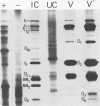
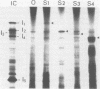
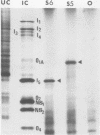
Abstract
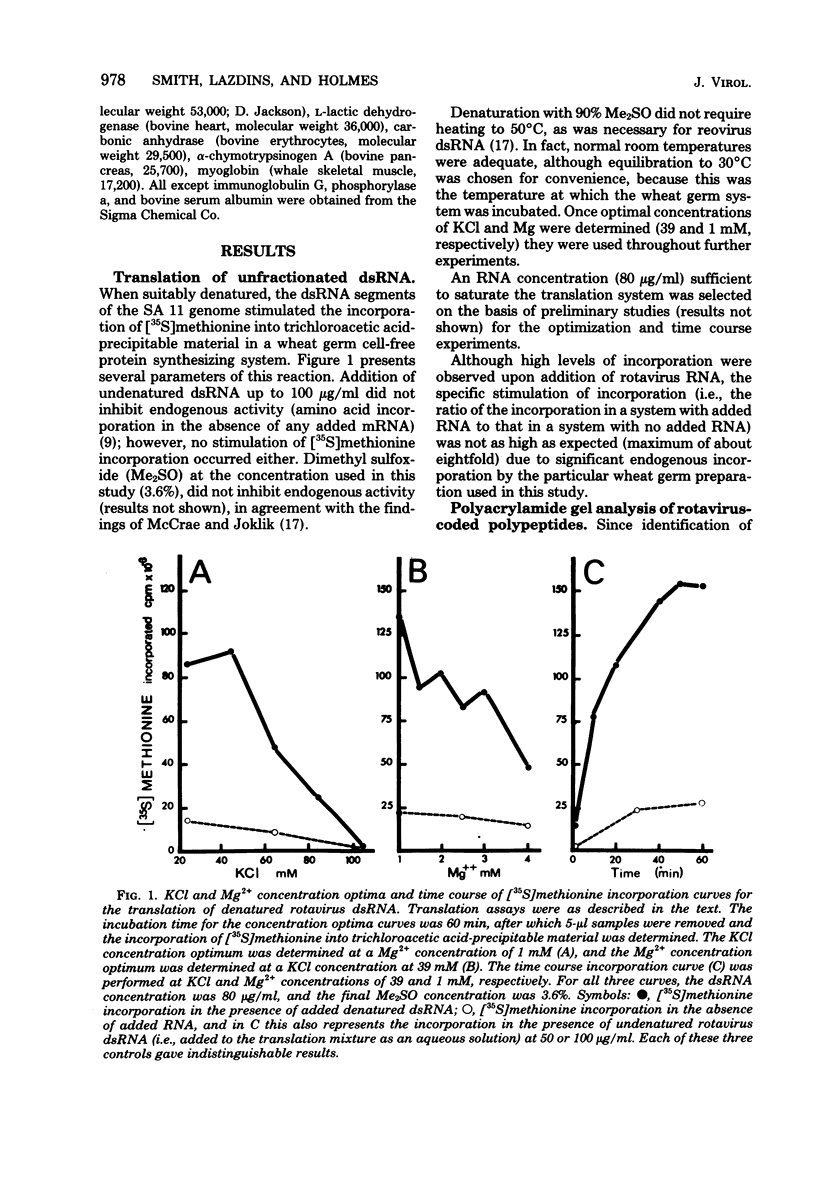
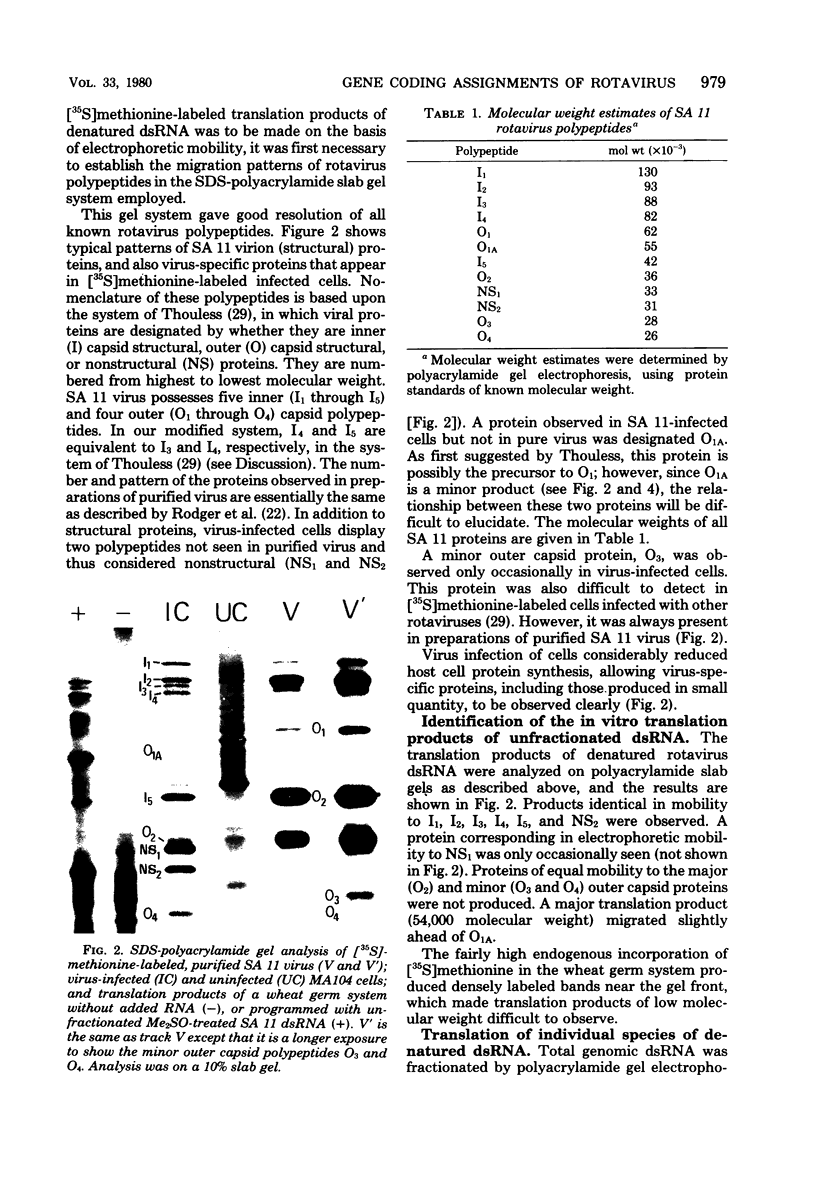
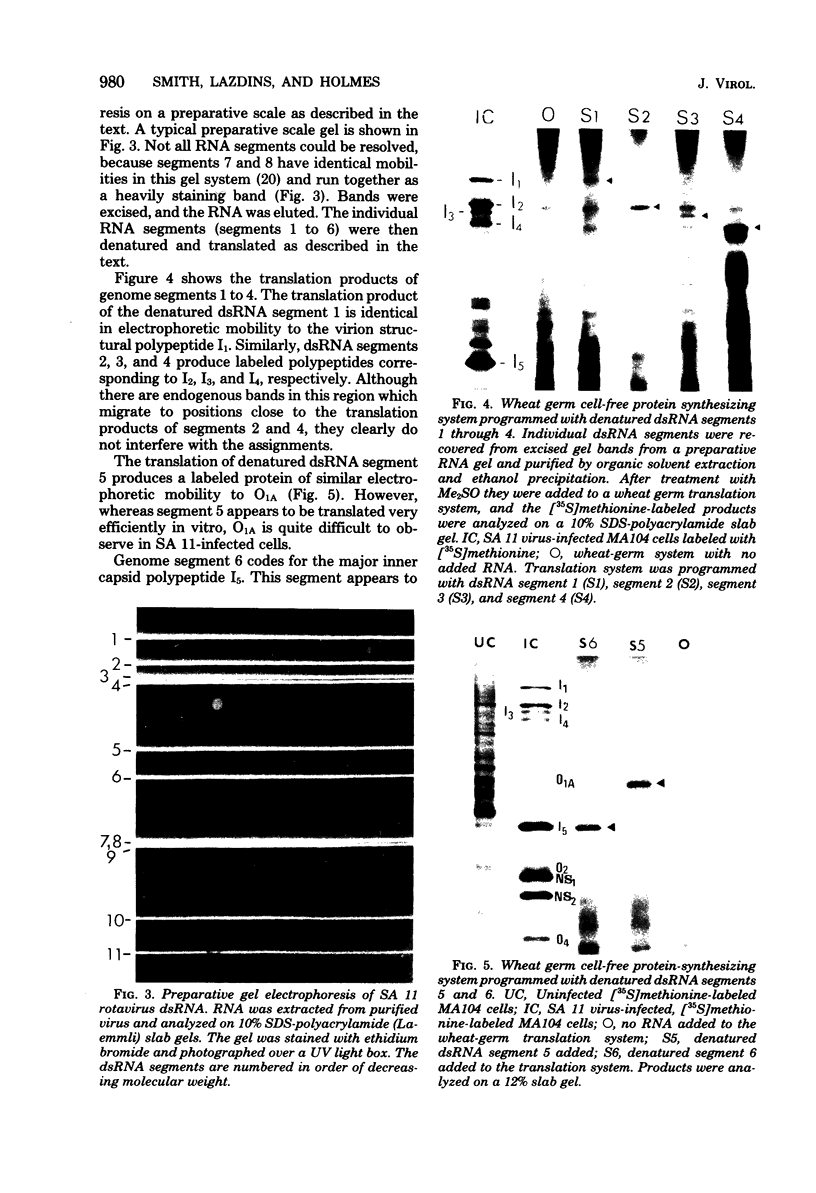
The segmented double-stranded (ds) RNA genome of the simian rotavirus SA 11, after denaturation, can be translated in a cell-free protein synthesizing system. Of the 11 genome segments, 9 can be resolved on polyacrylamide gels and thus could be individually isolated and translated, providing a means of identifying the polypeptide encoded by each segment. On the basis of electrophoretic mobility of products in sodium dodecyl sulfate-polyacrylamide gels, the probable gene-coding assignments of dsRNA segments 1 to 6 were determined. RNA segments 1 to 4 code for polypeptides I1, I2, I3, and I4, respectively; segment 5 codes for a polypeptide very similar in mobility to a minor polypeptide present in SA 11-infected cells, O1A; and segment 6 codes for the major inner-capsid polypeptide I5.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Dobos P. Cell free transcription and translation of rotavirus RNA. Biochem Biophys Res Commun. 1979 Jun 13;88(3):791–796. doi: 10.1016/0006-291x(79)91477-3. [DOI] [PubMed] [Google Scholar]
- Elder K. T., Bye J. M., Skehel J. J., Waterfield M. D., Smith A. E. In vitro synthesis, glycosylation, and membrane insertion of influenza virus haemagglutinin. Virology. 1979 Jun;95(2):343–350. doi: 10.1016/0042-6822(79)90489-6. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Calderón E., González N., Salomon A., Martuscelli A., Romero P. Presence of two distinct types of rotavirus in infants and young children hospitalized with acute gastroenteritis in Mexico City, 1977. J Infect Dis. 1979 Apr;139(4):474–477. doi: 10.1093/infdis/139.4.474. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill L. K., Sun J. D., Kandel J. Effect of double stranded RNA on protein synthesis in an in vitro wheat germ embryo system. Biochem Biophys Res Commun. 1976 Nov 8;73(1):149–156. doi: 10.1016/0006-291x(76)90509-x. [DOI] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnagl R. D., Holmes I. H. Characteristics of the genome of human infantile enteritis virus (Rotavirus). J Virol. 1976 Jul;19(1):267–270. doi: 10.1128/jvi.19.1.267-270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoub B. D., Lecatsas G., Prozesky O. W. Antigenic relationship between human and simian rotaviruses. J Med Microbiol. 1977 Feb;10(1):1–6. doi: 10.1099/00222615-10-1-1. [DOI] [PubMed] [Google Scholar]
- Schuerch A. R., Mitchell W. R., Joklik W. K. Isolation of intact individual species of single- and double-stranded RNA after fractionation by polyacrylamide gel electrophoresis. Anal Biochem. 1975 May 12;65(1-2):331–345. doi: 10.1016/0003-2697(75)90517-5. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. doi: 10.1073/pnas.54.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H. Serotypes of human rotavirus. Lancet. 1978 Jan 7;1(8054):39–39. doi: 10.1016/s0140-6736(78)90381-1. [DOI] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies on a reovirus-like agent (rotovirus) from lambs. J Virol. 1977 Mar;21(3):1215–1218. doi: 10.1128/jvi.21.3.1215-1218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Characterization of pig rotavirus RNA. J Gen Virol. 1976 Oct;33(1):147–150. doi: 10.1099/0022-1317-33-1-147. [DOI] [PubMed] [Google Scholar]
- Verly E., Cohen J. Demonstration of size variation of RNA segments between different isolates of calf rotavirus. J Gen Virol. 1977 Jun;35(3):583–586. doi: 10.1099/0022-1317-35-3-583. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]