Abstract
Human ether a go-go-related gene type 1 (hERG1) K+ channels conduct the rapid delayed rectifier K+ current and mediate action potential repolarization in the heart. Mutations in KCNH2 (the gene that encodes hERG1) causes LQT2, one of the most common forms of long QT syndrome, a disorder of cardiac repolarization that predisposes affected subjects to ventricular arrhythmia and increases the risk of sudden cardiac death. Hundreds of LQT2-associated mutations have been described, and most cause a loss of function by disrupting subunit folding, assembly, or trafficking of the channel to the cell surface. Loss-of-function mutations in hERG1 channels have also recently been implicated in epilepsy. A single gain-of-function mutation has been described that causes short QT syndrome and cardiac arrhythmia. In addition, up-regulation of hERG1 channel expression has been demonstrated in specific tumors and has been associated with skeletal muscle atrophy in mice.
Keywords: Cardiac electrophysiology, Cardiac potassium current, Ether-à-go-go, K+ channel, Ventricular fibrillation
Introduction
KCNH2, the gene encoding the human ether a go-go-related gene type 1 (hERG1) K+ channel, was discovered in 1994 [109]. Based on the biophysical properties of heterologously expressed channels, it was proposed that hERG1 channels conduct the rapid delayed rectifier K+ current, IKr in cardiomyocytes [83, 102]. IKr is one of several K+ currents that mediate repolarization of vertebrate cardiomyocytes and is distinguished by its disproportionately rapid rate of inactivation compared to activation, its association with an inherited form of arrhythmia, and by its clinically significant pharmacology.
A reduction of IKr caused by long QT syndrome (LQTS)-associated mutations in KCNH2 can induce ventricular arrhythmia and cause sudden cardiac death. As reviewed elsewhere [90], in noncardiac cells, ERG1 channels primarily contribute to maintenance of the resting potential. In addition to the heart, ERG1 expression has been identified in several regions of the brain [27], tumor cells [5, 10, 27], gastro-intestinal smooth muscle myocytes [21], pancreatic β-cells [79], lactotrophs [8], carotid body cells [68] and the inner ear [66]. However, with the exception of the infrequent association with epilepsy, KCNH2 mutations are not known to cause disease in organs other than the heart. Several excellent and comprehensive reviews of hERG1 channels have recently appeared [47, 70, 75]. Here, the role of hERG1 channels in human disease is reviewed.
hERG1 channel structure and accessory subunits
In humans, KCNH2 is located on chromosome 7q35–36, and the coding region comprises 16 exons spanning approximately 34 kb of genomic sequence. The full-length hERG1 subunit (hERG1a) is composed of 1,159 amino acids with a predicted molecular mass of 127 kDa and has six transmembrane domains (S1–S6). hERG1a has a long (376 amino acids) N-terminus and residues 1–135 comprise the so-called “eag domain” that was crystallized and found to be the first eukaryotic example of a protein– protein interaction structure called a Per–Arnt–Sim (PAS) domain [58]. The function of the PAS domain in hERG1a is uncertain; however, LQTS-associated mutations in this region disrupt channel trafficking and accelerate the rate of deactivation [14, 58], perhaps by disrupting its interaction with the S4–S5 linker of the channel [103]. The PAS domain can be phosphorylated [13, 18] and needs to be properly folded for normal trafficking of the channel complex from the endoplasmic reticulum (ER) to the Golgi and cell surface [69]. An alternatively spliced variant of hERG1 (hERG1b) was isolated from mouse and human heart [50, 51] and is composed of 819 amino acids with a predicted molecular mass of 94 kDa [39]. The N-terminus of hERG1b is only 36 amino acids and lacks the PAS domain but has an “RXR” ER retention signal sequence that prevents its trafficking to the surface membrane unless coassembled with hERG1a subunits [72]. In rodents, erg1a and erg1b has also been shown to coassemble in the brain [27]. The large C-terminus of both hERG1a and hERG1b contains a cyclic nucleotide binding domain (CNBD). Unlike cyclic-nucleotide gated channels, where binding of cyclic adenosine monophosphate (cAMP) to the CNBD is required for channel activation, cAMP has a relatively minor effect on hERG1 channel gating, causing a few millivolts shift in the voltage dependence of channel activation [18].
Similar to other Kv channels, functional hERG1 channels are tetrameric complexes formed by coassembly of four α-subunits, either hERG1a subunits alone or hERG1a plus hERG1b subunits [39, 71, 80]. In heterologous expression systems, hERG1 proteins can also coassemble with two ancillary β-subunits, MinK (KCNE1) and MiRP1 (KCNE2) [1, 55]. The KCNEs are small transmembrane proteins (123–129 amino acids) with a single transmembrane domain. Although MinK most likely functions as the accessory subunit for KCNQ1 to form IKs channels in the heart [7, 82], MinK can also modulate hERG1 channel density when overexpressed in heterologous expression systems [9, 55]. MiRP1 was initially reported to alter the pharmacology and gating kinetics of hERG1 [1], and LQTS-associated mutations in MiRP1 alter hERG1 currents differently from wild-type MiRP1 [1, 26, 34, 53]. However, the consequences and physiological significance of this interaction has been contested [110], and the effects of MiRP1 in heterologous expression studies are variable [3, 54, 110]. Moreover, it is not clear whether MiRP1 is expressed at high enough levels to affect hERG1 function throughout the heart as physiologically significant levels may be limited to pacemaker cells and Purkinje cells of the conduction system [73]. Most recently, it was reported that interaction of MiRP1 with KCNQ1 may be more physiologically significant than its association with hERG1. Along with MinK, MiRP1 can coassembly with KCNQ1 to form a heteromultimeric channel complex with a net result of decreased IKs conductance [36]. Moreover, targeted disruption of kcne2 in mice suggests that constitutively active KCNQ1/MiRP1 channels are expressed in thyrocytes and that these channels are required for normal thyroid hormone biosynthesis [77]. Altered thyroid function might have a role in LQTS associated with mutations in KCNE2. Linkage of KCNE2 mutations to ventricular arrhythmia and/or sudden cardiac death in a large kindred would go a long way towards substantiating the role of MiRP1 in LQTS.
Kinetics of channel gating determine role of hERG1 in action potential repolarization
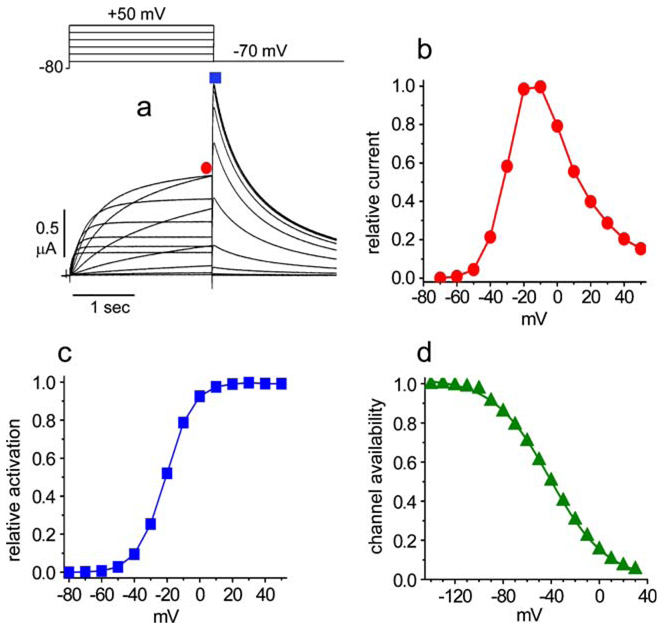
Similar to other Kv channels, hERG1 is activated by voltage. The threshold for channel opening is about −50 mV, and the voltage required for 1/2 activation is about −30 mV. The single-channel conductance (γ) of hERG1 channels is 12.4 pS between −60 and −120 mV when determined with a [K+]o of 120 mM [57, 122]. At physiological levels of [K+]o (5 mM), γ determined by nonstationary noise analysis is about 2 pS at 0 mV [44]. hERG1 channels also inactivate in a voltage-dependent manner with a half point of about −85 mV. Channel activation is slow, especially compared to the rapid onset of P-type inactivation that is proposed to be mediated by rearrangement of the selectivity filter [45]. The faster rate of inactivation results in a voltage-dependent reduction in whole cell conductance and a bell-shaped I−V relationship (Fig. 1). When the membrane is repolarized, channels recover from inactivation at a rate about ten times faster than the rate of deactivation. These kinetic features strongly affect the contribution of hERG1 to net current during the delayed repolarization phase typical of cardiac action potentials. Channel inactivation dominates at positive transmembrane potentials and greatly reduces the magnitude of hERG1 current at the peak of a cardiac action potential. During the plateau phase and continuing through phase 3 repolarization, channels recover from inactivation, and because deactivation is relatively slow, hERG1 current transiently increases in magnitude even as the electrochemical driving force for K+ efflux is reduced.
Fig. 1.
Biophysical properties of hERG1 channel currents. a Voltage clamp protocol (upper panel) and heterologously expressed hERG1 ionic currents (lower panel) recorded from a Xenopus oocyte. Currents were recorded at test potentials that ranged from −70 to +50 mV; deactivating (“tail”) currents were measured at −70 mV. b Current–voltage (I−V) relationship for hERG1 currents measured at the end of test pulses, as indicated by red circle in a. c Voltage dependence of hERG1 current activation. The peak of tail currents measured at −70 mV (indicated by blue square in a) were normalized to the largest value and plotted as a function of the test potential. d Voltage dependence of hERG1 inactivation. Channel availability is decreased at positive potentials, resulting in a decreased magnitude of peak outward currents and the bell-shaped I−V relationship depicted in b
Homotetrameric hERG1a channels deactivate slowly compared to homotetrameric hERG1b channels, whereas heteromultimeric hERG1a/1b channels deactivate at an intermediate rate that more closely matches the rate determined in native cardiomyocytes [50, 51]. Slow deactivation associated with the hERG1a subunits was proposed to be mediated by an interaction between its N-terminal PAS domain and the S4–S5 linker [104], a structure that couples voltage sensor movement to opening and closing of the activation gate [52]. Deletion of the PAS domain greatly accelerates deactivation [58, 95], and coexpression with an independent N-terminal domain (residues 1–135) can restore slow deactivation [28].
Discovery of the link between hERG1 channels and the long QT syndrome
The clinical condition known as “long QT syndrome” was initially reported in 1957 by Jervell and Lange-Nielsen [35] in children that were deaf–mute and had prolonged QT intervals, recurrent episodes of syncope, and a high incidence of sudden death. Six years later, Romano et al. [78] and Ward [108] independently described patients who also experienced syncope and sudden cardiac death but were not deaf. These initial studies indicated that at least some cases of ventricular arrhythmia associated with a prolonged QT interval were inherited. The clinical manifestations were described in detail, and treatment regimens were developed over the next 25 years, with the most significant contributions made by Moss, Schwartz, Vincent, and their colleagues [59–63, 85, 86, 89]. In 1991, molecular genetic studies began to zero in on the genetic basis responsible for congenital forms of this disorder. The initial study by Keating, Vincent, and colleagues [42] linked the LQTS phenotype of a large kindred to a small region of chromosome 11 that later was shown to contain KvLQT1 [105] (now called KCNQ1), a gene that encodes the α-subunits that coassemble to form IKs channels [7, 82]. By 1997, mutations in four different ion channel genes were linked with inherited LQTS [19, 98, 99, 105, 106]. The rapid progress in the description of the molecular basis of LQTS and other channelopathies was paralleled by the discovery of new ion channels. Low stringency screening of a human hippocampal cDNA library for transcripts homologous with eag, a K+ channel gene expressed in Drosophila and mammals [109], led to the discovery in 1994 of two new K+ channel gene subfamilies: eag-related gene (erg) and eag-like (elk). A year later, mutations in the human erg gene (HERG) were associated with LQTS in multiple families [19]; soon afterwards, it was discovered that HERG (now called KCNH2) encodes the α-subunits of the K+ channel that conducts the cardiac rapid delayed rectifier K+ current, IKr [83, 102]. In 1997, two new members of the erg gene subfamily were discovered and named erg2 and erg3 [91]; hence, the original HERG gene is now more properly referred to as HERG1. There are currently 12 different recognized forms of inherited arrhythmia that include prolonged QT interval as a clinical symptom. The common name for each “syndrome” (LQT1–LQT12) is based on the chronological order that the corresponding gene or chromosomal locus was discovered (Table 1). Long QT syndrome caused by mutations in KCNH2 was the second LQTS loci described and thus is called LQT2.
Table 1.
Congenital LQTS types, genes, and associated proteins. LQT1–LQT3 account for about 75% of “clinically robust” LQTS [101]. LQT9–LQT12 are extremely rare
| Type | Human gene (alternative name) | Protein |
|---|---|---|
| LQT1 | KCNQ1 (KVLQT1) | α-Subunit for slow delayed rectifier K channel |
| LQT2 | KCNH2 (HERG1) | α-Subunit for rapid delayed rectifier K channel |
| LQT3 | SCN5A | α-Subunit for Na channel (NaV1.5) |
| LQT4a | ANK2 | Ankyrin-B |
| LQT5 | KCNE1 | β-Subunit (“MinK”) for slow delayed rectifier K channel |
| LQT6 | KCNE2 | β-Subunit (“MiRP1”) for several K+ channels |
| LQT7a | KCNJ2 | α-Subunit for inward rectifier K channel (Kir2.1) |
| LQT8 | CACNA1C | α-Subunit for L-type Ca channel |
| LQT9a | CAV3 | Caveolin-3 |
| LQT10 | SCN4B | β-Subunit for Na channel (NaV1.5) |
| LQT11 | AKAP9 | A-Kinase anchoring protein type 9 (“Yotiao”) |
| LQT12 | SNTA1 | Alpha1-syntrophin |
These arrhythmia disorders are not always associated with prolonged QT intervals
Clinical aspects of LQT2
LQTS often presents as unexplained seizures or syncope, and clinical diagnosis is confirmed by measurement of a prolonged QT interval on the body surface electrocardiogram (ECG). The QT interval is a measure of the time required for electrical repolarization of the ventricles. In addition to a prolonged QT interval, the hallmark ECG pattern for LQT2 is a notched (bifid) T-wave [117], but this feature is not observed in all patients with a confirmed LQT2 genotype. Syncope associated with LQTS is caused by a specific ventricular tachyarrhythmia called torsade de pointes (“twisting of the points”) that is distinguished by sinusoidal twisting of the QRS axis around the isoelectric line of an ECG tracing. Torsade de pointes can either cease spontaneously or degenerate into ventricular fibrillation, a highly disorganized electrical activity that is life-threatening and is a major cause of sudden death. The incidence of congenital LQTS has not been accurately defined, but it has been estimated that as many as 1 in 2,500 people worldwide are affected [101]. Congenital LQTS is most often caused by dominant mutations in KCNH2 or KCNQ1. In the vast majority of LQTS patients, the heart is structurally normal. An exception to this generality is LQT8, where gain-of-function mutations in the cardiac L-type Ca2+ channel gene (CACNA1C) cause developmental defects in addition to a severely prolonged QT interval [96, 97].
For LQT2 patients, the most common trigger for arrhythmia appears to be emotional stress. In the most cited genotype–phenotype correlation study [88], Schwartz and colleagues reported that, in a sample of 177 symptomatic patients with LQT2, the majority of lethal or nonlethal cardiac events were associated with an increased sympathetic tone: 51% and 15% of the events were triggered by emotion and exercise, respectively. The other arrhythmia-related events (34%) occurred while the affected individuals were sleeping or resting with no obvious arousing stimulus. By contrast, the most common trigger for LQT1 (KCNQ1 mutations) is exercise (68% of events), while most events associated with LQT3 (SCN5A mutations) occurred while the patients were asleep (55% of events). A very common trigger for LQT2-associated arrhythmia is a loud and unexpected noise, such as the buzzing of an alarm clock, or the ringing of a telephone or doorbell [112]. In the ECG illustrated in Fig. 2, a subject with confirmed LQT2 (mutation: G604S) was asleep when an alarm clock triggered multiple ventricular extrasystoles followed by torsade de pointes. A recently described but uncommon trigger for LQT2 is fever [2].
Fig. 2.
ECG recording of a sleeping LQT2 subject who was startled awake by the sudden noise from an alarm clock. Note the appearance of several ventricular extrasystoles and the long–short intervals of the QRS complexes immediately before the onset of torsade de pointes. Reproduced with permission from [112]
LQTS can also be acquired, either in association with chronic heart failure or an undesirable side effect of a drug that blocks hERG1 channels or interferes with their trafficking from the ER to the cell surface [22]. Other significant risk factors for congenital or acquired LQTS are hypokalemia, bradycardia, and female gender [43]. Not surprisingly, drug-induced arrhythmia is probably far more likely in subjects with congenital LQTS, regardless of the underlying mutation, but this association has been difficult to confirm [115]. For drugs (e.g., terfenadine) that are otherwise metabolized to a hERG1-inactive metabolite, a compromised liver function due to disease or mutations in specific CYP isoforms is also an important risk factor [22]. Inhibitors of CYP3A4 (e.g., erythromycin and other macrolide antibacterial agents) can also prevent metabolism of coadministered drugs such as terfenadine and cisapride to their hERG1-inactive metabolites [22]. The consequences of drug-induced QT prolongation can be serious and closely resemble LQT2, with torsade de pointes arrhythmia, ventricular fibrillation, and sudden death [84]. Not long ago, drug-induced QT prolongation was a property actively sought as an antiarrhythmic mechanism, a so-called “class III” drug effect [93] that was later shown to be commonly mediated by blocking of hERG1 channels. Prolonged electrical refractoriness of the ventricle can prevent certain types of arrhythmia, but the proarrhythmic potential of this action is not trivial and prompted pharmaceutical companies to abandon discovery and development of drugs with this activity and to actively screen all noncardiac drugs for this unwanted side effect as a normal component of drug development.
The treatment of LQT2 is the same as for other forms of the disorder. The most common therapy is administration of beta-receptor blockers. The effectiveness of beta-blocker therapy was documented over a 5-year period in a large study with 869 LQTS patients, where syncope, aborted cardiac arrests, or death were reduced in the probands from 0.97 to 0.31 events per year, and in affected family members from 0.26 to 0.15 events per year [64]. Recently, activators of hERG1 channels have been discovered [29, 40, 119], but none of these compounds have been evaluated in a clinical trial to determine efficacy or safety. High-risk patients often receive left cardiac sympathetic denervation and/or an implantable cardioverter defibrillator. In the most comprehensive study involving 147 LQTS patients, left cardiac sympathetic denervation reduced cardiac events by ~90% [87].
Functional consequences of LQT2-associated hERG1 mutations
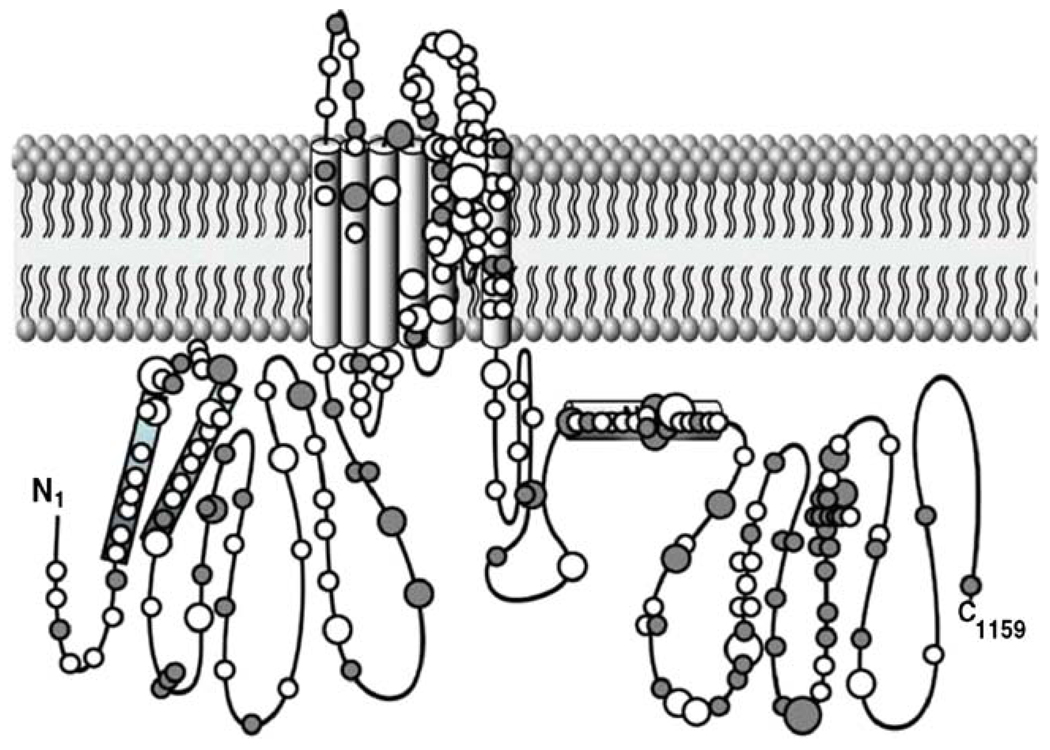
According to the on-line inherited arrhythmias database (http://www.fsm.it/cardmoc/), 291 different mutations in hERG1 were reported to cause LQT2 as of September 2009. However, this certainly underestimates the number of mutations that will eventually be discovered to cause LQT2, as evidenced by a report published in late September 2009 of an additional 159 unique KCNH2 mutations in a sample comprising 2,500 LQTS phenotype-positive individuals and 903 genotype-positive cases [41]. This increases the known LQT2-associated mutations in KCNH2 to a total of 450 and confirms that the location of the mutations in the hERG1 subunit is widespread (Fig. 3). In a sample of 226 genetically confirmed cases of LQT2, 62% were missense mutations, 24% were frameshift mutations, and the remaining 14% were nonsense, splice site, or inframe insertions or deletions in KCNH2 [41]. Only a small percentage of KCNH2 mutations have been characterized (and hence definitively verified) by a functional, in vitro electrophysiological analysis.
Fig. 3.
Diagram of a single hERG1 subunit, illustrating the widespread distribution in the location of LQT2-associated mutations. The subunit has six transmembrane segments and large intracellular N-terminal and C-terminal domains. Missense mutations are indicated by open circles; other types of mutations (i.e., frameshifts, deletions, splice site) are indicated as filled circles. The size of the symbol reflects the prevalence of a specific mutation (smallest symbol once; largest symbol >5 times). Reproduced with permission from [41]
The functional consequences of hERG1 mutations are usually determined by comparing the current magnitude and other biophysical properties of heterologously expressed mutant channels with wild-type channels using the whole-cell voltage clamp technique. In addition, channel density at the surface membrane is often assayed using Western blots and antibodies to untagged or epitope tagged subunits. hERG1 proteins are core glycosylated in the ER. The mature glycosylation pattern is achieved in the Golgi before the protein can be adequately trafficked to the plasma membrane, and the difference sizes (135 kDa for core-glycosylated versus 155 kDa for mature protein) are used to quantify defects in folding and/or trafficking [120]. Export of hERG1 proteins from the ER and trafficking to the Golgi is dependent on the small GTPase Sar1 which regulates the formation of coat-associated protein complex II vesicles used during ER export [20]. LQT2-associated mutations in hERG1 can cause loss of function by reducing channel trafficking to the plasma membrane. As first described by Zhou et al. [120], hERG1 subunits with the LQT2-associated mutations Y611H or V822M are misfolded and retained in the ER in the core-glycosylated form. ER-retained subunits are then rapidly degraded by the ubiquitin–proteasome pathway. It is now known that defective protein folding, retention in the ER, or disrupted trafficking to the Golgi and surface membrane is the primary mechanism of loss of function caused by missense mutations in hERG1 [4]. Many of these mutant channels can function, albeit with altered kinetic properties, if they can be induced to traffic to the membrane, either by reducing the incubation temperature during cell culture [69] or by pharmacological rescue with drugs that otherwise block the channel [24, 76, 116, 121]. Initial functional studies of mutant hERG1 channels were performed using heterologous expression in Xenopus oocytes incubated at low temperatures [81], leading to the erroneous conclusion that some mutations reduced current magnitude by affecting channel gating when actually the major defect was temperature-sensitive defective folding and reduced trafficking [69, 121]. Some drugs such as arsenic trioxide (As2O3) and pentamidine can also reduce hERG1 channel trafficking, specifically by disrupting the association between hERG1 channels and their chaperone proteins [23, 46]. Thus, drugs can reduce hERG1 function either by direct blocking of the channel pore or by reducing hERG1 protein trafficking to the cell membrane, prompting the development of assays for both activities [111].
Homozygous mutations in KCNH2 are very rare and, when confirmed genetically, result in intrauterine death or live birth with severe QT prolongation [33, 38]. Thus, heterozygous mutations in hERG1 are the norm for LQT2. For this reason, it is important to assay the defects caused by a specific heterozygous mutation in KCNH2 by coexpression of mutant and wild-type hERG1 subunits. This approach is useful to determine if a particular missense mutation causes a dominant-negative suppression of channel density and/or function. For example, hERG1 subunits with the pore mutation G638S act like a “poison pill” in that it freely associates with normal hERG1 subunits and destroys the function of tetrameric channels that contain even a single mutant subunit [81]. Transgenic rabbits with the G628S mutation exhibited a >50% incidence of sudden arrhythmic death [12]. Surprisingly, rather than the expected compensatory increase in a repolarizing current, the reduction in IKr in these rabbits was accompanied by a decreased IKs [12]. More disruptive mutations (e.g., frame-shifts, premature stops, or deletions that produce truncated proteins) and even some missense mutations can cause haploinsufficiency, where mutant and normal subunits do not interact and only 1/2 of the total number of hERG1 channels are likely to be dysfunctional or absent (rapidly degraded).
Although certainly not as common as folding and trafficking defects (estimated to be the mechanism for up to 90% of missense mutations), some mutations reduce IKr by altering the properties of hERG1 channel gating. Mutations that enhance inactivation (e.g., G584S) [118] or accelerate the rate of deactivation (e.g., M124R or other mutations in the PAS domain) [92] reduce the outward current contributed by IKr during repolarization of the action potential. A nonsense mutation (e.g., R1014X) causes nonsense-mediated mRNA decay rather than a production of truncated protein [25].
In 2002, Moss and colleagues [65] tested the hypothesis that the location of specific mutations in the hERG channel protein might correlate with severity of disease. In 201 subjects with 44 different mutations, 35 subjects had mutations that were located in the pore region. Subjects with pore mutations (14 in total) had more severe clinical symptoms and experienced a two times higher frequency of arrhythmia-related cardiac events than did subjects with nonpore mutations. Although not confirmed with electro-physiological analyses, the pore mutants are more likely to cause a strong dominant-negative effect, either increasing the rate of degradation of multimeric subunit assemblies in the ER/Golgi or by preventing ion conductance if the channels are successfully exported from the Golgi to the plasma membrane.
Disorders other than LQTS associated with hERG1 channels
Short QT syndrome
Gain-of-function mutations in cardiac K+ channel genes, including KCNQ1 [32] and KCNJ2 [74], can result in QT interval phenotype opposite to that of LQTS. Repolarization of cardiomyocytes is accelerated and is manifested as a short QT interval on the body surface ECG. To date, one missense mutation in hERG1 (N588K) has been associated with short QT syndrome [11]. Asn588 is located in the S5-pore linker of the hERG1 subunit, and its mutation to Lys causes a positive shift in the voltage dependence of P-type inactivation. This shift reduces the extent of inactivation, and the resulting increase in outward current during the plateau phase of the action potential hastens repolarization of the ventricle and shortens the QT interval. The finding that gain-of-function mutations in hERG1 or other K+ channels can cause lethal arrhythmia suggests that drugs or gene therapies designed to enhance repolarizing currents as a treatment for LQTS will have to be carefully tailored to prevent excess activity of the intervention.
Epilepsy
LQTS is frequently misdiagnosed as a seizure disorder or epilepsy, and patients are therefore often treated with antiepileptic drugs. As noted above, KCNH2 (HERG1) was initially discovered in the hippocampus [109], and it was later demonstrated that this gene is expressed in many regions of the central nervous system. Although it might seem intuitively obvious that mutations in KCNH2 might cause CNS disorders, it was not until very recently that a clear link was made between hERG1 mutations and epilepsy. A recent case history [67] provides what may become a more common finding in the near future. The EEG of a 60-year-old man with a 40-year history of epilepsy (tonic–clonic seizure and syncope) demonstrated paroxysmal slow waves in response to intermittent photic stimulation. His epilepsy was treated with clonazepam and zonisamide, but he suffered three bouts of seizure during times that he was off this medication. After his most recent seizure episode, he underwent a cardiac evaluation. During ECG monitoring, he suddenly developed tonic–clonic seizures that was preceded by bradycardia and QT prolongation and that coincided with torsades de pointes arrhythmia. Subsequent genetic testing revealed a missense mutation (R534C) in KCNH2 and a family history of sudden cardiac death. He has since been successfully treated with a beta-blocker and an implantable cardioverter defibrillator [67]. This example is not an isolated case. Johnson et al. [37] recently quantified the coexistence of LQTS and seizure activity in a well-defined cohort. In 343 unrelated LQTS probands, a history of seizures was more common in LQT2 (30 of 77, 39%) than all other subtypes of LQTS combined (11 of 106, 10%). Thus, an initial diagnosis of epilepsy in a patient experiencing syncope and seizure and subsequent prophylactic treatment with an antiepileptic drug (AED) may be inadequate—ECG monitoring could reveal the more complex clinical problem of epilepsy plus LQTS, requiring coadministration of an AED and a beta-blocker.
Based on the widespread distribution of hERG1 in the nervous system, it is somewhat surprising that mutations in KCNH2 have so rarely been associated with epilepsy and not to any other neurological disorder. Disease-causing mutations in hERG2 and hERG3 have also not been described. In rodents at least, erg2 and erg3 proteins are exclusively expressed in the nervous system [91], albeit sometimes in association with erg1 [27]. All three types of erg proteins can coassemble to form heteromultimeric channels in CHO cells [113, 114]. Moreover, single-cell reverse transcription polymerase chain reaction has revealed that all three erg channel types are expressed in rat embryonic rhombencephalon neurons, and voltage clamp studies suggest that currents are conducted by heteromultimeric channels [30]. It is likely that additional neuropathies may eventually be attributed to dysfunction of hERG1 as well as hERG2 and hERG3 channels.
Up-regulation of hERG1 in cancer and atrophic skeletal muscle
Enhanced expression of hEAG and hERG1 channels has been detected in several human cancer cell lines [6, 10], including neuroblastomas [56], rhabdomyosarcomas [94], monoblastic leukemias, mammary carcinomas, and colon carcinoma [17]. hERG1 is also expressed in lymphocytes [94] and primary tumors of several tissues including the endometrium [16] and colon [48]. The clinical significance of these findings are uncertain and represents a typical chicken or egg conundrum; which came first, enhanced channel expression or altered cell cycle progression? Nonetheless, pharmacological evidence suggests that up-regulated eag/erg channel expression may be important. Specific channel blockers can reduce cell proliferation [17], and the channels are involved in regulation of tumor cell migration [48] and adhesion-dependent signaling [15, 31].
Erg channel expression is also altered during muscle atrophy. Erg1a channels in mice are up-regulated in skeletal muscle 6 weeks after injection with Kb human esophageal cancer cells to induce muscle atrophy [107]. In the same study, erg1a was also up-regulated in the skeletal muscle of non-weight-bearing hind limbs of mice. Moreover, treatment of mice with astemizole (a potent hERG1 channel blocker) prevented muscle atrophy. Erg1a (but not erg1b) participates in the initiation of muscle atrophy in these mice by activating ubiquitin–proteasome proteolysis, but the molecular pathways involved in this regulation are not understood [107].
Developmental disorders
A specific LQT2-associated missense mutation in hERG1 (N629D) was originally discovered as a heterozygous mutation in a subject with typical LQT2. Heterologous expression revealed that the mutation alters ionic selectivity of the hERG1 channel [49], but the main dysfunction is reduced trafficking to the cell membrane [4]. Functional consequences of the N629D mutation other than LQTS have been investigated in transgenic mice. Homozygous N629D mutations cause cessation of spontaneous pacemaker activity and embryolethality at E11.5 [100]. Several developmental defects were also noted in these mice, including altered looping architecture of the heart, poorly developed bulbus cordis, distorted branchial arches, and enhanced apoptosis in the first branchial arch and cardiac outflow tract. It is unclear how the absence of functional hERG1 currents cause these developmental defects or if these changes are relevant to the embryolethality associated with other homozygous KCNH2 mutations.
Summary
Although hERG1 channels are highly expressed in several tissues, the pathology associated with congenital mutations in KCNH2 appear to be largely limited to the heart. Mutations in KCNH2 are a common cause of LQTS. Most mutations cause loss of channel function by causing the subunits to misfold and/or decrease their trafficking to the plasma membrane. The role of hERG1 channels in epilepsy, cancer, and skeletal muscle atrophy are poorly understood but deserve further inquiry.
Acknowledgments
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant HL055236.
References
- 1.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 2.Amin AS, Herfst LJ, Delisle BP, Klemens CA, Rook MB, Bezzina CR, Underkofler HA, Holzem KM, Ruijter JM, Tan HL, January CT, Wilde AA. Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118:2552–2561. doi: 10.1172/JCI35337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anantharam A, Lewis A, Panaghie G, Gordon E, McCrossan ZA, Lerner DJ, Abbott GW. RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+ channel function in oocyte expression studies. J Biol Chem. 2003;278:11739–11745. doi: 10.1074/jbc.M212751200. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 5.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–232. discussion 232-224. [PubMed] [Google Scholar]
- 6.Arcangeli A, Rosati B, Crociani O, Cherubini A, Fontana L, Passani B, Wanke E, Olivotto M. Modulation of HERG current and herg gene expression during retinoic acid treatment of human neuroblastoma cells: potentiating effects of BDNF. J Neurobiol. 1999;40:214–225. [PubMed] [Google Scholar]
- 7.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium channel. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 8.Bauer CK, Wulfsen I, Schafer R, Glassmeier G, Wimmers S, Flitsch J, Ludecke DK, Schwarz JR. HERG K+ currents in human prolactin-secreting adenoma cells. Pflugers Arch. 2003;445:589–600. doi: 10.1007/s00424-002-0980-0. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi L, Shen Z, Dennis AT, Priori SG, Napolitano C, Ronchetti E, Bryskin R, Schwartz PJ, Brown AM. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum Mol Genet. 1999;8:1499–1507. doi: 10.1093/hmg/8.8.1499. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells. Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- 11.Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 12.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayabyab FS, Schlichter LC. Regulation of an ERG K+ current by Src tyrosine kinase. J Biol Chem. 2002;277:13673–13681. doi: 10.1074/jbc.M108211200. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zou A, Splawski I, Keating MT, Sanguinetti MC. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- 15.Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, Di Stefano P, Degani S, Balzi M, Olivotto M, Wanke E, Becchetti A, Defilippi P, Wymore R, Arcangeli A. Human ether-a-go-go-related gene 1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signaling. Mol Biol Cell. 2005;16:2972–2983. doi: 10.1091/mbc.E04-10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L, Noci I, Borri P, Borrani E, Giachi M, Becchetti A, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer. 2000;83:1722–1729. doi: 10.1054/bjoc.2000.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, Wymore RS, Arcangeli A. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem. 2003;278:2947–2955. doi: 10.1074/jbc.M210789200. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Melman Y, Palma E, Fishman GI, McDonald TV. Cyclic AMP regulates the HERG K+ channel by dual pathways. Curr Biol. 2000;10:671–674. doi: 10.1016/s0960-9822(00)00516-9. [DOI] [PubMed] [Google Scholar]
- 19.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 20.Delisle BP, Underkofler HA, Moungey BM, Slind JK, Kilby JA, Best JM, Foell JD, Balijepalli RC, Kamp TJ, January CT. Small GTPase determinants for the Golgi processing and plasmalemmal expression of human ether-a-go-go related (hERG) K+ channels. J Biol Chem. 2009;284:2844–2853. doi: 10.1074/jbc.M807289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrelly AM, Ro S, Callaghan BP, Khoyi MA, Fleming N, Horowitz B, Sanders KM, Keef KD. Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol. 2003;284:G883–G895. doi: 10.1152/ajpgi.00394.2002. [DOI] [PubMed] [Google Scholar]
- 22.Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–495. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- 25.Gong Q, Zhang L, Vincent GM, Horne BD, Zhou Z. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation. 2007;116:17–24. doi: 10.1161/CIRCULATIONAHA.107.708818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon E, Panaghie G, Deng L, Bee KJ, Roepke TK, Krogh-Madsen T, Christini DJ, Ostrer H, Basson CT, Chung W, Abbott GW. A KCNE2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovasc Res. 2008;77:98–106. doi: 10.1093/cvr/cvm030. [DOI] [PubMed] [Google Scholar]
- 27.Guasti L, Cilia E, Crociani O, Hofmann G, Polvani S, Becchetti A, Wanke E, Tempia F, Arcangeli A. Expression pattern of the ether-a-go-go-related (ERG) family proteins in the adult mouse central nervous system: evidence for coassembly of different subunits. J Comp Neurol. 2005;491:157–174. doi: 10.1002/cne.20721. [DOI] [PubMed] [Google Scholar]
- 28.Gustina AS, Trudeau MC. A recombinant N-terminal domain fully restores deactivation gating in N-truncated and long QT syndrome mutant hERG potassium channels. Proc Natl Acad Sci U S A. 2009;106:13082–13087. doi: 10.1073/pnas.0900180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen S-P, Grunnet M. Activation of hERG potassium channels by the diphenylurea NS1643. Mol Pharmacol. 2005;69:266–277. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 30.Hirdes W, Schweizer M, Schuricht KS, Guddat SS, Wulfsen I, Bauer CK, Schwarz JR. Fast erg K+ currents in rat embryonic serotonergic neurones. J Physiol. 2005;564:33–49. doi: 10.1113/jphysiol.2004.082123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, Lastraioli E, Polvani S, Bartolozzi B, Solazzo V, Gragnani L, Defilippi P, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG K+ channels activation during beta (1) integrin-mediated adhesion to fibronectin induces an upregulation of alpha(v)beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem. 2001;276:4923–4931. doi: 10.1074/jbc.M005682200. [DOI] [PubMed] [Google Scholar]
- 32.Hong K, Piper DR, Diaz-Valdecantos A, Brugada J, Oliva A, Burashnikov E, Santos-de-Soto J, Grueso-Montero J, Diaz-Enfante E, Brugada P, Sachse F, Sanguinetti MC, Brugada R. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Hoorntje T, Alders M, van Tintelen P, van der Lip K, Sreeram N, van der Wal A, Mannens M, Wilde A. Homozygous premature truncation of the HERG protein: the human HERG knockout. Circulation. 1999;100:1264–1267. doi: 10.1161/01.cir.100.12.1264. [DOI] [PubMed] [Google Scholar]
- 34.Isbrandt D, Friederich P, Solth A, Haverkamp W, Ebneth A, Borggrefe M, Funke H, Sauter K, Breithardt G, Pongs O, Schulze-Bahr E. Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J Mol Med. 2002;80:524–532. doi: 10.1007/s00109-002-0364-0. [DOI] [PubMed] [Google Scholar]
- 35.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q–T interval and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 36.Jiang M, Xu X, Wang Y, Toyoda F, Liu XS, Zhang M, Robinson RB, Tseng GN. Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. J Biol Chem. 2009;284:16452–16462. doi: 10.1074/jbc.M808262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson WH, Jr, Yang P, Yang T, Lau YR, Mostella BA, Wolff DJ, Roden DM, Benson DW. Clinical, genetic, and biophysical characterization of a homozygous HERG mutation causing severe neonatal long QT syndrome. Pediatr Res. 2003;53:744–748. doi: 10.1203/01.PDR.0000059750.17002.B6. [DOI] [PubMed] [Google Scholar]
- 39.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004;279:44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Chen XL, Wang H, Ji J, Cheng H, Incardona J, Reynolds W, Viviani F, Tabart M, Rampe D. Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol. 2005;67:827–836. doi: 10.1124/mol.104.006577. [DOI] [PubMed] [Google Scholar]
- 41.Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2, 500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keating M, Atkinson D, Dunn C, Timothy K, Vincent GM, Leppert M. Linkage of a cardiac arrhythmia, the long QT syndrome, and the Harvey ras-1 gene. Science. 1991;252:704–706. doi: 10.1126/science.1673802. [DOI] [PubMed] [Google Scholar]
- 43.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 44.Kiehn J, Lacerda A, Wible B, Brown AM. Molecular physiology and pharmacology of HERG: single-channel currents and block by dofetilide. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- 45.Kiss L, Korn SJ. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys J. 1998;74:1840–1849. doi: 10.1016/S0006-3495(98)77894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang J, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 47.Lagrutta AA, Trepakova ES, Salata JJ. The hERG channel and risk of drug-acquired cardiac arrhythmia: an overview. Curr Top Med Chem. 2008;8:1102–1112. doi: 10.2174/156802608785700016. [DOI] [PubMed] [Google Scholar]
- 48.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, Bencini L, Calistri M, Messerini L, Scatizzi M, Moretti R, Wanke E, Olivotto M, Mugnai G, Arcangeli A. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- 49.Lees-Miller JP, Duan Y, Teng GQ, Thorstad K, Duff HJ. Novel gain-of-function mechanism in K(+) channel-related long-QT syndrome: altered gating and selectivity in the HERG1 N629D mutant. Circ Res. 2000;86:507–513. doi: 10.1161/01.res.86.5.507. [DOI] [PubMed] [Google Scholar]
- 50.Lees-Miller JP, Kondo C, Wang L, Duff HJ. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ Res. 1997;81:719–726. doi: 10.1161/01.res.81.5.719. [DOI] [PubMed] [Google Scholar]
- 51.London B, Trudeau MC, Newton KP, Beyer AK, Copeland NG, Gilbert DJ, Jenkins NA, Satler CA, Robertson GA. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1997;81:870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- 52.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Mahaut-Smith MP, Huang CL, Vandenberg JI. Mutant MiRP1 subunits modulate HERG K+ channel gating: a mechanism for pro-arrhythmia in long QT syndrome type 6. J Physiol. 2003;551:253–262. doi: 10.1113/jphysiol.2003.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazhari R, Greenstein JL, Winslow RL, Marban E, Nuss HB. Molecular interactions between two long-QT syndrome gene products, HERG and KCNE2, rationalized by in vitro and in silico analysis. Circ Res. 2001;89:33–38. doi: 10.1161/hh1301.093633. [DOI] [PubMed] [Google Scholar]
- 55.McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang K-W, Goldstein SAN, Fishman GI. A minK-HERG complex regulates the cardiac potassium current IKr. Nature. 1997;388:289–292. doi: 10.1038/40882. [DOI] [PubMed] [Google Scholar]
- 56.Meves H, Schwarz JR, Wulfsen I. Separation of M-like current and ERG current in NG108-15 cells. Br J Pharmacol. 1999;127:1213–1223. doi: 10.1038/sj.bjp.0702642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitcheson JS, Chen J, Sanguinetti MC. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J Gen Physiol. 2000;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morais Cabral JH, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 59.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 60.Moss AJ, McDonald J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N Engl J Med. 1971;285:903–904. doi: 10.1056/NEJM197110142851607. [DOI] [PubMed] [Google Scholar]
- 61.Moss AJ, Schwartz PJ, Crampton RS, Locati E, Carleen E. The long QT syndrome: a prospective international study. Circulation. 1985;71:17–21. doi: 10.1161/01.cir.71.1.17. [DOI] [PubMed] [Google Scholar]
- 62.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Jr, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 63.Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, Schwartz PJ, Towbin JA, Vincent GM, Lehmann MH, Keating MT, MacCluer JW, Timothy KW. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation. 1995;92:2929–2934. doi: 10.1161/01.cir.92.10.2929. [DOI] [PubMed] [Google Scholar]
- 64.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 65.Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori SG, Schwartz PJ, Vincent GM, Robinson JL, Andrews ML, Feng C, Hall WJ, Medina A, Zhang L, Wang Z. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 66.Nie L, Gratton MA, Mu KJ, Dinglasan JN, Feng W, Yamoah EN. Expression and functional phenotype of mouse ERG K+ channels in the inner ear: potential role in K+ regulation in the inner ear. J Neurosci. 2005;25:8671–8679. doi: 10.1523/JNEUROSCI.1422-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Omichi C, Momose Y, Kitahara S. Congenital long QT syndrome presenting with a history of epilepsy: Misdiagnosis or relationship between channelopathies of the heart and brain? Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02267.x. [DOI] [PubMed] [Google Scholar]
- 68.Overholt JL, Ficker E, Yang T, Shams H, Bright GR, Prabhakar NR. HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J Neurophysiol. 2000;83:1150–1157. doi: 10.1152/jn.2000.83.3.1150. [DOI] [PubMed] [Google Scholar]
- 69.Paulussen A, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem. 2002;277:48610–48616. doi: 10.1074/jbc.M206569200. [DOI] [PubMed] [Google Scholar]
- 70.Perrin MJ, Subbiah RN, Vandenberg JI, Hill AP. Human ether-a-go-go related gene (hERG) K+ channels: function and dysfunction. Prog Biophys Mol Biol. 2008;98:137–148. doi: 10.1016/j.pbiomolbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Phartiyal P, Jones EM, Robertson GA. Heteromeric assembly of human ether-a-go-go-related gene (hERG) 1a/1b channels occurs cotranslationally via N-terminal interactions. J Biol Chem. 2007;282:9874–9882. doi: 10.1074/jbc.M610875200. [DOI] [PubMed] [Google Scholar]
- 72.Phartiyal P, Sale H, Jones EM, Robertson GA. Endoplasmic reticulum retention and rescue by heteromeric assembly regulate human ERG 1a/1b surface channel composition. J Biol Chem. 2008;283:3702–3707. doi: 10.1074/jbc.M708999200. [DOI] [PubMed] [Google Scholar]
- 73.Pourrier M, Zicha S, Ehrlich J, Han W, Nattel S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ Res. 2003;93:189–191. doi: 10.1161/01.RES.0000084851.60947.B5. [DOI] [PubMed] [Google Scholar]
- 74.Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 75.Recanatini M, Cavalli A, Masetti M. Modeling HERG and its interactions with drugs: recent advances in light of current potassium channel simulations. ChemMedChem. 2008;3:523–535. doi: 10.1002/cmdc.200700264. [DOI] [PubMed] [Google Scholar]
- 76.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb Exp Pharmacol. 2006;171:349–355. doi: 10.1007/3-540-29715-4_14. [DOI] [PubMed] [Google Scholar]
- 77.Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, Fine E, Lerner DJ, Carrasco N, Abbott GW. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med. 2009;15:1186–1194. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romano C, Gemme G, Pongiglione R. Aritmie cardiache rare dell'eta pediatrica. Clin Pediatr (Bologna) 1963;45:656–683. [PubMed] [Google Scholar]
- 79.Rosati B, Marchetti P, Crociani O, Lecchi M, Lupi R, Arcangeli A, Olivotto M, Wanke E. Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: the role of HERG K+ channels in firing and release. Faseb J. 2000;14:2601–2610. doi: 10.1096/fj.00-0077com. [DOI] [PubMed] [Google Scholar]
- 80.Sale H, Wang J, O'Hara TJ, Tester DJ, Phartiyal P, He JQ, Rudy Y, Ackerman MJ, Robertson GA. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with long-QT syndrome. Circ Res. 2008;103:e81–e95. doi: 10.1161/CIRCRESAHA.108.185249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+ channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 83.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 84.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz P. Idiopathic long QT syndrome: progress and questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84:503–511. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz PJ, Zaza A, Locati E, Moss AJ. Stress and sudden death. The case of the long QT syndrome. Circulation. 1991;83:II71–II80. [PubMed] [Google Scholar]
- 90.Schwarz JR, Bauer CK. Functions of erg K+ channels in excitable cells. J Cell Mol Med. 2004;8:22–30. doi: 10.1111/j.1582-4934.2004.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi W, Wymore RS, Wang H-S, Pan Z, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shushi L, Kerem B, Goldmit M, Peretz A, Attali B, Medina A, Towbin JA, Kurokawa J, Kass RS, Benhorin J. Clinical, genetic, and electrophysiologic characteristics of a new PAS-domain HERG mutation (M124R) causing long QT syndrome. Ann Noninvasive Electrocardiol. 2005;10:334–341. doi: 10.1111/j.1542-474X.2005.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh BN, Ahmed R. Class III antiarrhythmic drugs. Curr Opin Cardiol. 1994;9:12–22. doi: 10.1097/00001573-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 94.Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528–18534. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- 95.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Splawski I, Timothy KW, Vincent GM, Atkinson DL, Keating MT. Molecular basis of the long-QT syndrome associated with deafness. N Engl J Med. 1997;336:1562–1567. doi: 10.1056/NEJM199705293362204. [DOI] [PubMed] [Google Scholar]
- 99.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 100.Teng GQ, Zhao X, Lees-Miller JP, Quinn FR, Li P, Rancourt DE, London B, Cross JC, Duff HJ. Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality. Circ Res. 2008;103:1483–1491. doi: 10.1161/CIRCRESAHA.108.177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tester DJ, Ackerman MJ. Cardiomyopathic and channelopathic causes of sudden unexplained death in infants and children. Annu Rev Med. 2009;60:69–84. doi: 10.1146/annurev.med.60.052907.103838. [DOI] [PubMed] [Google Scholar]
- 102.Trudeau M, Warmke JW, Ganetzky B, Robertson GA. HERG, A human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Trudeau MC, Zappia AM, Robertson GA. The mechanism of N-terminal regulation of deactivation in HERG potassium channels. Biophys J. 1998;74:A254. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Trudeau MC, Zappia AM, Robertson GA. Regulation of deactivation by an amino terminal domain in Human Ether-a-go-go-related Gene potassium channels. J Gen Physiol. 1998;112:637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Towbin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 106.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Hockerman GH, Green HW, III, Babbs CF, Mohammad SI, Gerrard D, Latour MA, London B, Hannon KM, Pond AL. Merg1a K+ channel induces skeletal muscle atrophy by activating the ubiquitin proteasome pathway. Faseb J. 2006;20:1531–1533. doi: 10.1096/fj.05-5350fje. [DOI] [PubMed] [Google Scholar]
- 108.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc. 1964;54:103–106. [PubMed] [Google Scholar]
- 109.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weerapura M, Nattel S, Chartier D, Caballero R, Hebert TE. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link? J Physiol. 2002;540:15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wible BA, Hawryluk P, Ficker E, Kuryshev YA, Kirsch G, Brown AM. HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol Methods. 2005;52:136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Wilde AA, Jongbloed RJ, Doevendans PA, Duren DR, Hauer RN, van Langen IM, van Tintelen JP, Smeets HJ, Meyer H, Geelen JL. Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1) J Am Coll Cardiol. 1999;33:327–332. doi: 10.1016/s0735-1097(98)00578-6. [DOI] [PubMed] [Google Scholar]
- 113.Wimmers S, Bauer CK, Schwarz JR. Biophysical properties of heteromultimeric erg K+ channels. Pflugers Arch. 2002;445:423–430. doi: 10.1007/s00424-002-0936-4. [DOI] [PubMed] [Google Scholar]
- 114.Wimmers S, Wulfsen I, Bauer CK, Schwarz JR. Erg1, erg2 and erg3 K channel subunits are able to form heteromultimers. Pflugers Arch. 2001;441:450–455. doi: 10.1007/s004240000467. [DOI] [PubMed] [Google Scholar]
- 115.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, Hohnloser SH, Shimizu W, Schwartz PJ, Stanton M, Murray KT, Norris K, George AL, Jr, Roden DM. Allelic variants in long-QT disease genes in patients with drug- associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 116.Yao Y, Teng S, Li N, Zhang Y, Boyden PA, Pu J. Aminoglycoside antibiotics restore functional expression of truncated HERG channels produced by nonsense mutations. Heart Rhythm. 2009;6:553–560. doi: 10.1016/j.hrthm.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 117.Zhang L, Timothy KW, Vincent GM, Lehmann MH, Fox J, Giuli LC, Shen J, Splawski I, Priori SG, Compton SJ, Yanowitz F, Benhorin J, Moss AJ, Schwartz PJ, Robinson JL, Wang Q, Zareba W, Keating MT, Towbin JA, Napolitano C, Medina A. Spectrum of ST-T-wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation. 2000;102:2849–2855. doi: 10.1161/01.cir.102.23.2849. [DOI] [PubMed] [Google Scholar]
- 118.Zhao JT, Hill AP, Varghese A, Cooper AA, Swan H, Laitinen-Forsblom PJ, Rees MI, Skinner JR, Campbell TJ, Vandenberg JI. Not All hERG pore domain mutations have a severe phenotype: G584S has an inactivation gating defect with mild phenotype compared to G572S, which has a dominant negative trafficking defect and a severe phenotype. J Cardiovasc Electrophysiol. 2009;20:923–930. doi: 10.1111/j.1540-8167.2009.01468.x. [DOI] [PubMed] [Google Scholar]
- 119.Zhou J, Augelli-Szafran CE, Bradley JA, Chen X, Koci BJ, Volberg WA, Sun Z, Cordes JS. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol Pharmacol. 2005;68:876–884. doi: 10.1124/mol.105.014035. [DOI] [PubMed] [Google Scholar]
- 120.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem. 1998;273:21061–21066. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 121.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 122.Zou A, Curran ME, Keating MT, Sanguinetti MC. Single HERG delayed rectifier K+ channels in Xenopus oocytes. Am J Physiol. 1997;272:H1309–H1314. doi: 10.1152/ajpheart.1997.272.3.H1309. [DOI] [PubMed] [Google Scholar]