Abstract
Transmembrane TNF-α, a precursor of the soluble form of TNF-α, is expressed on activated macrophages and lymphocytes as well as other cell types. After processing by TNF-α-converting enzyme (TACE), the soluble form of TNF-α is cleaved from transmembrane TNF-α and mediates its biological activities through binding to Types 1 and 2 TNF receptors (TNF-R1 and -R2) of remote tissues. Accumulating evidence suggests that not only soluble TNF-α, but also transmembrane TNF-α is involved in the inflammatory response. Transmembrane TNF-α acts as a bipolar molecule that transmits signals both as a ligand and as a receptor in a cell-to-cell contact fashion. Transmembrane TNF-α on TNF-α-producing cells binds to TNF-R1 and -R2, and transmits signals to the target cells as a ligand, whereas transmembrane TNF-α also acts as a receptor that transmits outside-to-inside (reverse) signals back to the cells after binding to its native receptors. Anti-TNF agents infliximab, adalimumab and etanercept bind to and neutralize soluble TNF-α, but exert different effects on transmembrane TNF-α-expressing cells (TNF-α-producing cells). In the clinical settings, these three anti-TNF agents are equally effective for RA, but etanercept is not effective for granulomatous diseases. Moreover, infliximab induces granulomatous infections more frequently than etanercept. Considering the important role of transmembrane TNF-α in granulomatous inflammation, reviewing the biology of transmembrane TNF-α and its interaction with anti-TNF agents will contribute to understanding the bases of differential clinical efficacy of these promising treatment modalities.
Keywords: Cytokine, Tumour necrosis factor-α, TNF, Outside-to-inside signal, Transmembrane, Infliximab, Etanercept, Adalimumab
Introduction
TNF-α is a potent pro-inflammatory cytokine exerting pleiotropic effects on various cell types and plays a critical role in the pathogenesis of chronic inflammatory diseases, such as RA [1, 2]. Accumulating evidence suggests that not only soluble TNF-α, but also its precursor form, transmembrane TNF-α, is involved in the inflammatory response. Transmembrane TNF-α exerts its biological function in a cell-to-cell contact fashion, which is distinct from the feature of soluble TNF-α, which acts at sites remote from the TNF-α-producing cells [3]. In transgenic mice, transmembrane TNF-α was shown to be sufficient to induce arthritis with synovial hyperplasia and inflammation [4, 5].
Transmembrane TNF-α acts as a ligand by binding to TNF-α receptors as well as functioning as a receptor that transmits outside-to-inside (reverse) signals back into the transmembrane TNF-α-bearing cells (TNF-α-producing cells) [6]. It is therefore considered that transmembrane TNF-α plays a critical role in local inflammation [7–10]. Anti-TNF agents have been successfully introduced for the treatment of chronic inflammatory diseases. However, clinical features against granulomatous inflammation are not similar among these agents. For example, all the anti-TNF agents are effective against RA, but not all of them against Crohn’s disease [1, 2]. The binding and neutralizing activities against soluble TNF-α are the critical and common mechanisms of action of these anti-TNF-agents. On the other hand, recent studies have shown that these agents have differential effects against transmembrane TNF-α and TNF-α-producing cells [7–10]. In the light of a growing body of evidence for the involvement of transmembrane TNF-α in inflammation, such as granulomatous inflammation, it would be important to summarize the biology of transmembrane TNF-α in health and disease as well as its interaction with anti-TNF agents. We would like to review the following issues: (i) biological function of transmembrane TNF-α as a ligand, (ii) biological function of transmembrane TNF-α as a receptor and (iii) different effects of anti-TNF agents on transmembrane TNF-α-bearing cells (TNF-α-producing cells) that would help to understand the different clinical effects of the anti-TNF agents.
Biology of transmembrane TNF-α and soluble TNF-α
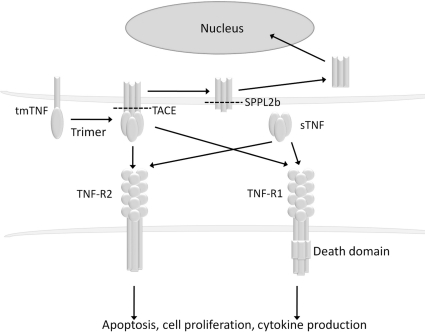
TNF-α is generated as a precursor form called transmembrane TNF-α that is expressed as a cell surface type II polypeptide consisting of 233 amino acid residues (26 kDa) on activated macrophages and lymphocytes as well as other cell types [11–13] (Fig. 1). After being processed by such metalloproteinases as TNF-α-converting enzyme (TACE) between residues alanine76 and valine77, the soluble form of TNF-α of 157 amino acid residues (17 kDa) is released and mediates its biological activities through Type 1 and 2 TNF receptors (TNF-R1 also known as TNFRSF1A, CD120a and TNF-R2 also known as TNFRSF1B, CD120b, respectively) [14–17]. Soluble TNF-α is a homotrimer of 17-kDa cleaved monomers and transmembrane TNF-α also exists as a homotrimer of 26-kDa uncleaved monomers [18]. Transmembrane TNF-α also binds to TNF-R1 and -R2, but its biological activities are supposed to be mediated mainly through TNF-R2 [19]. Transmembrane TNF-α is palmitoylated at a specific cysteine residue located just at the boundary between the transmembrane and the cytoplasmic domains [20]. In addition, serine residues of the intracellular domain of transmembrane TNF-α are phosphorylated [21]. These kinds of post-translational modification may be important for the regulation of transmembrane TNF-α function. After releasing soluble TNF-α by TACE cleavage, the residual cytoplasmic domain of transmembrane TNF-α migrated back into the nucleus of the transmembrane TNF-α-bearing cells [22].
Fig. 1.
Biology of transmembrane TNF-α and soluble TNF-α.
Transmembrane TNF-α is a precursor form of soluble TNF-α that is expressed on TNF-α-producing cells as a homotrimer. After processing by TACE, soluble TNF-α is generated and binds to TNF-R1 or -R2. Transmembrane TNF-α also binds to TNF-R1 and -R2. Upon binding to TNF receptors, both transmembrane and soluble TNF-α mediate pleiotropic effects (apoptosis, cell proliferation and cytokine production). The remaining transmembrane TNF-α after cleavage with TACE is further processed by SPPL2b and the intracellular domain is translocated into the nucleus and is supposed to mediate cytokine production. tmTNF: transmembrane TNF-α; sTNF: soluble TNF-α.
TNF-R1 and -R2 are expressed on almost all nucleated cells [17] in the form of pre-assembled trimers [23]. Both TNF receptors are capable of binding intracellular adaptor proteins that lead to activation of complex intracellular signalling processes and mediate the pleiotropic effects of TNF-α [2, 24]. The signalling pathways initiated by TNF-R2, which may be the preferential receptor for transmembrane TNF-α, are less characterized compared with those of TNF-R1. However, TNF-R2 appears to have both shared and opposing effects to TNF-R1 and may be actively involved in the pathogenesis of inflammatory diseases [25]. Although controversial, a functional M196R polymorphism of TNF-R2 [26] is associated with an increased risk of a number of inflammatory diseases, such as RA [27, 28], SLE [26, 29] and ulcerative colitis [30]. A meta-analysis revealed the association of the 196R polymorphism of TNF-R2 and SLE [31].
Biological activities of transmembrane TNF-α as a ligand
Transmembrane TNF-α on the cell surface of TNF-α-producing cells binds to TNF receptors on the target cells and exerts various biological functions that will contribute to the modulation of local inflammation in a cell-to-cell contact manner as well as in a cell-type-specific fashion. Expression of transmembrane TNF-α on various cell types would contribute to the physiological as well as pathological responses in health and diseases (Table 1).
Table 1.
Biological activities of transmembrane TNF-α as a ligand
| Target | Function | References |
|---|---|---|
| Tumour cells (various types) | Cytotoxicity | [32–35, 38, 39, 43] |
| HIV-infected lymphocyte | Cell death | [44] |
| Intracellular parasite-infected macrophage | Inhibit the growth of intracellular pathogens | [45–49] |
| Mycobacterium infection | T-cell and macrophage migration, granuloma formation | [52–55] |
| Monocyte | IL-10 production | [70] |
| B cell | Proliferation, Ig production | [58–64] |
| T cell | HLA-DR and CD25 expression, GM-CSF production | [19] |
| NK cell | Enhancement of cytotoxic activity | [65] |
| Endothelial cell | Cell death, induction of pro-coagulant agents, adhesion molecules and pro-inflammatory cytokines | [42, 19, 56, 57] |
| Adipose tissue | Inhibition of adipocyte differentiation, local insulin resistance | [66, 67] |
| Heart | Concentric cardiac hypertrophy | [68, 69] |
| Lung | Interstitial inflammation | [36, 37] |
| Liver | Hepatitis | [40, 41] |
Cytotoxic activity
In the late 1980s, a number of reports showed the cytotoxic effects mediated by transmembrane TNF-α. Human macrophages and lymphocytes stimulated with such agents as lipopolysaccharide (LPS), IFN-γ or phorbol myristate acetate express transmembrane and soluble TNF-α. Tumour cells were lysed by incubating with transmembrane TNF-α on paraformaldehyde-fixed activated monocytes [32–34], paraformaldehyde-fixed activated lymphocytes [33] and microsomes [12]. This cytotoxic activity is mediated by TNF receptors [34]. Freshly isolated human NK cells constitutively express transmembrane TNF-α that mediates cytotoxic activity [35]. In patients with HIV infection and acute respiratory distress syndrome, functionally active, cytotoxic transmembrane TNF-α was expressed on the alveolar macrophages [36, 37], which is supposed to be a mechanism for TNF-α-mediated lung injury. CD8+ T cells in SLE patients express an increased amount of transmembrane TNF-α upon activation and exerts cytotoxic activity when incubated with L929 cells [38]. Monocytes primed with cytokines demonstrated increased killing of tumour cell lines as well as primary acute myeloid leukaemia blasts by a mechanism dependent on transmembrane TNF-α [39]. In experimental Con A-induced or melphalan-induced hepatitis [40, 41], transmembrane TNF-α is involved in the pathogenesis through both TNF-R1 and -R2. Melphalan inhibited TACE and induced Kupffer cells to express transmembrane TNF-α, which leads to hepatocyte injury. In endothelial programmed cell death by ionizing radiation and LPS, transmembrane TNF-α played a critical role through TNF-R1 [42]. Lipid rafts participate in the cytotoxicity of transmembrane TNF-α through intercellular adhesion molecule-1 (ICAM-1) clustering and consequent enhancement of the cell-to-cell contact in Raji cells [43].
Host defence against intracellular pathogens
One of the major biological roles of TNF-α is in the host defence to bacterial, viral and parasitic infections [2]. The importance of transmembrane TNF-α in the inhibition of intracellular organisms is beginning to be elucidated.
HIV-infected T-cell line or HIV-infected peripheral blood lymphocytes were induced to cell death when co-cultured with cells expressing transmembrane TNF-α through cooperative signalling of TNF-R1 and -R2 [44]. The contact mechanism mediated by transmembrane TNF-α on CD4+ T cells activated Leishmania major-infected macrophages to inhibit the growth of intracellular Leishmania, an effect it exerts more strongly than soluble TNF-α [45]. By using transmembrane TNF-α-knock-in mice, which express functional transmembrane TNF-α but do not release soluble TNF-α, transmembrane TNF-α was shown to be sufficient to control infection due to L. major [46]. In vitro tissue co-culture system revealed that T-cell-expressed transmembrane TNF-α is necessary and sufficient for memory T-cell responses to intracellular pathogen Francisella tularensis, and is particularly important for intramacrophage control of bacterial growth by CD8+ T cells [47]. IFN-γ induces monocyte apoptosis and Coxiella burnetii killing through β2-integrin-mediated cell clustering, which allows transmembrane TNF-α to deliver a death signal to infected monocytes. Both TNF-R1 and -R2 are involved in this process [48]. Transmembrane TNF-α participates in cell-mediated immunity to Listeria monocytogenes as shown in transgenic mice. In the absence of secreted TNF-α, transmembrane TNF-α endows macrophages with enhanced capacity to kill L. monocytogenes [49].
Protective immune response to Mycobacterium tuberculosis is regulated by T cells, macrophages and cytokines, such as INF-γ, IL-12 and TNF-α [50, 51]. A critical role of TNF-α has been extensively reported in neutralizing or gene-deletion experiments in mice infected with mycobacterial species with varying virulence. The importance of transmembrane TNF-α for protection from M. tuberculosis or less virulent M. bovis bacillus Calmette–Guerin infection was demonstrated in transgenic mice expressing transmembrane TNF-α [52, 53]. Transmembrane TNF-α is sufficient to initiate T cell and macrophage migration as well as granuloma formation, and effective against acute, but not long-term M. tuberculosis infection [54, 55].
Activation of endothelial cells
Human umbilical vein endothelial cells (HUVECs) co-cultured with transmembrane TNF-α-expressing Chinese hamster ovary (CHO) cells expressed tissue factor with synergistic action of both TNF-R1 and -R2 in an adhesion molecule (E-selectin/ICAM-1)-dependent manner [19, 56]. In addition, plasma membranes isolated from stimulated T lymphocytes up-regulated the expression of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1) and E-selectin on isolated human brain microvascular endothelial cells (HB-MEC) and their IL-6 expression [57], which was partly diminished by inhibitors of TNF-α. Induction of pro-coagulant agents, adhesion molecules and pro-inflammatory cytokine by transmembrane TNF-α may reflect the inflammation of microvessels mediated by direct cell-to-cell contact between inflammatory cells and endothelial cells.
B-cell proliferation and immunoglobulin production
Transmembrane TNF-α is expressed on HIV-infected CD4+ T cells and markedly stimulated proliferation and immunoglobulin (Ig) production by both autologous and allogeneic B cells in an antigen-non-specific, MHC-unrestricted, contact-dependent manner [58, 59]. Likewise, B-cell activation was induced by transmembrane TNF-α on HTLV type I (HTLV-I)-infected CD4+ T cells and herpesvirus saimiri-transformed CD4+ T cells [60–62]. It is thus considered that human CD4+ T-cell clones, when infected by certain viruses, can provide abnormal B cell help and explain at least in part the hypergammaglobulinaemia and other phenomena related to polyclonal B-cell activation seen in patients infected with these viruses [63]. In healthy individuals, transmembrane TNF-α on Con A-activated CD4+ T-cell clones provided a co-stimulatory signal for human B-cell activation and Ig production through TNF-R1, but not by TNF-R2 [64].
T-cell/thymocyte activation
Transmembrane TNF-α expressed on CHO cells stimulated human peripheral T cells to express HLA-DR [19]. Thymocytes from TNF-R2 transgenic mice induced proliferation, CD25 expression and GM-CSF production when co-cultured with transmembrane TNF-α-expressing CHO cells [19].
NK cell stimulation
Transmembrane TNF-α is an important mediator for NK cell–dendritic cell (DC) crosstalk [65]. In mouse, proliferation and cytotoxic activity of NK cells were enhanced by transmembrane TNF-α on DCs through NK cell-surface TNF-R2.
Adipocyte differentiation
Expression of transmembrane TNF-α on adipocytes resulted in inhibition of differentiation by selectively activating TNF-R1 [66]. This result might indicate that transmembrane TNF-α is a local mediator of insulin resistance. Supporting evidence was demonstrated in transgenic mice. Mice specifically expressing transmembrane TNF-α in adipocytes showed a decreased whole body adipose mass, and local, but not systemic, insulin resistance [67]. These data demonstrate that exclusive action of TNF-α in adipose tissue strongly inhibits insulin action at this site and leads to reduced adiposity in mice.
Cardiac hypertrophy
There is a growing body of evidence that the short-term and self-limited expression of TNF-α plays an important homeostatic role in the heart [68]. Transgenic mice with cardiac-restricted overexpression of transmembrane TNF-α provoke a concentric hypertrophic cardiac phenotype [69].
Cytokine production from monocytes
Transmembrane TNF-α expressed on glutaraldehyde-fixed pre-stimulated human T-cells induced monocytes to secrete IL-10 in a cell–cell contact manner [70]. TNF-R1 and -R2 on the monocyte surface are stimulated by transmembrane TNF-α on glutaraldehyde-fixed pre-stimulated human CD4+ T cells to produce TNF-α. Extracellular signal-regulated kinase, a member of mitogen-activated protein kinases, was involved in the downstream signalling [71]. It is thus considered that transmembrane TNF-α plays an important role for monocyte cytokine production in T-cell–monocyte cognate interaction.
Biological activities of transmembrane TNF-α as a receptor
Transmembrane TNF-α-bearing cells show their biological activity when transmembrane TNF-α on their cell surface is bound to its receptor, TNF-R1 or -R2. The biological activity is induced by the transmembrane TNF-α-mediated signal, also called an ‘outside-to-inside signal’ or ‘reverse signal’. In contrast to the well-characterized functions of transmembrane TNF-α as a ligand, the biological functions elicited by outside-to-inside (reverse) signal have not completely been clarified. However, it is supposed that outside-to-inside signalling mediated by transmembrane TNF-α contributes to the pleiotropy of this pro-inflammatory cytokine and its fine-tuning of immune response [6]. The biological activities of transmembrane TNF-α as a receptor have been demonstrated in T cells, monocytes/macrophages and NK cells in humans [72–76]. The elevation of intracellular calcium concentration in both human T-cell line and mouse macrophage cell line [62, 77] was induced through transmembrane TNF-α (Table 2).
Table 2.
Biological activities of transmembrane TNF-α as a receptor
| tmTNF-expressing cells | Function | Reference |
|---|---|---|
| T cell | E-selectin expression, | [72] |
| Production of IL-2 and IFN-γ | [62] | |
| Alloresponse against endothelial cells | [73] | |
| Monocyte/macrophage | Down-regulation of LPS-induced soluble TNF-α, IL-6, IL-1 and IL-10 | [74] |
| Sensitization to soluble TNF-induced activation (pre-stimulation) or reduction of mRNA stability of IL-1β and IL-8 (post-stimulation) | [75] | |
| TNF-α production | [71] | |
| NK cell | Increased cytotoxicity by up-regulation of perforin and granzyme B | [76] |
tmTNF: transmembrane TNF-α.
Modulation of T-cell function
Harashima et al. [72] reported that activation by polyclonal anti-TNF-α antibody against transmembrane TNF-α on phytohemagglutinin-activated normal human CD4+ T cells resulted in the induction of an adhesion molecule, E-selectin (CD62E). In addition, Jurkat T cells or HeLa cells stably expressing transmembrane TNF-α up-regulated E-selectin when brought into cell-to-cell contact with TNF-R2-expressing HeLa cells [72]. Transmembrane TNF-α was involved in the alloresponse of T cells against human microvascular endothelial cells (HMECs) [73]. CD4+ T cells proliferated upon stimulation with HMECs were down-regulated by reverse signalling through transmembrane TNF-α. In addition, stimulation of transmembrane TNF-α on CD8+ T cells increased their cytotoxic potential against HMECs, although the stimulation was not by native cell-surface TNF-R, but by polyclonal anti-TNF-α or soluble TNF-R production of IL-2 and IFN-γ in human T-cell line [62].
Modulation of monocyte/macrophage function
In human monocytes/macrophages pre-incubated with TNF-R1-expressing human endothelial cells, reverse signalling through transmembrane TNF-α mediated LPS resistance as indicated by the down-regulation of LPS-induced soluble TNF-α and IL-6 as well as IL-1 and -10 [74]. Pre-treatment with soluble TNF-R1 for inducing reverse signalling through transmembrane TNF-α sensitized human monocyte cell line U937 cells to soluble TNF-α-induced activation, whereas stimulation of transmembrane TNF-α after soluble TNF-α-induced activation of U937 cells reduced mRNA stability of IL-1β and IL-8 [75]. In contrast to these findings that transmembrane TNF-α may inhibit sustained activation of monocytes, transmembrane TNF-α played a positive role in the activation of monocytes. Ligation of transmembrane TNF-α on monocytes by TNF-R2 on T cells or soluble TNF-R2:Ig receptor construct (etanercept) induced TNF-α production due to outside-to-inside signalling through transmembrane TNF-α [71].
Activation of NK cell function
Such a positive effect by transmembrane TNF-α was also reported in NK cells. Pre-stimulation of transmembrane TNF-α with soluble TNF-R1 resulted in increased cytotoxicity of NK92 cells, a human NK cell line [76]. This increased cytotoxicity of NK92 cells was accompanied by augmented mRNA production of two cytotoxic molecules, perforin and granzyme B.
Other TNF ligand family members and outside-to-inside signal
These lines of evidence indicate that transmembrane TNF-α transmits outside-to-inside signals back to the cell by a cell-to-cell contact manner in local inflammation. Outside-to-inside (reverse) signals transmitted by other members of TNF ligand family have also been reported. CD40L co-stimulation is important in the regulation of IL-4 production from T cells [78]. CD30L on the neutrophil transmits reverse signal to induce IL-8 expression and a rapid respiratory burst [79]. In addition, outside-to-inside signal transmitted by CD27L or FasL leads to T-cell proliferation [80–82]. Enhancement of IgG production of B cells and promotion of maturation of DCs were shown to be reverse signalling by OX40L [83]. Outside-to-inside signals have been studied relatively well for the CD137L. A variety of biological functions, such as cytokine induction and cell proliferation, in different cell types have been reviewed recently [84].
Binding of anti-TNF agents to soluble and transmembrane TNF-α
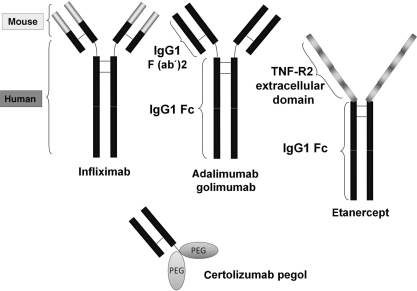
Anti-TNF agents have been successfully applied to the treatment of Crohn’s disease and RA as well as other chronic inflammatory diseases like psoriasis, AS and Behçet’s disease [1, 85]. Three anti-TNF agents, infliximab, adalimumab and etanercept, are approved worldwide for the treatment of these diseases and there are ample data on the clinical profile. Other anti-TNF agents, certolizumab pegol and golimumab, have just been approved for clinical use. Infliximab, adalimumab and golimumab are mAbs against human TNF-α and etanercept is engineered from human TNF receptors (Fig. 2). Infliximab is a chimeric mouse–human anti-TNF-α mAb composed of a murine variable region and a human IgG1 constant region. Adalimumab and golimumab are fully humanized anti-TNF-α mAbs, which are indistinguishable from the normal human IgG1. Etanercept is composed of the extracellular portion of the two human TNF-R2 (p75 TNF receptor) linked to the Fc portion (CH2 and CH3 domains) of human IgG1. Certolizumab is a Fab' fragment of an anti-TNF-α IgG1 mAb and is lacking the Fc portion. The hinge region of certolizumab is covalently linked to two cross-linked chains of 20 kDa of polyethylene glycol, giving certolizumab pegol [86].
Fig. 2.
Structures of anti-TNF agents.
Infliximab is a mouse–human chimeric monoclonal anti-TNF antibody of IgG1 isotype. Adalimumab and golimumab are fully human IgG1 monoclonal anti-TNF antibodies. Etanercept is a fusion protein of the extracellular domain of TNF-R2 and the Fc region of IgG1. Certolizumab pegol is a PEGylated Fab′ fragment of humanized monoclonal anti-TNF antibody.
Infliximab binds to both monomer and trimer forms of soluble TNF-α, whereas etanercept binds only to the trimer form [87]. Infliximab formed stable complexes with soluble TNF-α, while etanercept formed relatively unstable complexes [87]. Each infliximab molecule is capable of binding to two TNF-α molecules, and up to three infliximab molecules can bind to each TNF-α homotrimer. In contrast, etanercept is supposed to form 1 : 1 complex with the TNF-α trimer [87]. In fact, the mAbs, but not TNF-R2:Ig soluble receptor, form large protein complexes in vitro [88]. Overall, all three anti-TNF agents have similar intrinsic binding properties for soluble TNF [10]. Although these kinds of analysis at the molecular level have not been performed, certolizumab pegol showed similar potency in neutralizing soluble TNF-α to infliximab, adalimumab and etanercept [89].
Infliximab, adalimumab, etanercept and certolizumab pegol bind to transmembrane TNF-α on transmembrane TNF-α-transfected cells [7, 9, 89] with similar affinities that were lower (weaker) than for soluble TNF-α [10]. As in the case of soluble TNF-α, up to three molecules of infliximab can bind one transmembrane TNF-α, one etanercept can bind one molecule of transmembrane TNF-α [87].
Functional properties of anti-TNF agents on transmembrane TNF-α
Among the five anti-TNF agents, infliximab, adalimumab and etanercept are approved and have been clinically introduced worldwide for years. Here, we would like to describe the functional properties of anti-TNF agents with emphasis on these three widely used TNF antagonists. Infliximab, adalimumab and etanercept are effective for the treatment of RA, PsA and AS; however, etanercept is not effective for Crohn’s disease, Wegener’s granulomatosis and sarcoidosis [86, 90]. This difference in the clinical efficacy may be explained by the differences in pharmacokinetics, tissue distribution and functional properties of these anti-TNF agents. Considering the important role of transmembrane TNF-α in health and diseases, differential effects of anti-TNF agents on transmembrane TNF-α may explain the difference in these clinical efficacies. A number of groups have reported head-to-head comparison of the functional properties for these anti-TNF agents on transmembrane TNF-α [7, 8, 10, 87, 89] (Fig. 3).
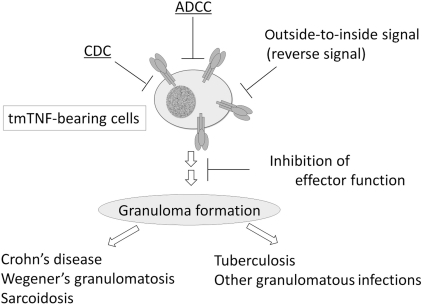
Fig. 3.
Inhibition of TNF-α-bearing cells by anti-TNF agents.
Transmembrane TNF-α plays an important role in granuloma formation, which is essential for the development of granulomatous diseases such as Crohn’s disease, and the host defence against tuberculosis. There are at least four distinct mechanisms for the inhibition of TNF-α-bearing cells by anti-TNF agents: (i) inhibition of transmembrane TNF-α-mediated effector function, (ii) destruction of TNF-α-bearing cells by CDC, (iii) destruction of TNF-α-bearing cells by ADCC and (iv) destruction of TNF-α-bearing cells by outside-to-inside signal (reverse signal).
Inhibition of ligand activity of transmembrane TNF-α
Infliximab was significantly more potent than etanercept at blocking transmembrane TNF-α-mediated E-selectin expression in HUVECs [87]. In a bioassay using human lung carcinoma cell line A549, infliximab, adalimumab and certolizumab pegol similarly inhibited transmembrane TNF-α-mediated cell death; however, etanercept showed ∼2-fold less activity [89]. Taken together, effector function of transmembrane TNF-α is inhibited by any of the anti-TNF agents, although the activity of etanercept is weaker than the other antagonists.
Inhibition of transmembrane TNF-α-bearing cells
Complement-dependent cytotoxicity
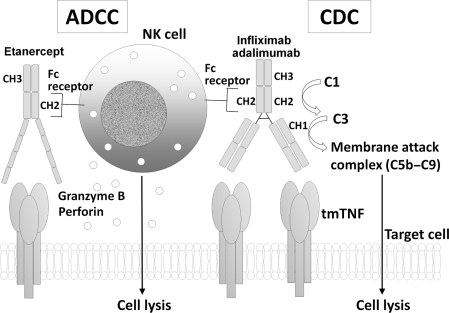
In a system using human Jurkat T cells [8], mouse NS0 myeloma cells [89], mouse Sp2/0 myeloma cells [10] or CHO cells [9], complement-dependent cytotoxicity (CDC) was analysed for the anti-TNF agents. All the reports were in agreement that infliximab and adalimumab induced CDC much more potently than etanercept. In contrast, certolizumab pegol did not have any CDC activity [89], which reflects its absence of the Fc portion of IgG1. From the structural point of view, lack of activation of the complement system by etanercept seems to be reasonable as well. Infliximab, adalimumab and etanercept commonly possess the Fc portion of IgG1, whose CH2 domain activates the first component of complement (C1) activation (Fig. 4). However, etanercept does not carry the CH1 domain of IgG1. A narrow region of 23 amino acid residues within the CH1 domain serves as a platform for complement C3 activation [91]; it was later confirmed that three amino acid residues within the specific 23 amino acids are involved in the covalent attachment with C3 [92, 93]. Etanercept is structurally impaired in the appropriate activation of C3, the most important step in complement activation. Moreover, lack of a hinge region in the Fc portion of etanercept resulted in rigidity compared with the natural antibody and eventually culminated in conformational hindrance to the proper access of complement proteins. It is thus difficult for etanercet to make a membrane attack complex of complement proteins (C5b–C9) for CDC at least in vitro. When activated human peripheral blood mononuclear cells were studied as target cells, none of these three anti-TNF agents induced CDC [10], which may be due to the use of different cell types from the above-mentioned experiments.
Fig. 4.
CDC and ADCC by anti-TNF agents.
Infliximab, adalimumab and etanercept commonly possess the Fc portion of IgG1, whose CH2 domain activates complement C1. Activation of C1 leads to complement C3 activation and subsequent formation of a membrane attack complex (C5b–C9) and lysis of the target cells. However, etanercept does not carry the CH1 domain of IgG1 which is important for the activation of C3. Infliximab, adalimumab and etanercept carry CH2 and CH3 domains of the Fc domain of IgG1 that mediate the binding to Fc receptors, which culminates in granzyme B and perforin release from NK cells and lysis of the target cells.
Antibody-dependent cell-mediated cytotoxicity
Infliximab, adalimumab and etanercept showed similar antibody-dependent cell-mediated cytotoxicity (ADCC) activitiy using mTNF-transfected Jurkat T cells as target [8], while infliximab and adalimumab showed much more potent ADCC than etanercept in NS0 cells [89] or in CHO cells [9]. Certolizumab pegol did not show any ADCC activity [89]. The discrepancy in etanercept-induced ADCC is not clear, but may be explained by the different experimental conditions, such as difference in the species of target cell, in the expression level of transmembrane TNF-α. From the structural viewpoint, infliximab, adalimumab and etanercept carry CH2 and CH3 domains of the Fc domain of IgG1, whereas certolizumab pegol does not (Fig. 4). These domains of IgG1 are involved in the binding to Fc receptors of NK cells [94], which leads to the lysis of target cells by granzyme B and perforin. The presence or absence of soluble TNF-α in the assay system may also affect ADCC activities. Both mAbs and etanercept weakly bound to Fcγ receptors in the absence of soluble TNF-α, but in the presence of soluble TNF-α, there was a marked increase in binding only by mAbs infliximab and adalimumab [9]. As for infliximab, induction of both CDC and ADCC has been reported by others [95].
Outside-to-inside signalling (reverse signalling)
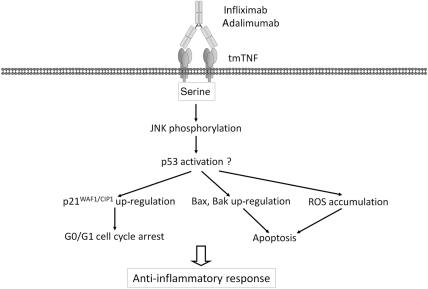
This is a novel function of anti-TNF agents for the inhibition of TNF-α-producing cells, which is mediated by mechanisms independent of CDC and ADCC [8, 96] (Fig. 5). Infliximab and adalimumab, but not etanercept, induced apoptosis and cell cycle G0/G1 arrest upon binding to transmembrane TNF-α-expressing Jurkat T cells. Cross-linking of etanercept bound to the cell-surface transmembrane TNF-α resulted in increased apoptosis [96], which indicates that multimer formation with mAbs and transmembrane TNF-α may be essential for the initiation of the subsequent intracellular signals. IL-10 production was induced by infliximab, but not by etanercept, in transmembrane TNF-alpha-expressing Jurkat T cells [96]. c-Jun NH2-terminal kinase activation followed by up-regulation of p21WAF1/CIP1, Bax and Bak as well as reactive oxygen species (ROS) accumulation are important intracellular signalling events for apoptosis and cell cycle arrest [96]. In addition, site-directed mutagenesis revealed that three serine residues in the cytoplasmic domain of transmembrane TNF-α are essential for these biological effects. The amino acid sequence of the intracellular domain of transmembrane TNF-α is well conserved in different species [77] (Fig. 6), and is thus considered to play an important role. All three serine residues were conserved among different species. A casein kinase I (CKI) consensus sequence in the cytoplasmic domain may be involved in outside-to-inside signalling as well [77]. This domain is dephosphorylated upon activation of transmembrane TNF-α in mouse macrophage cell line RAW264.7, and is accompanied by an increase in intracellular calcium levels [77]. Increase in intracellular calcium levels by outside-to-inside signal of transmembrane TNF-α has also been reported by others [62]. For the outside-to-inside signalling, the amino-terminal intracellular domain of transmembrane TNF-α cleaved by signal peptide peptidase-like 2b (SPPL2b) may play an additional role [97, 98]. This cleaved intracellular domain triggers expression of the pro-inflammatory cytokine IL-12 in human DCs [97]. Consistent with these findings, the amino-terminal intracellular domain of transmembrane TNF-α contains a putative nuclear localizing signal (KKTGGPQGSRR; one-letter amino acid code), localizes in the nucleus and seems to be associated with IL-1β expression in human HeLa cells [22, 99].
Fig. 5.
Outside-to-inside signal by adalimumab and infliximab.
This is a novel mechanism for the inhibition of transmembrane TNF-α-bearing cells by anti-TNF antibodies. In the absence of NK cells or complement, adalimumab or infliximab induces G0/G1 cell cycle arrest and apoptosis, which inhibits TNF-α-producing cells and leads to an anti-inflammatory response. A number of molecules (p21WAF1/CIP1, Bax, Bak and ROS) were involved in these intracellular signalling events through the intracellular domain of transmembrane TNF-α. These signalling molecules are supposed to be associated with p53 activation. Three serine residues in the intracellular domain of transmembrane TNF-α are essential for the activities. Bak and Bax are proapoptotic multidomain molecules; tmTNF: transmembrane TNF-α.
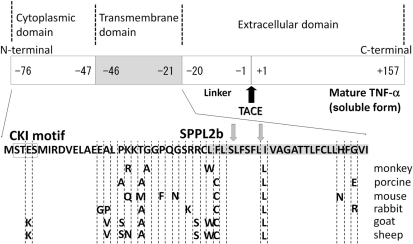
Fig. 6.
Structure of transmembrane TNF-α.
Transmembrane TNF-α is a type II polypeptide composed of a extracellular domain (177 amino acid residues), a transmembrane domain (26 amino acid residues, shaded) and an intracellular domain (30 amino acid residues). Mature TNF-α (soluble TNF-α) of 157 amino acid residues is cleaved from transmembrane TNF-α by TACE (black arrow). The remaining part is further cleaved by SPPL2b in the transmembrane domain (two grey arrows), and the intracellular domain is translocated into the nucleus to possibly modulate gene expression of the TNF-α-bearing cells. The intracellular domain contains CKI motif (boxed) and three serine residues. These serine residues are conserved among different species and are essential for the outside-to-inside signal transmitted by transmembrane TNF-α upon binding to anti-TNF antibody. Amino acid residues are shown in the one-letter code. The transmembrane domain of transmembrane TNF-α is shaded.
Granulomatous diseases and transmembraneTNF-α
The three widely used anti-TNF agents, infliximab, adalimumab and etanercept, show different clinical efficacy. Infliximab and adalimumab, but not etanercept, are effective against such diseases as Crohn’s disease, WG and sarcoidosis [86, 90]. These are granulomatous inflammatory disorders. In addition, side effects are different between these anti-TNF agents. Post-marketing surveillance in the USA (from January 1998 through September 2002) has identified that infliximab was associated with a 2- to 8-fold greater risk of such granulomatous infections as tuberculosis, listeriosis and histoplasmosis compared with etanercept [100]. The increased incidence of tuberculosis in patients treated with infliximab or adalimumab compared with etanercept was also reported in other European countries [101]. It has become apparent that efficacy in granulomatous inflammatory diseases and risk of granulomatous infections seems to reflect the anti-granuloma function of anti-TNF agents. In fact, specimens from RA patients who developed tuberculosis after treatment with infliximab lack granuloma formation [102]. The presence or absence of an anti-granuloma effect would be the most prominent difference between the currently available anti-TNF agents.
Transmembrane TNF-α has recently been shown to contribute to the host defence against acute M. tuberculosis infection in humans. CD8+CCR7-CD45RA+ effector memory T cells express granulysin and mediate antimicrobial activity against M. tuberculosis [103]. This T-cell subset expressed transmembrane TNF-α and bound infliximab, making itself susceptible to complement-mediated lysis and the resultant reduced antimicrobial activity.
In patients with Crohn’s disease, treatment with infliximab induced a rapid increase of the number of apoptotic CD3+ lamina propria T cells, without detectable changes in peripheral blood T lymphocyte phenotype or markers of apoptosis [104]. Moreover, transmembrane TNF-α, in the absence of soluble TNF-α, induces colitis in a mouse model [105]. In the clinical setting, it is likely that infliximab induces apoptosis at least in part by transmembrane TNF-α-mediated effects: CDC, ADCC and/or outside-to-inside signalling.
Considering that infliximab and adalimumab induce CDC, ADCC and outside-to-inside signalling through transmembrane TNF-α, these anti-TNF mAbs seem to be more potent than etanercept in the elimination of transmembrane TNF-α-bearing macrophages and transmembrane TNF-α-bearing T cells. Thus, infliximab and adalimumab may more strongly inhibit granuloma formation by these cells in tuberculosis or in Crohn’s disease, as compared with etanercept. Different effects of these anti-TNF agents on transmembrane TNF-α might at least partly explain their different clinical efficacies. Although available information is limited for the new anti-TNF agents, certolizumab pegol and golimumab, it is important to further analyse both the basic and clinical data of these new agents and put the pieces together to more precisely understand the similarities and dissimilarities of mechanism of action of anti-TNF agents. Moreover, it is of note that the clinical efficacy profiles are not solely dependent on the mode of action on transmembrane TNF-α. These anti-TNF agents are not similar with respect to doses, routes and frequency of administration, pharmacokinetics or immunogenicity. There might be other indirect effects in the inflammatory network different between the anti-TNF agents. The mechanisms of action of these anti-TNF agents are more complex in patients than in in vitro experiments.
Conclusion
Transmembrane TNF-α is a bipolar molecule that transmits signals as a ligand and as a receptor back to the cell. It is thus considered that transmembrane TNF-α plays an important role in local inflammation in a cell-to-cell contact manner. Infliximab, adalimumab and etanercept similarly bind to transmembrane TNF-α on TNF-α-producing cells, and the former two mAbs seem to transmit stronger inhibitory signals through transmembrane TNF-α. Understanding the mechanism of action of anti-TNF agents and their relationship with clinical effects will contribute to appropriate prediction of the clinical efficacy of forthcoming anti-TNF agents, the application of the agents to new disease targets and the development of new anti-TNF agents.
Acknowledgement
Funding: This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.H.).
Disclosure statement: The authors have declared no conflict of interest.
References
- 1.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 2.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 3.Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–8. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- 4.Georgopoulos S, Plows D, Kollias G. Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J Inflamm. 1996;46:86–97. [PubMed] [Google Scholar]
- 5.Alexopoulou L, Pasparakis M, Kollias G. A murine transmembrane tumor necrosis factor (TNF) transgene induces arthritis by cooperative p55/p75 TNF receptor signaling. Eur J Immunol. 1997;27:2588–92. doi: 10.1002/eji.1830271018. [DOI] [PubMed] [Google Scholar]
- 6.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353–66. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-α. Gastroenterology. 2004;126:934–5. doi: 10.1053/j.gastro.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Mitoma H, Horiuchi T, Tsukamoto H, et al. Mechanisms for cytotoxic effects of anti-TNF agents on transmembrane TNF-expressing cells: comparison among infliximab, etanercept and adalimumab. Arthritis Rheum. 2008;58:1248–57. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 9.Arora T, Padaki R, Liu L, et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine. 2009;45:124–31. doi: 10.1016/j.cyto.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Kaymakcalan Z, Sakorafas P, Bose S, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009;131:308–16. doi: 10.1016/j.clim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Pennica D, Nedwin GE, Hayflick JS, et al. Human tumor necrosis factor: precursor structure, cDNA cloning, expression, and homology to lymphotoxin. Nature. 1984;312:724–9. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 12.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 13.Luettiq B, Decker T, Lohmann-Matthes ML. Evidence for the existence of two forms of membrane tumor necrosis factor: an integral protein and a molecule attached to its receptor. J Immunol. 1989;143:4034–8. [PubMed] [Google Scholar]
- 14.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 15.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 16.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 17.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–9. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 18.Tang P, Hung M-C, Klostergaard J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry. 1996;35:8216–25. doi: 10.1021/bi952182t. [DOI] [PubMed] [Google Scholar]
- 19.Grell ME, Douni E, Wajant H, et al. The transmembrane form of tumour necrosis factor is the prime activating ligand of the 80 kDa tumour necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 20.Utsumi T, Takeshige T, Tanaka K, et al. Transmembrane TNF (pro-TNF) is palmitoylated. FEBS Lett. 2001;500:1–6. doi: 10.1016/s0014-5793(01)02576-5. [DOI] [PubMed] [Google Scholar]
- 21.Pocsik E, Duda E, Wallach D. Phosphorylation of the 26 kDa TNF precursor in monocytic cells and in transfected HeLa cell. J Inflamm. 1995;45:152–60. [PubMed] [Google Scholar]
- 22.Domonkos A, Udvardy A, Laszlo L, Nagy T, Duda E. Receptor-like properties of the 26 kDa transmembrane form of TNF. Eur Cytokine Netw. 2001;12:411–9. [PubMed] [Google Scholar]
- 23.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–4. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 24.MacEwan DJ. TNF ligands and receptors - a matter of life and death. Br J Pharmacol. 2002;135:855–75. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem. 2004;11:2205–12. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 26.Morita C, Horiuchi T, Tsukamoto H, et al. Association of tumor necrosis factor type II polymorphism 196r with systemic lupus erythematosus in the Japanese: molecular and functional analysis. Arthritis Rheum. 2001;44:2817–27. doi: 10.1002/1529-0131(200112)44:12<2819::aid-art469>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Constantin A, Dieude P, Lawers-Cances V, et al. Tumor necrosis factor receptor II gene polymorphism and severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:742–7. doi: 10.1002/art.20113. [DOI] [PubMed] [Google Scholar]
- 28.Goeb V, Dieude P, Vittecoq O, et al. Association between the TNFRII 196R allele and diagnosis of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1056–62. doi: 10.1186/ar1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komata T, Tsuchiya N, Matsushita M, Hagiwara K, Tokunaga K. Association of tumor necrosis factor receptor 2 (TNFR2) polymorphism with susceptibility to systemic lupus erythematosus. Tissue Antigens. 1999;53:527–33. doi: 10.1034/j.1399-0039.1999.530602.x. [DOI] [PubMed] [Google Scholar]
- 30.Pierik M, Vermeire S, Steen KV, et al. Tumour necrosis factor-a receptor type 1 and 2 polymorphisms in inflammatory bowel disease and their association with response to infliximab. Aliment Pharmacol Ther. 2004;20:303–10. doi: 10.1111/j.1365-2036.2004.01946.x. [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi T, Kiyohara C, Tsukamoto H, et al. A functional M196R polymorphism of tumour necrosis factor receptor type 2 is associated with systemic lupus erythematosus: a case-control study and a meta-analysis. Ann Rheum Dis. 2007;66:320–4. doi: 10.1136/ard.2006.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker T, Lohmann-Matthes M-L, Gifford GE. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987;138:957–62. [PubMed] [Google Scholar]
- 33.Peck R, Brockhaus M, Frey JR. Cell surface tumor necrosis factor (TNF) accounts for monocyte- and lymphocyte-mediated killing of TNF-resistant target cells. Cell Immunol. 1989;122:1–10. doi: 10.1016/0008-8749(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 34.Fishman M. Cytolytic activities of activated macrophages versus paraformaldehyde-fixed macrophages; soluble versus membrane-associated TNF. Cell Immunol. 1991;137:164–74. doi: 10.1016/0008-8749(91)90066-k. [DOI] [PubMed] [Google Scholar]
- 35.Caron G, Delneste Y, Aubry JP, et al. Human NK cells constitutively express membrane TNF-alpha (mTNFalpha) and present mTNFalpha-dependent cytotoxic activity. Eur J Immunol. 1999;29:3588–95. doi: 10.1002/(SICI)1521-4141(199911)29:11<3588::AID-IMMU3588>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Agostini C, Sancetta R, Cerutti A, Semenzato G. Alveolar macrophages as a cell source of cytokine hyperproduction in HIV-related interstitial lung disease. J Leukoc Biol. 1995;58:495–500. doi: 10.1002/jlb.58.5.495. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong L, Thickett DR, Christie SJ, Kendall H, Millar AB. Increased expression of functionally active membrane-associated tumor necrosis factor in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2000;22:68–74. doi: 10.1165/ajrcmb.22.1.3728. [DOI] [PubMed] [Google Scholar]
- 38.Horiuchi T, Morita C, Tsukamoto H, et al. Increased expression of membrane TNF-α on activated peripheral CD8+ T cells in systemic lupus erythematosus. Int J Mol Med. 2006;17:875–9. [PubMed] [Google Scholar]
- 39.Williams MA, Newland AC, Kelsey SM. Cytokine modulated cell-membrane bound tumour necrosis factor expression is associated with enhanced monocyte-mediated killing of human leukaemic targets. Leuk Res. 2000;24:317–30. doi: 10.1016/s0145-2126(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Küsters S, Tiegs G, Alexopoulou L, et al. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol. 1997;27:2870–5. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- 41.Kresse M, Latta M, Künstle G, et al. Kupffer cell-expressed membrane-bound TNF mediates melphalan hepatotoxicity via activation of both TNF receptors. J Immunol. 2005;175:4076–83. doi: 10.4049/jimmunol.175.6.4076. [DOI] [PubMed] [Google Scholar]
- 42.Eissner G, Kohlhuber F, Grell M, et al. Critical involvement of transmembrane tumor necrosis factor-alpha in endothelial programmed cell death mediated by ionizing radiation and bacterial endotoxin. Blood. 1995;86:4184–93. [PubMed] [Google Scholar]
- 43.Zhang S, Liu T, Liang H, et al. Lipid rafts uncouple surface expression of transmembrane TNF-alpha from its cytotoxicity associated with ICAM-1 clustering in Raji cells. Mol Immunol. 2009;46:1551–60. doi: 10.1016/j.molimm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Lazdins JK, Grell M, Walker MR, Woods-Cook K, Scheurich P, Pfizenmaier K. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. J Exp Med. 1997;185:81–90. doi: 10.1084/jem.185.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birkland TP, Sypek JP, Wyler DJ. Soluble TNF and membrane TNF expressed on CD4+ T lymphocytes differ in their ability to activate macrophage antileishmanial defense. J Leukoc Biol. 1992;51:296–9. doi: 10.1002/jlb.51.3.296. [DOI] [PubMed] [Google Scholar]
- 46.Allenbach C, Launois P, Mueller C, Tacchini-Cottier F. An essential role for transmembrane TNF in the resolution of the inflammatory lesion induced by Leishmania major infection. Eur J Immunol. 2008;38:720–31. doi: 10.1002/eji.200737662. [DOI] [PubMed] [Google Scholar]
- 47.Cowley SC, Sedgwick JD, Elkins KL. Differential requirements by CD4+ and CD8+ T cells for soluble and membrane TNF in control of Francisella tularensis live vaccine strain intramacrophage growth. J Immunol. 2007;179:7709–19. doi: 10.4049/jimmunol.179.11.7709. [DOI] [PubMed] [Google Scholar]
- 48.Dellacasagrande J, Ghigo E, Raoult D, Capo C, Mege JL. IFN-gamma-induced apoptosis and microbicidal activity in monocytes harboring the intracellular bacterium Coxiella burnetii require membrane TNF and homotypic cell adherence. J Immunol. 2002;169:6309–15. doi: 10.4049/jimmunol.169.11.6309. [DOI] [PubMed] [Google Scholar]
- 49.Torres D, Janot L, Quesniaux VF, et al. Membrane tumor necrosis factor confers partial protection to Listeria infection. Am J Pathol. 2005;167:1677–87. doi: 10.1016/S0002-9440(10)61250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 51.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 52.Olleros ML, Guler R, Corazza N, et al. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guérin infection in the absence of secreted TNF and lymphotoxin-alpha. J Immunol. 2002;168:3394–401. doi: 10.4049/jimmunol.168.7.3394. [DOI] [PubMed] [Google Scholar]
- 53.Olleros ML, Guler R, Vesin D, et al. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-Guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–20. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saunders BM, Tran S, Ruuls S, Sedgwick JD, Briscoe H, Britton WJ. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–9. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- 55.Fremond C, Allie N, Dambuza I, et al. Membrane TNF confers protection to acute mycobacterial infection. Respir Res. 2005;6:136. doi: 10.1186/1465-9921-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid EF, Binder K, Grell M, Scheurich P, Pfizenmaier K. Both tumor necrosis factor receptors, TNFR60 and TNFR80, are involved in signaling endothelial tissue factor expression by juxtacrine tumor necrosis factor alpha. Blood. 1995;86:1836–41. [PubMed] [Google Scholar]
- 57.Lou J, Dayer JM, Grau GE, Burger D. Direct cell/cell contact with stimulated T lymphocytes induces the expression of cell adhesion molecules and cytokines by human brain microvascular endothelial cells. Eur J Immunol. 1996;26:3107–13. doi: 10.1002/eji.1830261242. [DOI] [PubMed] [Google Scholar]
- 58.Macchia D, Parronchi P, Piccinni MP, et al. In vitro infection with HIV enables human CD4+ T cell clones to induce noncognate contact-dependent polyclonal B cell activation. J Immunol. 1991;146:3413–8. [PubMed] [Google Scholar]
- 59.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993;363:464–6. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 60.Del Prete G, De Carli M, D’Elios MM, et al. Polyclonal B cell activation induced by Herpesvirus saimiri-transformed human CD4+ T cell clones. Role for membrane TNF-alpha/TNF-alpha receptors and CD2/CD58 interactions. J Immunol. 1994;153:4872–9. [PubMed] [Google Scholar]
- 61.Saha K, Ware R, Yellin MJ, Chess L, Lowy I. Herpesvirus saimiri-transformed human CD4+ T cells can provide polyclonal B cell help via the CD40 ligand as well as the TNF-alpha pathway and through release of lymphokines. J Immunol. 1996;157:3876–85. [PubMed] [Google Scholar]
- 62.Higuchi M, Nagasawa K, Horiuchi T, et al. Membrane tumor necrosis factor-alpha (TNF-alpha) expressed on HTLV-I-infected T cells mediates a costimulatory signal for B cell activation–characterization of membrane TNF-alpha. Clin Immunol Immunopathol. 1997;82:133–40. doi: 10.1006/clin.1996.4291. [DOI] [PubMed] [Google Scholar]
- 63.Maggi E, Almerigogna F, Del Prete G, Romagnani S. Abnormal B cell helper activity by virus-infected human CD4+ T cells. Semin Immunol. 1993;5:449–55. doi: 10.1006/smim.1993.1051. [DOI] [PubMed] [Google Scholar]
- 64.Aversa G, Punnonen J, de Vries JE. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J Exp Med. 1993;177:1575–85. doi: 10.1084/jem.177.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Chakrabarti AK, Tan JL, Ge L, Gambotto A, Vujanovic NL. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell-natural killer cell crosstalk. Blood. 2007;109:3333–41. doi: 10.1182/blood-2006-06-026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J Biol Chem. 1999;274:26287–95. doi: 10.1074/jbc.274.37.26287. [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–11. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 68.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Ann Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 69.Dibbs ZI, Diwan A, Nemoto S, et al. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–8. doi: 10.1161/01.CIR.0000085203.46621.F4. [DOI] [PubMed] [Google Scholar]
- 70.Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J Immunol. 1997;158:3673–81. [PubMed] [Google Scholar]
- 71.Rossol M, Meusch U, Pierer M, et al. Interaction between transmembrane TNF and TNFR1/2 mediates the activation of monocytes by contact with T cells. J Immunol. 2007;179:4239–48. doi: 10.4049/jimmunol.179.6.4239. [DOI] [PubMed] [Google Scholar]
- 72.Harashima S, Horiuchi T, Hatta N, et al. Outside-to-inside signal through the membrane TNF-α induces E-selectin (CD62E) expression on activated human CD4+ T cells. J Immunol. 2001;166:130–6. doi: 10.4049/jimmunol.166.1.130. [DOI] [PubMed] [Google Scholar]
- 73.Vudattu NK, Holler E, Ewing P, et al. Reverse signalling of membrane-integrated tumour necrosis factor differentially regulated alloresponses of CD4+ and CD8+ T cells against human mirovascular endothelial cells. Immunology. 2005;115:536–43. doi: 10.1111/j.1365-2567.2005.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eissner G, Kirchner S, Lindner H, et al. Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J Immunol. 2000;164:6193–8. doi: 10.4049/jimmunol.164.12.6193. [DOI] [PubMed] [Google Scholar]
- 75.Xin L, Wang J, Zhang H, et al. Dual regulation of soluble tumor necrosis factor-α induced activation of human monocytic cells via modulating transmembrane TNF-α-mediated “reverse signaling”. Int J Mol Med. 2006;18:885–92. [PubMed] [Google Scholar]
- 76.Yu M, Shi W, Zhang J, et al. Influence of reverse signaling via membrane TNF-α on cytotoxicity of NK92 cell. Eur J Cell Biol. 2009;88:181–91. doi: 10.1016/j.ejcb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Watts AD, Hunt NH, Wanigasekara Y, et al. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling’. EMBO J. 1999;18:2119–26. doi: 10.1093/emboj/18.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blotta MH, Marshall JD, DeKruyff RH, Umetsu DT. Cross-linking of the CD40 ligand on human CD4+ T lymphocytes generates a costimulatory signal that up-regulates IL-4 synthesis. J Immunol. 1996;156:3133–40. [PubMed] [Google Scholar]
- 79.Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–9. [PubMed] [Google Scholar]
- 80.Bowman MR, Crimmins MA, Yetz-Aldape J, Kriz R, Kelleher K, Herrmann S. The cloning of CD70 and its identification as the ligand for CD27. J Immunol. 1994;152:1756–61. [PubMed] [Google Scholar]
- 81.Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–8. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun M, Ames KT, Suzuki I, Fink PJ. The cytoplasmic domain of Fas ligand costimulates TCR signals. J Immunol. 2006;177:1481–91. doi: 10.4049/jimmunol.177.3.1481. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Chen Y, Ge Y, et al. Characterization and functional study of five novel monoclonal antibodies against human OX40L highlight reverse signaling: enhancement of IgG production of B cells and promotion of maturation of DCs. Tissue Antigens. 2004;64:566–74. doi: 10.1111/j.1399-0039.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 84.Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol. 2005;77:281–6. doi: 10.1189/jlb.0904558. [DOI] [PubMed] [Google Scholar]
- 85.Rigby WF. Drug insight: different mechanisms of action of tumor necrosis factor antagonists—passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007;3:227–33. doi: 10.1038/ncprheum0438. [DOI] [PubMed] [Google Scholar]
- 86.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Therapeut. 2008;117:244–79. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–26. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 88.Kohno T, Tam LT, Stevens SR, Louie JS. Binding characteristics of tumor necrosis factor receptor-Fc fusion proteins vs anti-tumor necrosis factor mAbs. J Investig Dermatol Symp Proc. 2007;12:5–8. doi: 10.1038/sj.jidsymp.5650034. [DOI] [PubMed] [Google Scholar]
- 89.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–32. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 90.Ramos-Casals M, Brito-Zeron P, Munoz S, Soto MJ. BIOGEAS STUDY Group. A systematic review of the off-label use of biological therapies in systemic autoimmune diseases. Medicine. 2008;87:345–64. doi: 10.1097/MD.0b013e318190f170. [DOI] [PubMed] [Google Scholar]
- 91.Shohet JM, Pemberton P, Carroll MC. Identification of a major binding site for complement C3 on the IgG1 heavy chain. J Biol Chem. 1993;268:5866–71. [PubMed] [Google Scholar]
- 92.Sahu A, Pangburn MK. Covalent attachment of human complement C3 to IgG. Identification of the amino acid residue involved in ester linkage formation. J Biol Chem. 1994;269:28997–9002. [PubMed] [Google Scholar]
- 93.Vidarte L, Pastor C, Mas S, et al. Serine 132 is the C3 covalent attachment point on the CH1 domain of human IgG1. J Biol Chem. 2001;276:38217–23. doi: 10.1074/jbc.M104870200. [DOI] [PubMed] [Google Scholar]
- 94.Siberil S, Dutertre C-A, Boix C, et al. Molecular aspects of human FcγR interactions with IgG: functional and therapeutic consequences. Immunol Lett. 2006;106:111–8. doi: 10.1016/j.imlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine. 1995;7:251–9. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 96.Mitoma H, Horiuchi T, Hatta N, et al. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-α. Gastroenterology. 2005;128:376–92. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 97.Friedmann E, Hauben E, Maylandt K, et al. SPPL2a and SPPL2b promote intramembrane proteolysis of TNF-α in activated dendritic cells to trigger IL-12 production. Nat Cell Biol. 2006;8:843–8. doi: 10.1038/ncb1440. [DOI] [PubMed] [Google Scholar]
- 98.Fluhrer R, Grammer G, Israel L, et al. A γ-secretase-like intramembrane cleavage of TNF-α by the GxGD aspartyl protease SPPL2b. Nat Cell Biol. 2006;8:894–6. doi: 10.1038/ncb1450. [DOI] [PubMed] [Google Scholar]
- 99.Jans DA, Xiao C-Y, Lam MHC. Nuclear targeting signal recognition: a key control point in nuclear transport? BioEssays. 2000;22:532–44. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 100.Wallis RS, Broder MS, Wong JY, Hanson JY, Beenhouwer DO. Granulomatous infectious diseases associated with TNF antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 101.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–11. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 102.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 103.Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–77. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.ten Hove TC, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut. 2002;50:206–11. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corazza N, Brunner T, Buri C, et al. Transmembrane tumor necrosis factor is a potent inducer of colitis even in the absence of its secreted form. Gastroenterology. 2004;127:816–25. doi: 10.1053/j.gastro.2004.06.036. [DOI] [PubMed] [Google Scholar]