a defect in mast cells. Additionally, while IFN-γ may alter mast cell cytokine or NO production, it is not known to have a major impact on degranulation or other mast cell functions (50). However, this aspect of the MIIG mice may be useful for further studies examining the biology of these cells.
While IFN-γ has long been appreciated as an important cytokine for the control of multiple protozoan parasites, including T. gondii, T. cruzi, and L. major, the current studies have added to our understanding of its role in each of these infections. Overall, these studies have strengthened the broad hypothesis that IFN-γ is critical for control of many different protozoan parasites because it primes macrophages (which are critical host cells), leading to the destruction of these intracellular pathogens. In the case of T. gondii infection, our results suggest that macrophages are a far more important target cell type for both acute parasitic infection and IFN-γ activation than has been previously recognized. While T. gondii can infect almost any cell type, and control of this parasite is known to be profoundly dependent on IFN-γ (51), the rapidity with which MIIG mice succumbed to infection closely mimics the phenotype IFN-γ−/−mice, which is surprising considering the narrowly focused defect of IFN-γ signaling in these mice. However, when considering how these findings apply to human health, it should also be noted that human patients who are heterozygous for similar mutations in IFN-γR1 do not appear to suffer from susceptibility to T. gondii.(52) This difference could be due to either a fundamental biologic difference between mice and men, or it could be due to the fact that inhibition in IFN-γ signaling in human patients with partial IFN-γ receptor deficiency (as the heterozygous state for these mutations is called) is not as complete as in the macrophages of our transgenic animals. Similarly, while T. cruzi may certainly infect macrophages, it has not been previously demonstrated that this cell type may have such a profound effect in vivo on acute parasite control or on host survival. Finally, while macrophages are the classic cell type infected by L. major and IFN-γ is clearly critical for control of this infection, this report is the first direct in vivo demonstration that this cytokine must act directly and specifically on these cells in order to control infection. Future studies involving MIIG mice and other parasitic or bacterial pathogens, such as Plasmodium, Salmonella, Listeria, and mycobacteria are likely to reveal other notable or unexpected findings.
The MIIG mice may also help to answer complex questions regarding the biology of IFN-γ in other contexts. In multiple model systems, IFN-γ can play either a positive or negative (or combination) role in promoting immune responses and/or immunopathology (53) (54) (44) (55) (56) (4). Even in a ‘classic’ context, such as mycobacterial infection, IFN-γ may influence various cell types in unexpected ways, such as suppressing protective T cell responses (54). This ‘inflammatory’ cytokine appears to have a protective role in experimental allergic encephalitis (EAE) as well as collagen induced arthritis (CIA) (57-60) (56, 61-63). However, this effect is complicated and some data are contradictory (53). Additionally, IFN-γ has been identified as a cytokine with profound effects on T cell expansion, differentiation, and contraction (5-7, 64). Though much of this effect appears to be due to its direct action on T cells, not all observations are explained by this mechanism of action, and some uncertainty remains. In each of these circumstances, complex mechanisms and sites of action of IFN-γ are still being clarified and new tools, such as the MIIG mice, are needed. Indeed, the MIIG mice have already helped to define a role for IFN-γ activated macrophages in production of inflammatory cytokines and recruitment of inflammatory cells to the CNS, in the context of LCMV infection.(65) Thus, the direct in vivo effects of IFN-γ on macrophages in multiple inflammatory contexts may be further elucidated by MIIG mice.
Acknowledgements
The authors would like to thank Dr. Peter Murray for generously providing the CD68 promoter construct and Dr. Robert Schreiber for his kind gift of the dominant-negative IFN-γ receptor construct. We also wish to thank Drs David Hildeman and Jose Cancelas for reagents and helpful discussions; and Alyssa Sproles for assistance with cytokine determinations.
Footnotes
This work was supported by generous start-up funds from the Division of Immunobiology (CCHMC), a grant from USIDNET, and by NIH-R01HL091769 (to M.J.) and by NIH-R01AI057992 (to C.K.).
Nonstandard abbreviations used in this manuscript: MIIG, macrophages insensitive to interferon gamma; WT, wild type; LCMV, lymphocytic choriomeningitis virus
References
- 1.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 2.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Strehl B, Seifert U, Kruger E, Heink S, Kuckelkorn U, Kloetzel PM. Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol Rev. 2005;207:19–30. doi: 10.1111/j.0105-2896.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 4.Muhl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 6.Haring JS, Harty JT. Aberrant contraction of antigen-specific CD4 T cells after infection in the absence of gamma interferon or its receptor. Infect Immun. 2006;74:6252–6263. doi: 10.1128/IAI.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray HW. Gamma interferon, cytokine-induced macrophage activation, and antimicrobial host defense. In vitro, in animal models, and in humans. Diagn Microbiol Infect Dis. 1990;13:411–421. doi: 10.1016/0732-8893(90)90012-k. [DOI] [PubMed] [Google Scholar]
- 10.Shtrichman R, Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001;4:251–259. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 11.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locksley RM, Louis JA. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoneto T, Yoshimoto T, Wang CR, Takahama Y, Tsuji M, Waki S, Nariuchi H. Gamma interferon production is critical for protective immunity to infection with blood-stage Plasmodium berghei XAT but neither NO production nor NK cell activation is critical. Infect Immun. 1999;67:2349–2356. doi: 10.1128/iai.67.5.2349-2356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke ED, Hoffman SL. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 15.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 16.McCabe RE, Meagher SG, Mullins BT. Endogenous interferon-gamma, macrophage activation, and murine host defense against acute infection with Trypanosoma cruzi. J Infect Dis. 1991;163:912–915. doi: 10.1093/infdis/163.4.912. [DOI] [PubMed] [Google Scholar]
- 17.Culshaw RJ, Bancroft GJ, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–3079. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salat J, Sak B, Le T, Kopecky J. Susceptibility of IFN-gamma or IL-12 knock-out and SCID mice to infection with two microsporidian species, Encephalitozoon cuniculi and E. intestinalis. Folia Parasitol (Praha) 2004;51:275–282. [PubMed] [Google Scholar]
- 19.Salata RA, Murray HW, Rubin BY, Ravdin JI. The role of gamma interferon in the generation of human macrophages cytotoxic for Entamoeba histolytica trophozoites. Am J Trop Med Hyg. 1987;37:72–78. doi: 10.4269/ajtmh.1987.37.72. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi I, Suzuki R, Waki S, Tagawa Y, Seng S, Tum S, Omata Y, Saito A, Nagasawa H, Iwakura Y, Suzuki N, Mikami T, Toyoda Y. Roles of CD4(+) T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect Immun. 1999;67:4143–4148. doi: 10.1128/iai.67.8.4143-4148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, Shellito JE. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol. 1999;162:2890–2894. [PubMed] [Google Scholar]
- 22.Singer SM, Nash TE. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun. 2000;68:170–175. doi: 10.1128/iai.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan CF, Prendergast TJ, Wiebe ME, Stanley ER, Platzer E, Remold HG, Welte K, Rubin BY, Murray HW. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984;160:600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Taniguchi T, Tanaka N. The interferon system and interferon regulatory factor transcription factors -- studies from gene knockout mice. Cytokine Growth Factor Rev. 2001;12:133–142. doi: 10.1016/s1359-6101(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 25.Greaves DR, Quinn CM, Seldin MF, Gordon S. Functional comparison of the murine macrosialin and human CD68 promoters in macrophage and nonmacrophage cell lines. Genomics. 1998;54:165–168. doi: 10.1006/geno.1998.5546. [DOI] [PubMed] [Google Scholar]
- 26.Gough PJ, Gordon S, Greaves DR. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 2001;103:351–361. doi: 10.1046/j.1365-2567.2001.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, Nemazee D. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 28.Dighe AS, Campbell D, Hsieh CS, Clarke S, Greaves DR, Gordon S, Murphy KM, Schreiber RD. Tissue-specific targeting of cytokine unresponsiveness in transgenic mice. Immunity. 1995;3:657–666. doi: 10.1016/1074-7613(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 29.Dighe AS, Farrar MA, Schreiber RD. Inhibition of cellular responsiveness to interferon-gamma (IFN gamma) induced by overexpression of inactive forms of the IFN gamma receptor. J Biol Chem. 1993;268:10645–10653. [PubMed] [Google Scholar]
- 30.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 31.Machado FS, Esper L, Dias A, Madan R, Gu Y, Hildeman D, Serhan CN, Karp CL, Aliberti J. Native and aspirin-triggered lipoxins control innate immunity by inducing proteasomal degradation of TRAF6. J Exp Med. 2008;205:1077–1086. doi: 10.1084/jem.20072416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Aliberti JC, Machado FS, Gazzinelli RT, Teixeira MM, Silva JS. Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzi-infected macrophages and mediates resistance to parasite infection in mice. Infect Immun. 1999;67:2810–2814. doi: 10.1128/iai.67.6.2810-2814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 34.Noben-Trauth N, Lira R, Nagase H, Paul WE, Sacks DL. The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. J Immunol. 2003;170:5152–5158. doi: 10.4049/jimmunol.170.10.5152. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr Protoc Immunol. 2003 doi: 10.1002/0471142735.im0628s54. Chapter 6:Unit 6 28. [DOI] [PubMed] [Google Scholar]
- 38.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova JL. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 40.Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 41.Horny HP, Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of normal and neoplastic human tissue mast cells with macrophage-associated antibodies, with special reference to the recently developed monoclonal antibody PG-M1. Hum Pathol. 1993;24:355–358. doi: 10.1016/0046-8177(93)90081-q. [DOI] [PubMed] [Google Scholar]
- 42.Lohman BL, Welsh RM. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol. 1998;72:7815–7821. doi: 10.1128/jvi.72.10.7815-7821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utermohlen O, Dangel A, Tarnok A, Lehmann-Grube F. Modulation by gamma interferon of antiviral cell-mediated immune responses in vivo. J Virol. 1996;70:1521–1526. doi: 10.1128/jvi.70.3.1521-1526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 45.Pien GC, Nguyen KB, Malmgaard L, Satoskar AR, Biron CA. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J Immunol. 2002;169:5827–5837. doi: 10.4049/jimmunol.169.10.5827. [DOI] [PubMed] [Google Scholar]
- 46.Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, Aliberti J. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol Res. 2003;27:521–528. doi: 10.1385/IR:27:2-3:521. [DOI] [PubMed] [Google Scholar]
- 47.Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 48.de Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. 1999;66:740–746. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- 49.Romao PR, Da Costa Santiago H, Ramos CD, De Oliveira CF, Monteiro MC, De Queiroz Cunha F, Vieira LQ. Mast cell degranulation contributes to susceptibility to Leishmania major. Parasite Immunol. 2009;31:140–146. doi: 10.1111/j.1365-3024.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 50.Kulka M, Befus AD. The dynamic and complex role of mast cells in allergic disease. Arch Immunol Ther Exp (Warsz) 2003;51:111–120. [PubMed] [Google Scholar]
- 51.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 52.Janssen R, Van Wengen A, Verhard E, De Boer T, Zomerdijk T, Ottenhoff TH, Van Dissel JT. Divergent role for TNF-alpha in IFN-gamma-induced killing of Toxoplasma gondii and Salmonella typhimurium contributes to selective susceptibility of patients with partial IFN-gamma receptor 1 deficiency. J Immunol. 2002;169:3900–3907. doi: 10.4049/jimmunol.169.7.3900. [DOI] [PubMed] [Google Scholar]
- 53.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Furlan R, Bergami A, Cantarella D, Brambilla E, Taniguchi M, Dellabona P, Casorati G, Martino G. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur J Immunol. 2003;33:1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 58.Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- 59.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 60.Willenborg DO, Staykova M, Fordham S, O’Brien N, Linares D. The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:16–25. doi: 10.1016/j.jneuroim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 62.Guedez YB, Whittington KB, Clayton JL, Joosten LA, van de Loo FA, van den Berg WB, Rosloniec EF. Genetic ablation of interferon-gamma up-regulates interleukin-1beta expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001;44:2413–2424. doi: 10.1002/1529-0131(200110)44:10<2413::aid-art406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Kageyama Y, Koide Y, Yoshida A, Uchijima M, Arai T, Miyamoto S, Ozeki T, Hiyoshi M, Kushida K, Inoue T. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-gamma receptor. J Immunol. 1998;161:1542–1548. [PubMed] [Google Scholar]
- 64.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 65.Lin AA, Tripathi PK, Sholl A, Jordan MB, Hildeman DA. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol. 2009;83:8604–8615. doi: 10.1128/JVI.02477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]