Abstract
Advances in the understanding of the biology of renal cell carcinoma have led to recent approval of several new agents including drugs that target vascular endothelial growth factor. Sunitinib is an oral tyrosine kinase inhibitor which interferes with multiple intracellular tumorogenic pathways, and has demonstrated impressive antitumor activity in phase II and subsequently improvement in progression free survival in phase III renal cancer trials. We review the unique side effects of sunitinib therapy with emphasis on establishing effective patient education for anticipation and early management of therapy-related side effects.
Keywords: sunitinib, renal cell carcinoma, side effects, tyrosine kinase inhibitor
Introduction
Kidney cancer accounts for 2% of all adult cancers.1,2 The median age of diagnosis is 65 years of age, with a slightly less than 2:1 male:female predominance. Although the classical clinical presentation included the triad of flank pain, abdominal mass, and hematuria, in the current era, most renal masses are detected as incidental findings on radiological imaging obtained for unrelated reasons. Approximately 30% of patients with renal cell carcinomas (RCC) are metastatic at the time of diagnosis.3 Forty percent of localized renal cell cancers at diagnosis become recurrent.4 The estimated 5-year survival rate of metastatic RCC (mRCC) is less than 30%. Risk factors associated with the incidence of kidney cancer include polycystic kidney disease, cysts occurring with hemodialysis, obesity, smoking, and hypertension.5 Certain genetic syndromes such as von Hippel-Lindau (VHL) have increased incidence of renal cancer. Clear cell RCC is the most common form of kidney cancer accounting for more than 85% of all kidney cancer.6
Historically, RCC has been viewed as a therapy-resistant cancer.3 Until the recent development of targeted agents, the therapeutic paradigm consisted of biological response modifier therapy including a variety of doses, combinations and schedules of interleukin-2 and interferon-alpha. The development of new and active agents followed scientific advances in the understanding of renal cancer biology. Dramatic improvements in renal cell cancer treatment and outcomes have occurred over the past few years. This progress has been driven in large part due to the improvement in understanding of the biology of RCC. Identification of the VHL gene as a tumor suppressor gene, whose inactivation by mutation or methylation leads to the formation of RCC, has elucidated the biology of sporadic RCCs.7 The protein product of the VHL functions in its intracellular pathway as a tumor suppressor by targeting hypoxia-inducible factor for proteosomal destruction. Without VHL, in the presence of uninhibited hypoxia-inducible factor, the cascade of intranuclear events leads to transcription of a number of genes, including vascular endothelium growth factor (VEGF) and platelet-derived growth factor (PDGF), leading to increased growth and vascular angiogenesis.8
Efforts to target the unique molecular derangements have focused on therapy which can interrupt these tumorogenic pathways. One multitarget agent in this emerging antineoplastic category includes sunitinib, which acts as an intracellular tyrosine kinase inhibitor (TKI) of multiple factors, including the intracellular domain of VEGF receptor (VEGFR-1, VEGFR-2), PDGF receptor (PDGFR-alpha and PDGF-beta), fetal liver tyrosine kinase receptor 3 (FLT3), and KIT (stem-cell factor receptor).9,10
The initial phase I trial of sunitinib was conducted in a dose-escalating fashion using a 4 weeks on drug followed by 2 weeks off drug regimen.11 The time off period was initially chosen to allow for recovery from potential toxicities of bone marrow suppression or adrenal suppression which were seen in preclinical animal studies. Dosing recommendations based on safety and toxicity were determined by phase I experience of 28 patients which used various dosing schedules and established a starting dose of 50 mg qd for 28 days followed by a 14-day period off drug. Dose limiting toxicities included fatigue, hypertension, and hand-foot syndrome (HFS).11 With this regimen, mucositis, peripheral edema, thrombocytopenia and neutropenia represented the majority of side effects. Asthenia and laboratory toxicities including thrombocytopenia and neutropenia improved after 2-week rest period.
Two phase II trials of mRCC patients demonstrated partial responses to sunitinib in patients who had failed prior cytokine therapy. In the first phase II trial of 63 patients, 40% of patients achieved partial responses with a median time to progression of 8.7 months.12 A second phase II trial conducted of 106 clear cell mRCC patients demonstrated a partial response in 34% of patients with a median progression-free survival of 8.3 months.13 A pivotal phase III trial of previously untreated patients with RCC comparing sunitinib with interferon-alpha demonstrated increased progression free survival of 11 vs 5 months (p < 0.001) and objective response rate 31% vs 6% (p < 0.001).14 At the time of analysis and publication, the original results of the phase III trial comparing sunitinib with interferon, the median duration of treatment was 6 months; an updated analysis of this data, after a median sunitinib treatment duration of 11 months, demonstrated an overall response rate of 47% (95% CI: 42, 52) for sunitinib vs 12% (95% CI: 9, 16) for interferon-alpha (p < 0.000001).15
Prospective data of side effects of sunitinib come primarily from the experience of these two phase II trials12,13 and one phase III trial (Table 1).14 Most toxicities were grade 1 or 2, with common adverse events including fatigue, diarrhea, neutropenia and anemia, gastrointestinal, skin and hair, and cardiac. Diarrhea was the most common side effect, with 53% of patients having any grade of diarrhea, of which 5% were grade 3 and there were no grade 4 toxicities. Overall, in this phase III study 38% of patients had a dose interruption of therapy and 32% of patients required dose reduction; however, only 10% discontinued drug due to adverse events.
Table 1.
Selected adverse events and laboratory abnormalities from phase II and III studies
| Adverse event |
Phase III study14N = 375 |
Phase II study12* N = 63 |
Phase II study13* N = 106 |
|||
|---|---|---|---|---|---|---|
| Grades 1–4 | Grades 3/4 | Grades 2–4 | Grades 3/4 | Grades 2–4 | Grades 3/4 | |
| Signs and symptoms | ||||||
| Diarrhea | 53% | 5% | 24% | 3% | 20% | 3% |
| Fatigue | 51% | 7% | 38% | 11% | 28% | 11% |
| Nausea | 44% | 3% | 19% | 3% | 13% | 0% |
| Stomatitis | 25% | 1% | 19% | 2% | 13% | 5% |
| Vomiting | 24% | 4% | 13% | 3% | 10% | 0% |
| Hypertension | 24% | 8% | 5% | 2% | 16% | 6% |
| Hand-foot syndrome | 20% | 5% | 8% | 2% | 15% | 7% |
| Laboratory abnormality | ||||||
| Neutropenia | 72% | 12% | 45% | 13% | 42% | 16% |
| Anemia | 71% | 4% | 37% | 10% | 26% | 6% |
| Increased creatinine | 66% | 1% | 14% | 0% | Not reported | Not reported |
| Thrombocytopenia | 65% | 8% | 18% | 0% | 21% | 6% |
Grade 1 toxicities not reported in study.
Graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0.40
Criteria for entry into the phase III trial which compared sunitinib with interferon-alpha included previously untreated patients with histology of clear cell component, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, no prior brain metastases, no uncontrolled hypertension, and no cardiac events the 12 months prior to entry into the study.14 However, subsequently after regulatory approval in the United States, the use of sunitinib has expanded to patients with prior therapy and unselected patients with unfavorable risk factors, as well as patients who have not undergone nephrectomy. An expanded access trial of approximately 4000 patients included patients with prior cytokine treatment (71%), ECOG score of 2 or better, 7.5% of patients had prior brain metastases, and 13% did not have clear cell histology.16 Notably, with increased time on sunitinib, 69% of patients who had been on treatment for more than 6 months experienced adverse events, compared with 25% of patients who were on sunitinib for less than 6 months. Grade 3 or 4 events which occurred in 39% of patients on sunitinib for longer than 6 months included nausea (1%), mucosal inflammation (2%), diarrhea (4%), fatigue (6%), thrombocytopenia (6%), and neutropenia (5%). The incidence of grade 3 or 4 cardiac adverse events was less than 1% which was not increased over time in extended use of sunitinib, demonstrating no increased cardiac toxicity over extended time periods. In this analysis, 8% of patients who were on sunitnib longer than 6 months discontinued therapy due to adverse events compared with 13% of patients on therapy less than 6 months.16 In a separate analysis of the 82 evaluated patients, 43% of sunitinib-treated RCC patients required dose reductions for treatment-related adverse events.17 Stomatitis, fatigue and HFS were the most frequent reasons for dose reductions. There was a correlation between increased adverse events and low body surface area, increased age, and female gender in the 49% of patients who underwent dose reductions or discontinuation.17 Further prospective evaluation of these and other patient characteristics predictive of toxicity is needed.
Adequate drug exposure is required to optimize sunitinib anti-tumor effect. Pharmacokinetic analyses demonstrate that sunitinib plasma concentrations correlate significantly with the probability of a partial response and time to progression in cytokine refractory patients.18 Since increased exposure to sunitinib is associated with improved clinical efficacy, the therapeutic goal should be to administer sunitinib at the highest tolerated dose for each patient, and to minimize dose delays, reductions, or discontinuation secondary to treatment related adverse side effects. This is best done through effective communication, early recognition of anticipated side effects, early intervention, and ongoing assessment of management strategies. The remainder of this article will focus on specific toxicities unique to sunitinib with emphasis on the incidence, purported mechanism when known, management and surveillance of each side effect.
Based on the phase II and III clinical trials, the recommended starting dose of sunitinib is 50 mg orally once daily for 28 days followed by a 14 day drug rest. The capsules are manufactured in strengths of 50, 25, and 12.5 mg. Dose limiting toxicities should result in initial dose reductions by 12.5 mg to 37.5 mg and then 25 mg daily. Dose reductions of sunitinib less than 25 mg daily may result in lower serum levels which may reduce therapeutic efficacy for the majority of patients and alternate therapy should be considered.11,18
Prior to initiating treatment, a focused review of past and current medical conditions should be conducted with emphasis on evaluation and optimization of clinical parameters which may be affected by sunitinib-related toxicities. These include aggressively managing uncontrolled hypertension, assessing for nutritional deficiencies, and detecting undiagnosed hypothyroidism. An evaluation of mucosal and skin surfaces with emphasis on foot examination is recommended. Consultation with podiatry or dermatology should be considered to treat pre-existing dermal pathology and optimize foot health prior to initiation of therapy. A multidisciplinary approach is recommended to provide an environment to facilitate good communication strategies with patients of the understanding, awareness, prevention, early detection, and management of side effects of sunitinib.
Drug interactions
Sunitinib is metabolized in the liver by cytochrome P450 (CYP3A4) pathway; thus, drugs that affect the CYP3A4 pathway may alter the metabolism of sunitinib and affect serum levels (Table 2). Caution should be used when sunitinib is administered in patients also receiving CYP3A4 inhibitors, as this may result in increased serum concentrations of both drugs. Conversely, coadministration with CYP3A4 inducers may lead to lower sunitinib levels and potentially decrease the efficacy and response of tumor to therapy. A thorough drug history should include medication list, prescription and nonprescription, and herbal remedies. St. John’s Wort, a common herbal remedy, is a CYP3A4 inducer, while grapefruit-containing products inhibit the CYP3A4 enzyme. Patients should be instructed to notify their oncology health care team of any new medications to minimize the potential for serious drug interactions. Coumadin is metabolized in the liver primarily by the P450 CYP2C9 cytochrome; however, a minor metabolic pathway involves metabolism via the CYP3A4 pathway, and thus theoretically, there may be alterations in warfarin metabolism.19 Patients on warfarin, or in whom warfarin is initiated while on sunitinib, should have close monitoring of their anticoagulation therapy with INR. Additionally, the treatment regimen of 4 weeks on therapy and 2 weeks off sunitinib each cycle may result in variable warfarin metabolism resulting in fluctuations of INR which should be anticipated and coumadin dosing should be individualized and adjusted accordingly.
Table 2.
Selected drug interactions41
| CYP3A4 Inhibitors (may raise sunitinib levels) |
|---|
| Atanzanavir |
| Clarithromycin |
| Grapefruit |
| Indinavir |
| Itraconazole |
| Ketoconazole |
| Nefazodone |
| Nelfinavir |
| Ritonavir |
| Saquinavir |
| Telithromycin |
| Voriconazole |
| CYP3A4 Inducers (may lower sunitinib levels) |
| Carbamazepine |
| Dexamethasone |
| Phenobarbital |
| Phenytoin |
| Rifabutin |
| Rifampin |
| Rifapentin |
| St John’s Wort |
Fatigue
Review of the available studies and clinical experience identifies fatigue as one of the most common toxicities sunitinib, but the degree of fatigue and its effect on quality of life is variable. Many patients carry on their normal activities of daily living and maintain similar activity schedules, while others experience fatigue and asthenia which ultimately are dose limiting. Management of fatigue is primarily supportive; however, it is important to identify correctable causes which may also contribute to fatigue, such as anemia and hypothyroidism. The presence of fatigue may also be a manifestation of psychosocial issues. Patients should be assessed for signs and symptoms of clinical depression and appropriate treatment measures should be introduced to optimize emotional and social support with pharmacologic treatment introduced when necessary. Identification and appropriate management of pain can help reduce debility. Ensuring proper nutrition and prevention of malnutrition, anorexia, and dehydration may also help reduce fatigue. Despite these interventions, dose reductions or treatment delays may be needed to allow patients to recover their quality of life and performance status.
Hypothyroidism
Multiple studies have identified thyroid dysfunction as a toxicity of sunitinib.20–22 In a retrospective review of the records of 66 patients receiving sunitinib for RCC, 85% of patients who were initially euthyroid developed one or more abnormal thyroid function tests.20 Thirty percent of patients were initiated on treatment for biochemical and clinical hypothyroidism resulting in a biochemical resolution of thyroid function abnormalities.
Subsequent prospective evaluation of 59 patients undergoing treatment with sunitinib for RCC or gastrointestinal stromal tumor determined that 27% of patients developed subclinical or clinical hypothyroidism.21 The percentage of RCC patients requiring treatment for hypothyroidism was 33%. The need for treatment was defined as persistent TSH greater than 10 mU/liter occurring on day one for two consecutive cycles and and typical symptoms of hypothyroidism. The median time to abnormal TSH for the RCC patients treated with sunitnib was 4 weeks, with a range of 2 to 22 months. Interestingly, most patients showed a pattern of elevated TSH level on day 28 of treatment cycle with a trend towards normalization 2 weeks later on the first day of the next treatment cycle after 2 weeks off sunitinib. Based on these observations and studies, the occurrence of thyroid abnormalities is high enough to warrant baseline thyroid function studies prior to initiation of sunitinib and every 2 cycles and thereafter when clinical symptoms develop. Clinical symptoms of hypothyroidism include fatigue, weakness, muscle aches and cramps, cold intolerance, bradycardia, depression, decreased deep tendon reflexes and myxedematous changes. Thyroid hormone replacement is indicated for severe biochemical abnormalities, such as TSH above 10 mU/liter, and/or if clinical symptoms of hypothyroidism develop. Mild elevations of TSH without other biochemical or clinical abnormalities may warrant only continued monitoring.
Cardiovascular side effects
VEGF inhibitors appear to have a class effect on the cardiovascular system including hypertension and cardiac toxicity. While the precise mechanism is not clear, hypotheses include pressor stimulation, increased extracellular volume, decreased vascular compliance, increased vascular resistance, endothelial dysfunction and altered nitrous oxide metabolism.23 In vitro evaluations of mice, rat, and human cardiomyocytes and coronary artery smooth muscle cells treated with sunitinib demonstrated toxicities, including myofibrillar disorganization, mitochondrial injury, and alteration of intracellular signaling pathways.24 Hypoxia inducible factor-1 related products are involved in myocardial response to ischemia, cardiac remodeling and revascularization, and thus inhibition of these products might be expected to hinder normal cardiac repair mechanisms.25,26
With increased clinical experience and widespread use of sunitinib in patients who may have increased cardiac risk factors or prior cardiac events, information on cardiac toxicities, including heart failure and cardiomyopathy, associated with sunitinib therapy is accumulating (Table 3). In the pivotal phase III study of sunitinib in RCC, 10% of patients experienced a decline in ejection fraction, with most changes being grade 1 or 2 and not dose limiting.14 It is important to note that these patients were prescreened (with inclusion criteria of left ventricular ejection fraction of greater than or equal to the lower limit of normal) and those who had cardiac dysfunction, defined as myocardial infarction, unstable angina, coronary or peripheral artery bypass graft, symptomatic congestive heart failure, transient ischemic attack, cerebrovascular accident or pulmonary edema within the past 12 months, were excluded from participation. Other cardiac adverse event data come from experience with sunitinib in patients with gastrointestinal stromal tumors (GIST) in which 11% patients had cardiovascular events including myocardial infarction or congestive heart failure.27 In this phase I/II study of patients with GIST, reductions of at least 10% in ejection fraction occurred in 28% patients treated with sunitinib. A separate retrospective review of 224 patients receiving sunitinib for 10 different malignancies identified 6 patients (3%), 4 of whom had RCC, who developed clinically significant congestive heart failure.28
Table 3.
Studies reporting cardiovascular side effects in patients treated with sunitinib
| Author | Motzer14 | Chu27 | Khakoo28 | Telli42 | Schmidiger29a |
|---|---|---|---|---|---|
| Study design | Prospective | Retrospective | Retrospective | Retrospective | 10 retrospective |
| 64 prospective | |||||
| Number of patients | 375 | 75 | 224 | 48 | 74 |
| % of patients with mRCC | 100% | 0% (100% GIST) | 77% (23% other solid tumors) | 85% (15% GIST) | 100% |
| Pre-existing HTNb | NR | 29% | 54% | 50% | 52% |
| Prior CAD | N/A | 5% | 13% | 6% | 9% |
| Prior CHF or cardiomyopathyc | N/A | 0% | NR | 6% | 7% |
| HTN during therapyd | 24% | 47% | NR | 67% | NR |
| >10% ejection fraction decline | <8% | 28% | 3%e | 15% | 14% |
| Symptomatic CHF | 2% | 8% | 3% | 15%f | NR |
Study included sunitinib and sorafenib.
Hypertension not defined in most studies, Chu27 defined hypertension as systolic >150 mmHg or diastolic >100 mmHg.
CHF or cardiomyopathy defined as prior symptomatic heart failure or ejection fraction below lower limit of normal.
Patients on therapy who had hypertension (includes pre-existing and on treatment) as determined by each study.
Study reported patients with symptomatic CHF, all of whom had >10% ejection fraction decline with therapy. Additional patients with >10% decline in ejection fraction without development of symptomatic CHF were not clearly defined.
Study identified patients at the time of presentation with grade 3/4 left ventricular dysfunction (ejection fraction < 40%) and symptomatic CHF requiring intervention.
Abbreviations: GIST, gastrointestinal stromal tumors; RCC, renal cell carcinoma; HTN, hypertension; CAD, coronary artery disease; CHF, congestive heart failure; N/A, not included if within past year; NR, not reported.
An observational study of sunitinib or sorafenib, another multi-targeted tyrosine kinase inhibitor, for treatment of metastatic RCC identified 34% of patients as having a cardiac event, defined as increased cardiac enzymes (both symptomatic and asymptomatic), symptomatic arrhythmia, left ventricular dysfunction, or acute coronary syndrome, and 41% having electrocardiogram (ECG) changes.29 The investigators prospectively evaluated 74 patients receiving sunitinib or sorafenib for mRCC with cardiac enzymes drawn with routine blood sampling bimonthly and performed ECGs monthly in asymptomatic patients and at any time patients clinically appeared to have cardiac symptoms. Thirteen of the 25 patients with a cardiac event had cardiac symptoms, defined as dyspnea on exertion, angina, or dizziness. Cardiac enzymes drawn with routine bloodwork or at the time of cardiac symptoms were elevated in 21 patients; 12 of these patients with elevated cardiac enzymes had no clinical symptoms. Of these asymptomatic patients with elevated cardiac enzymes, some underwent dose reduction or discontinuation, with subsequent resolution of enzymes. Other patients, showed fluctuations of cardiac enzymes which were felt to be related to the cyclic nature of sunitinib dosing. ECG changes were found in 12 of 25 symptomatic patients and in 18 (24%) asymptomatic patients. All patients recovered after cardiac medical event management and were considered eligible for reinitiation of TKI therapy. While ECGs are generally recommended at the time clinical concerns for cardiac symptoms arise, the significance of screening for asymptomatic ECG changes is unclear at this time.
It is important to assess a patient’s cardiac risk factors and cardiovascular history, and if concern for heart failure is identified, to obtain appropriate cardiac studies and monitor closely for changes in symptoms or functional decline with repeat cardiac evaluation. Congestive heart failure and left ventricular dysfunction generally, though not always, are reversible after discontinuation of sunitinib. Thus patient education and clinical vigilance are paramount to ensure early identification of symptoms with timely clinical assessment and appropriate laboratory and cardiac evaluation in order to initiate aggressive medical intervention when clinically indicated.
Most studies have demonstrated sunitinib-induced increases in mean systolic and diastolic blood pressure, with variable incidences of hypertension ranging from 28% to 47% reported in studies.14,16,27 Blood pressure should be evaluated as part of toxicity reviews and patients should be instructed to perform daily home blood pressure monitoring. Standard lifestyle recommendations for hypertension should be followed including regular exercise, weight control, and sodium restriction. Pharmacologic interventions include most classes of antihypertensives and no specific class has been proven more effective for sunitinib-induced hypertension. The non-dihydropyridine calcium channel blockers, verapamil and diltiazem, are both metabolized by the CYP3A4 cytochrome and should be used with caution. Patients should be given instructions regarding systolic and diastolic blood pressure parameters, for example systolic blood pressure greater than 150 or diastolic blood pressure greater than 90, which would necessitate notification of their oncology health care team. Sunitinib induced hypertension is usually reversible and improves with discontinuation, thus it is important to review antihypertensive medications and make appropriate adjustments if sunitinib regimen is reduced or treatment is interrupted. Additionally, blood pressure should be monitored during the scheduled 2 weeks off sunitinib in each cycle as improvements in blood pressure may require a temporary decrease in antihypertensive therapy.
Sunitinib can cause QT prolongation and should be given with caution in patients with history of QT prolongation or taking other medications which can prolong the QT interval. Medications affecting the QT interval include, but are not limited to, antiarrhythmics, antipsychotics, some antidepressants, certain antibiotics, some antiemetics, and methadone. Baseline electrocardiograms should be obtained in patients with relevant history or symptoms of arrhythmia. These patients should then be monitored with repeat ECG in each cycle of therapy and in whom clinical symptoms develop.
Gastrointestinal toxicity
Patients on sunitinib may experience anorexia, dyspepsia, flatulence, bloating, nausea or vomiting, most often mild to moderate in severity. Appropriate intervention with dietary changes such as avoidance of irritating or spicy foods and increasing the frequency of small meals may provide improvement of symptoms. Pharmacologic intervention with antiemetics or proton pump inhibitors as needed to avoid decreased oral intake. Initial patient education should place emphasis on the importance of maximizing oral hydration strategies to avoid dehydration and the potential for therapy interruption. Patients and family members should be instructed to call the office if they develop lightheadedness or dizziness or a decrease in blood pressure, allowing early assessment and intervention for dehydration with intravenous hydration.
Oral changes include taste alterations, mouth sensitivity, and functional and clinical stomatitis. These toxicities are usually grade 2 or less and are experienced by up to 30% of patients. In general, the oral toxicities increase throughout the active drug period of each cycle and improve during the 2 week off period. Oral care changes should include gentle brushing of teeth with toothpaste and mouthwash which are non-alcohol based, such as pediatric toothpaste (Table 4). Symptomatic relief can be tailored to symptoms such as special mouthwash preparations containing viscous lidocaine for mucositis, or lip balm for cheilitis. If necessary, pain medication may be needed to prevent dysphagia and decrease risk of dehydration or malnutrition. For grade 3 and 4 mucositis, interruption in therapy or dose reduction should be considered to prevent clinical deterioration in nutritional status.
Table 4.
Oral care products
| Cleansers and rinses | Product information |
|---|---|
| Salt water rinse | |
| Salt and baking soda rinse | |
| Biotine® rinse | Bioactive enzymes |
| Chlorhexidine gluconate 0.12% | Antimicrobial mouth rinse |
| Toothpastes | |
| Sensodyne® toothpaste | Potassium nitrate to reduce painful sensitivity |
| Children’s toothpaste | Flavoring is milder and burns less |
| Rinses for comfort and mucosal protection | |
| Rincinol™ PRN | Aloe vera rinse |
| Gelclair® bioadherent oral gel | Hydrates, soothes, and protects oral mucosa |
| Lidocaine Viscous | 2% lidocaine hydrocholoride solution |
| Lip protectants | |
| Blistex® | Dimethicone |
| Burt’s Bees® | Beeswax, oils, lanolin |
| Carmex® | Menthol, camphor, phenol |
| Zim’s Crack Creme® | Arnica |
Approximately half of sunitinib-treated patients with metastatic RCC experience diarrhea, usually grade 1 or 2 which is defined as an increase of up to 6 stools a day and/or requirement of less than 24 hours of intravenous hydration secondary to diarrhea.14 Diarrhea is usually characterized as loose, but not watery, and associated with urgency. Management of symptoms of diarrhea includes attention to dietary intake and avoidance of aggravating factors which can contribute to diarrhea. Aggressive oral fluid intake should be encouraged to avoid dehydration. Bananas, rice, and fiber-containing foods are among the dietary additions which can be recommended for patients. Pharmaceutical management options include loperamide or diphenoxylate. Patients should be encouraged to initiate these medications at the first signs of diarrhea. If diarrhea is severe and worsened with eating, loperamide or diphenoxylate can be taken 30 minutes prior to a meal. Cholestyramine taken prior to meals provides another alternative treatment option. Early intravenous hydration may be indicated for patients who develop dehydration from inadequately controlled diarrhea, and may minimize the need for treatment interruption or dose reduction. Detailed review with patient regarding adherence to recommended anti-diarrheal agent regimen for control of diarrhea is important to determine if changes in medications or dosing guidelines should be made.
Dermatologic changes
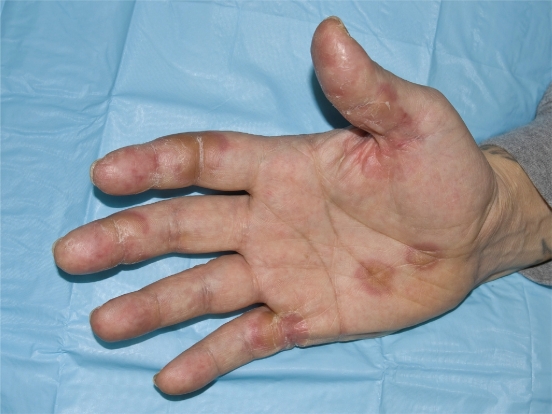
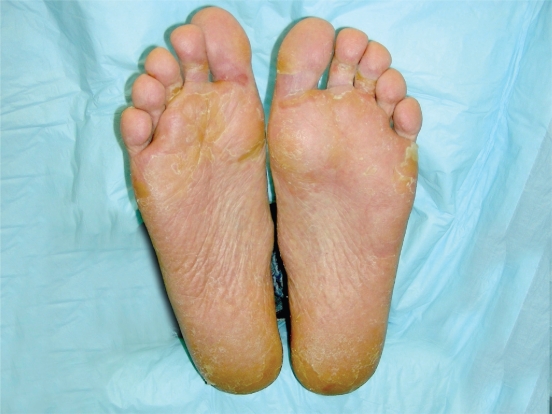
Skin and hair changes are frequent in patients taking sunitinib. The constellation of dermatological changes is unique and the management of side effects has largely been based on expert opinion and clinical experience. Palmar plantar erythrodysesthesia, also known as HFS, is a dermatologic condition which involves painful blister and callus formation of areas which are subjected to pressure or friction, such as the palms, thumbs and fingers, and soles of feet (Figures 1 and 2).30–32 Patients may also experience severe dry skin, cracking, and desquamation. The severity of the painful calluses can interfere with the patient’s ability to maintain their normal schedule of activities and lead to a decline in function and quality of life; in severe cases, patients are unable to bear weight on their feet secondary to pain. Hand-foot symptoms usually improve during the 2 week off drug period during each cycle; however, grade 3 HFS (pain interfering with activities of daily living) requires dose delays and possibly dose reductions. Patients should wear comfortable shoes with extra support. Management of acral erythema includes creams, lotion, and pedicure. Rashes may develop on any body surface and may be macular or papular in appearance. Pruritis may occur with dry skin and is managed by providing aggressive symptomatic relief with topical emollients or steroids, as well as consideration or oral antihistamine (Table 5). Hyperkeratosis can be treated with urea-based creams. Cotton socks, gel insole liners, and shoes designed for extra comfort and cushion may minimize the extent and severity of hand foot syndrome for some patients. Effective management of dermatologic toxicities can improve tolerance and often symptoms do not recur following resolution of dermatologic reaction. Emphasis on initiation of skin care at first signs of dermatologic toxicity and early notification of oncology health care team will allow assessment and changes to clinical regimen as indicated.
Figure 1.
Grade 3 hand-foot syndrome including acral erythema, hyperkeratotic areas, and desquamation. Considered grade 3 due to pain interfering with function.
Figure 2.
Grade 2 hand-foot syndrome including erythema, desquamation, and skin discoloration. Considered grade 2 because mild discomfort did not interfere with function.
Table 5.
Skin care products for hand-foot syndrome
| Skin care products | Product information |
|---|---|
| Cetaphil skin cleanser, Aveeno® shower gel | Non-deodorant, non-fragrant body washes |
| Udderly Smooth® udder cream | Allantoin, dimethicone |
| Udderly Smooth® extra care cream | Allantoin, dimethicone, 10% urea |
| Aveeno® skin relief moisturizing cream | Natural colloidal oatmeal; dimethicone |
| Norwegian Formula: soothing relief anti-itch moisturizer by Neutrogena | Dimethicone 1%, camphor 0.1%, and lidocaine |
| Norwegian Formula: foot cream by Neutrogena | Ceterayl alcohol, dimethicone, menthol and urea |
| Bag Balm® | 8-hydroxyquinoline sulfate 0.3% in a petrolatum lanolin base; eucalyptus |
| Eucerin® cream | Mineral oil, lanolin |
| Eucerin® dry skin therapy | Urea and alpha hydroxy acid |
| Aquaphor® healing ointment | 41% petrolatum |
| Xenaderm® ointment | Balsam peru, castor oil, trypsin |
| Corn Huskers Lotion® | Calcium alginate; non-oily moisturizer |
| Biafine® cream | Water-based emulsion for radiation dermatitis or abrasions |
| Gold Bond® triple action relief cream | 5% dimethicone, 0.15% menthol, aloe, vitamin E |
| Gold Bond® ultimate healing lotion | 5% dimethicone, jojoba esters, aloe |
| Gold Bond® anti-itch cream | 1% pramoxine HCL, 1% menthol |
| Kerasal™ | 5% salicylic acid: softens skin 10% urea: exfoliates and moisturizes |
| Keralac™ cream | 50% urea: exfoliates and moisturizes vitamin E, lactic acid, zinc |
| Keralac™ lotion | 35% urea: exfoliates and moisturizes vitamin E, lactic acid, zinc |
| Carmol® 40 | 40% urea |
| Miracle Foot Repair® cream | 60% aloe, 0.1% menthol |
| Regenecare® HA | 2% lidocaine, humectanct (moisturizer), aloe vera extract, hyaluronic acid (anti-inflammatory agent) |
| Lidocaine topical | 2% lidocaine |
| Lidamantle® | 3% lidocaine HCL |
| Lidamantle HC® | 3% lidocaine HCL, 0.5% hydrocortisone acetate |
| Pramocaine HCL | 1% pramoxine hydrochloride |
| Tetracaine jelly | 2% lidocaine |
Sunitinib commonly results in a yellowish color of patient’s skin which is related to this drug. Color changes can also be seen in the urine. These color changes can be confused with jaundice, but are not related to elevated bilirubin as evidenced by lack of yellow conjunctivae. Hair discoloration involves depigmentation and a resulting grayish hair color which reverses after discontinuation of sunitinib. Asymptomatic subungual hemorrhages occasionally occur with sunitinib, but do not require any intervention or change in treatment plan.
Toxicity in patients with brain metastases
As previously discussed, initial studies of sunitinib in RCC excluded prior or current brain metastases. Subsequently, with expanded access and increasing use of sunitinib in the community, the use of sunitinib in patients with brain metastases has increased. A few retrospective analyses have reviewed the outcomes of patients with metastatic RCC involving brain metastases undergoing targeted therapy treatment. The incidence of intracranial bleeding or hemorrhage in these studies with sunitinib or sorafenib ranged between 0% and 7%.33,43 The importance of blood pressure control was highlighted in a retrospective series which reported a 7% incidence of intracerebral hemorrhage; 80% of these patients had uncontrolled hypertension at the time of diagnosis.33 While most brain metastases pretreated with surgical resection or radiotherapy prior to sunitinib therapy did not result in any adverse intracranial bleeding events, close clinical observation and good blood pressure control are recommended. Sunitinib therapy may be held for several days prior to and following palliative radiation therapy to minimize overlapping toxicities.
Posterior reversible leukoencephalopathy syndrome
Posterior reversible leukoencephalopathy syndrome (PRES) has been reported in a patient with RCC 2 weeks after starting the first cycle of treatment at 50 mg daily.35 PRES is characterized by acute hypertension, seizures, impaired vision, and classic findings on MRI or CT imaging of the brain. As in the reported case, PRES is reversible, if diagnosed early in the course and treated with discontinuation of sunitinib and appropriate control of hypertension and seizures.
Tumor lysis syndrome
As evidenced by sunitinib’s efficacy in producing robust overall response rates, large bulky tumors may respond dramatically to treatment and tumor lysis syndrome has been reported as a rare complication. Prophylaxis should be considered in those patients who may be at risk.36
Hematologic toxicity
In the initial phase III trial comparing sunitinib with interferon-alpha, all grade leukopenia, neutropenia, anemia, and thrombocytopenia occurred in 60% to 70% of all patients treated with sunitinib.14 According to the expanded access analysis, in patients on sunitinib for 6 months or longer, transient grade 3 or 4 neutropenia and thrombocytopenia occurred in less than 6% and 8% of patients, respectively.16 Despite reductions in absolute neutrophil count, neutropenic fever or infection was not reported in the phase II or phase III trials.12–14,37 Less than 4% of patients experience grade 3 or 4 toxicity.14 Interestingly, macrocytosis has been observed in some patients but was not associated with decreased folate or cobalamin levels and improved in patients after discontinuation of sunitinib.38 Blood counts usually recover during the 2 week off period or with drug interruptions. Complete blood counts should be obtained immediately prior to each cycle in patients with documented cytopenias in prior cycles or at the end of the 4-week dosing period when cytopenias are likely to nadir. If grade 3 or 4 neutropenia or thrombocytopenia are present at the time of initiation of a new cycle, dose delay is recommended until toxicity is grade 2 or less. If the same toxicity occurs in subsequent cycles, dose reduction in sunitinib should be considered.
The same antiangiogenesis effect that contributes to the potency of sunitinib by preventing adequate blood flow to malignant tumors, also leads to the potential for significant adverse events by preventing adequate blood flow to normal tissues at a time of injury. Bleeding has been noted in the form of epistaxis, gingival, or gastrointestinal bleeding.14 The use of saline nasal spray and a bedside humidifier may reduce the incidence and severity of dryness in nasal passages resulting in risk of epistaxis.
Theoretically, patients on angiogenesis inhibitors are at risk of poor surgical wound healing, and sunitinib should be held in the perioperative period. The half-life of sunitinib is 40 hours, thus discontinuation of sunitinib for 1 week, or approximately 5 half-lives, prior to major surgical procedures would allow time for adequate drug elimination in order to prevent interference with angiogenesis, hemostasis, and wound healing.
Secondary to the increased risks of bleeding and poor wound healing, the use of anticoagulants may compound bleeding risks and potential complications when combined with sunitinib therapy. Coumadin should be used with caution in patients on sunitinib due prolonged effect on the coagulation cascade. As mentioned previously, coumadin also undergoes metabolism primarily by the hepatic P450 enzyme and thus the interaction of sunitinib can potentially alter levels of both drugs.39 Since most patients on sunitinib therapy are administered cycles of 4 weeks of sunitinib and 2 weeks without sunitinib, this planned alternation in therapy can lead to variability in coumadin metabolism and requires close attention to the potential for alterations in INR levels.
Conclusion
Since the approval of sunitinib for advanced RCC, there has been a fundamental shift in the management of patients with kidney cancer. In many ways, the breakthroughs in the understanding of the mechanisms of RCC and its targeted treatment options provide a model system to understand the future of cancer care. The shifting paradigm of cancer treatment no longer solely relies on intermittent physician-directed administration of therapy in a healthcare facility; instead, it increasingly entails the management of patients self-administering potent oral daily medication. Maximizing clinical outcomes of sunitinib therapy requires clear, effective communication, anticipation of side effects, and early intervention to avoid treatment delays and dose-limiting toxicities.
Footnotes
Disclosures
None of the authors disclose conflicts of interest.
References
- 1.McLaughlin JK, Lipworth L, Tarone RE. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33(5):527–533. doi: 10.1053/j.seminoncol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 4.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23(3):202–212. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 5.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: The multiethnic cohort. Am J Epidemiol. 2007;166(8):932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 6.Karumanchi SA, Merchan J, Sukhatme VP. Renal cancer: Molecular mechanisms and newer therapeutic options. Curr Opin Nephrol Hypertens. 2002;11(1):37–42. doi: 10.1097/00041552-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Latif F, Tory K, Gnarra J, et al. Identification of the von hippel-lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23(5):1028–1043. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 9.Chow LQ, Eckhardt SG. Sunitinib: From rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 10.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 11.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 15.Figlin RA, Hutson TE, Tomczak P, et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC) J Clin Oncol (Meeting Abstracts) 2008;26(15 suppl):5024. [Google Scholar]
- 16.Porta C, Szczylik C, Bracarda S, et al. Short- and long-term safety with sunitinib in an expanded access trial in metastatic renal cell carcinoma (mRCC) J Clin Oncol (Meeting Abstracts) 2008;26(15 suppl):5114. [Google Scholar]
- 17.van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. 2008;99(2):259–265. doi: 10.1038/sj.bjc.6604456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houk BE, Bello CL, Michaelson MD, et al. Exposure-response of sunitinib in metastatic renal cell carcinoma (mRCC): A population pharmacokinetic/pharmacodynamic (PKPD) approach. ASCO Meeting Abstracts. 2007;25(18 suppl):5027. [Google Scholar]
- 19.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(6 suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99(1):81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 21.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: A prospective evaluation. Br J Cancer. 2008;99(3):448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17(4):351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 23.Sica DA. Angiogenesis inhibitors and hypertension: An emerging issue. J Clin Oncol. 2006;24(9):1329–1331. doi: 10.1200/JCO.2005.04.5740. [DOI] [PubMed] [Google Scholar]
- 24.Dallabrida SM, Ismail N, Pravda EA, et al. Abstract 1505: Sunitinib-induced cardiotoxicity is mediated in part via direct effects on cardiac myocytes and smooth muscle cells Circulation 2007116(16 MeetingAbstracts):II 311. [Google Scholar]
- 25.Kido M, Du L, Sullivan CC, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46(11):2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Parisi Q, Biondi-Zoccai GG, Abbate A, et al. Hypoxia inducible factor-1 expression mediates myocardial response to ischemia late after acute myocardial infarction. Int J Cardiol. 2005;99(2):337–339. doi: 10.1016/j.ijcard.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: A multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112(11):2500–2508. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 29.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum SE, Wu S, Newman MA, West DP, Kuzel T, Lacouture ME. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer. 2008;16(6):557–566. doi: 10.1007/s00520-008-0409-1. [DOI] [PubMed] [Google Scholar]
- 31.Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering pandora’s vase: The growing problem of new toxicities from novel anticancer agents. the case of sorafenib and sunitinib. Clin Exp Med. 2007;7(4):127–134. doi: 10.1007/s10238-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6(7):491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 33.Pouessel D, Culine S. High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur Urol. 2008;53(2):376–381. doi: 10.1016/j.eururo.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 34.Unnithan JS, Choueiri T, Garcia J, et al. Safety of VEGF-targeted tyrosine kinase inhibitors in patients (pts) with metastatic renal cell carcinoma (mRCC) and central nervous system (CNS) metastases. J Clin Oncol (Meeting Abstracts) 2007;25(18 suppl):5047. [Google Scholar]
- 35.Martin G, Bellido L, Cruz JJ. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol. 2007;25(23):3559. doi: 10.1200/JCO.2007.12.8710. [DOI] [PubMed] [Google Scholar]
- 36.Nicholaou T, Wong R, Davis ID. Tumour lysis syndrome in a patient with renal-cell carcinoma treated with sunitinib malate. Lancet. 2007;369(9577):1923–1924. doi: 10.1016/S0140-6736(07)60903-9. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178(5):1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Rini BI, Choueiri TK, Elson P, et al. Sunitinib-induced macro-cytosis in patients with metastatic renal cell carcinoma. Cancer. 2008;113(6):1309–1314. doi: 10.1002/cncr.23711. [DOI] [PubMed] [Google Scholar]
- 39.Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics. 2003;13(5):247–252. doi: 10.1097/00008571-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Common Terminology Criteria for Adverse Events (CTCAE version 30) National Institutes of Health 2003. Accessed on the Cancer Therapy Evaluation Program (CTEP). Available at http://ctep.info.nih.gov
- 41.Sunitinib (Sutent®) [package insert]. 2007. New York, NY: Pfizer Labs, Division of Pfizer Pharmaceuticals; 2007 [Google Scholar]
- 42.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19(9):1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]