Abstract
The ubiquitin–proteasome system has become a promising molecular target in cancer therapy due to its critical role in cellular protein degradation, interaction with cell cycle and apoptosis regulation, and unique mechanism of action. Bortezomib (PS-341) is a potent and specific reversible proteasome inhibitor, which has shown strong in vitro antitumor activity as single agent and in combination with other cytotoxic drugs in a broad spectrum of hematological and solid malignancies. In preclinical studies, bortezomib induced apoptosis of malignant cells through the inhibition of NF-|B and stabilization of pro-apoptotic proteins. Bortezomib also promotes chemo- and radiosensitization of malignant cells in vitro and inhibits tumor growth in murine xenograft models. The proteasome has been established as a relevant target in hematologic malignancies and bortezomib has been approved for the treatment of multiple myeloma. This review summarizes recent data from clinical trials in solid tumors.
Keywords: proteasome, bortezomib, NF-κB, clinical studies, solid tumors
Introduction
Proteasome inhibitors represent a class of drugs that have anticancer activity through a variety of cellular mechanisms including induction of apoptosis, interference with cell cycle progression, inhibition of angiogenesis, and suppression of nuclear transcription factor kappa B (NF-κB).1 The proteasome is a multicatalytic enzyme complex that degrades several intracellular proteins by a targeted and controlled mechanism.2–4 The activity of proteasome in degradation of tumor-suppressing and proapoptotic protein targets known to be dysregulated in many human malignancies provides the rationale for its selection as a target for cancer therapy. The first-in-class proteasome inhibitor, bortezomib (PS-341; Velcade®; Millennium Pharmaceuticals), a boronic acid dipeptide derivative, received approval in the United States (2003) and Europe (2004) for relapsed and refractory multiple myeloma.5–7 In the APEX phase III study, comparing bortezomib and dexamethasone in patients with multiple myeloma, the median time to progression was significantly increased from 106 days with dexamethasone to 189 days with bortezomib and the 1-year overall survival was also higher in the bortezomib arm (80% vs 66%).7 Recently, the FDA approved bortezomib in relapsed mantle cell lymphoma.8
Bortezomib has also shown activity in preclinical studies of a variety of solid tumors, and this has paved the way for several phase I/II clinical studies of bortezomib either as single agent or in combination with cytotoxic and biologic agents.
This review aims to highlight current knowledge of the anticancer effects of bortezomib with emphasis on recent clinical studies.
Mechanism of action of proteasome
The ubiquitin-mediated proteasome pathway regulates a group of intracellular proteins that govern cell cycle, tumor growth, and survival. Proteasome participates in the turnover and degradation of several pathways, or short-lived cellular regulatory proteins, including p53, cyclins and the cyclin-dependent kinase (CDK) inhibitors p21 and p27, the estrogen receptor, and the inhibitor (IκB) of NF-κ.9–13 26S proteasome consists of a multisubunit, cylindrical complex including a 20S core catalytic component and 19S regulatory particles that contain polyubiquitin-binding sites and isopeptidase activity for the cleavage and release of ubiquitin from the protein substrate.14 The proteasome requires adenosine triphosphate (ATP) hydrolysis and regulates multicatalytic protease that selectively degrades polyubiquinated proteins. These proteins get marked for degradation by a multistep process. Prior to degradation, polyubiquitinated moieties are covalently attached to the target proteins in a multistep process involving three distinct enzymes: the ubiquitin-activating enzyme E1 binds ubiquitin and transfers it to the ubiquitin-conjugating enzyme E2. In the following step the ubiquitin ligase E3 catalyzes the transfer of the polyubiquitinated tails from E2 to lysine residues of the target protein, specifically marking them for proteolysis protein into small fragments in an ATP-dependent manner.4,15 Activated p53 arrests cells in the G1-phase and promotes apoptosis to allow elimination of damaged cells through induction of the proapoptotic protein Bax, which, in turn, is also a proteasomal substrate. The final outcome of this process is proteasome inhibitor-induced stabilization of p53, p21Cip1, p27Kip1, and Bax, dysregulation of cell-cycle progression and, finally, apoptosis.16
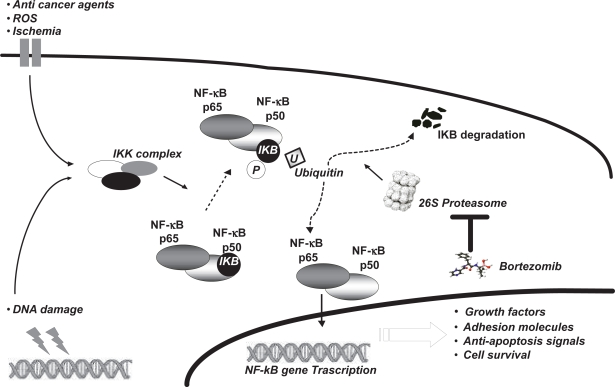
The proteasome modulates also the activity of the transcription factor nuclear NF-κB. The NF-κB pathway is activated by a variety of cellular stress signals, and chemo- and radiotherapy, which lead to phosphorylation of a serine residue on IκB, which targets it for ubiquitination and proteasomal degradation. This process allows activated the NF-κB subunit to translocate into the nucleus, where it induces expression of a variety of genes encoding cell adhesion molecules and antiapoptotic factors (Figure 1).17–19 More details have been provided in our previous review.20
Figure 1.
Several intra- and extracellular factors induce the intracellular increase of the IKK complex which phosphorylates the IKB protein. Phosphorylation of IKB causes its ubiquitination and degradation by 26S proteasome. NF-κB complex is then able to interact with its DNA ligand site, stimulating transcription of several genes which prompt apoptosis inhibition, growth factor increases, and cell survival. Bortezomib acts by inhibiting 26S proteasome.
Abbreviation: ROS, reactive oxygen species.
Preclinical studies of proteasome inhibitors
Proteasome inhibitors have been shown to be cytotoxic against a variety of cancer cell lines in vitro and in in vivo models. The activity of bortezomib in solid tumors in vivo has been evaluated in a variety of xenograft models.21,22
Bortezomib also increases the sensitivity of tumor cells to chemotherapy and radiation and reverses chemoresistance. Bortezomib was two times more potent in inhibiting the growth of chemoresistant multiple myeloma cells compared with chemosensitive cells, in direct correlation with NF-κB activity.23 In colon carcinoma cells, bortezomib inhibited the radiation-induced increase in NF-κB and enhanced radiosensitivity.24
Many chemotherapeutics induce NF-κB and thereby activate an antiapoptotic program that, if inhibited, can enhance the antitumor activity of the chemotherapeutic.25 Inhibition of the proteasome was shown initially to increase the efficacy of CPT-11 (irinotecan) through blockade of NF-κB in a model of colon cancer.26 In another study, gemcitabine caused a 59% reduction of pancreatic cancer volume compared with control, while the combination of gemcitabine and bortezomib increased growth inhibition to 75%.27
Taken together, results from preclinical studies show that bortezomib can induce apoptosis in a number of otherwise resistant tumor cells and can sensitize cancer cells to other cytotoxic agents and radiation therapy.
Clinical studies
The clinical feasibility of using bortezomib for treating solid malignancies has been explored in a number of phase I and II studies, the main of which are summarized below.
Phase I studies
A number of phase I trials have been carried out with different schedules of bortezomib.
A phase I clinical study evaluated the dose-limiting toxicity (DLT) and maximum-tolerated dose (MTD) of bortezomib as single agent administered as an intravenous bolus once-weekly for 4 out of 5 weeks in 53 patients, 48 of whom had advanced androgen-independent prostate cancer. The DLT was seen in 2 of 5 patients treated with a dose of 2.0 mg/m2, and it included grade 3 diarrhea in both patients and grade 3 syncope and hypotension in one patient; so, the recommended phase II dose of bortezomib was 1.6 mg/m2. Two patients with prostate cancer had prostate-specific antigen response, whereas 2 patients had partial response in lymph nodes. The biologic activity, such as inhibition of NF-κB related markers, was seen at tolerated doses of bortezomib. The maximum level of 20S inhibition was 70% to 75%, which suggests that the inhibition of proteasome is saturable.28 Another phase I study tested two different schedules (schedule 1: twice weekly for 4 out 6 weeks; schedule 2: twice weekly for 2 out of 3 weeks) of bortezomib in 44 patients with advanced cancers. The most common toxicity was thrombocytopenia, which was dose limiting at 1.7 mg/m2 (schedule 1) and 1.6 mg/m2 (schedule 2), whereas the MTD was 1.5 mg/m2 for both schedules. A patient with multiple myeloma had a partial response.29 Another schedule of bortezomib (starting dose: 1.0 mg/m2 on days 1, 4, 8, 11, every 3 weeks) was tested in a phase I/II study in 18 patients with unresectable hepatocellular carcinoma. Grade 2/3 toxicities included thrombocytopenia, fatigue, and neuropathy. MTD was considered to be 1.3 mg/m2. In 7/15 evaluable patients, stable disease was observed.30 The phase I single-agent studies with bortezomib are summarized in Table 1.
Table 1.
Phase I single agent studies of bortezomib
| Patient population | Prostate cancer | Advanced others solid tumors | Hepatocarcinoma |
|---|---|---|---|
| Number of patients | 53 | 46 | 14 |
| Schedule | Starting dose of 0.13 mg/m2 iv once weekly for 4 weeks q5 weeks | Arm 1: starting dose of 0.13 mg/m2 iv twice weekly for 4 weeks q6 weeks Arm 2: starting dose of 0.13 mg/m2 iv twice weekly for 2 weeks q3 weeks |
Starting dose of 1.0 mg/m2 iv on days 1, 4, 8, 11 q3 weeks |
| Best response obtained | 2 PR 2 SD | 1 PR | 7 SD |
| Toxicity observed | Diarrhea and hypotension | Neurotoxicity and fatigue | Thrombocytopenia, neurotoxicity and fatigue |
| MTD | 1.6 mg/m2 | Arm 1: 1.7 mg/m2 Arm 2: 1,6 mg/m2 | 1.3 mg/m2 |
Abbreviations: PR, partial response; SD, stable disease; MTD, maximum-tolerated dose.
Following preclinical studies which highlighted the synergy between bortezomib and taxanes,31,32 a phase I trial of twice-weekly bortezomib and weekly docetaxel was carried out; the recommended doses were 0.8 mg/m2 and 25 mg/m2, respectively, every 21 days. The DLT were thrombocytopenia and febrile neutropenia. Clinical activity was low in this pretreated patient population, since only 4 patients had stable disease as best observed response.33 In another phase I study, the combination of paclitaxel and bortezomib revealed no response rates.34 Another phase I study was carried out to evaluate the combination between 5-fluorouracil (5-FU) 500 mg/m2 and leucovorin (LV) 20 mg/m2 with starting dose of bortezomib 0.5 mg/ m2 twice weekly for 4 weeks, with 2 weeks rest. One partial response, 8 stable disease, and 10 progressive disease were achieved in 19 patients.35 Another phase I clinical trial evaluated the safety and biologic effects of bortezomib and irinotecan coadministered in 51 patients. The MTD for the combination regimen was bortezomib 1.3 mg/m2 twice a week and irinotecan 125 mg/m2 on days 1, 8, followed by a 1-week rest.36 In another study, escalating doses of bortezomib were administered along with standard FOLFOX-4 doses, in order to evaluate the DLT, toxicity profile, and activity of the combination. Among 13 evaluable patients, 5 had a partial response, 5 had stable disease, and 3 progressed. Two patients are long-term survivors after a combined chemosurgical approach.37
Findings from preclinical studies38 prompted a phase I trial to determine the MTD of escalating doses of gemcitabine (1000 mg/m2 given once a week for 2 weeks) with bortezomib (1.0 mg/m2 given twice a week) every 21 days, in 31 patients with advanced solid tumors. This combination was well tolerated and a partial response was observed in a patient with advanced nonsmall-cell lung cancer (NSCLC), who had been pretreated with gemcitabine.39 In another phase I study, a combination of bortezomib, gemcitabine, and carboplatin induced 4 partial responses and 5 stable diseases in 16 patients with advanced NSCLC.40
In addition to these studies, the administration of gemcitabine via fixed-dose rate (FDR) infusion at 10 mg/m2/min has been shown to increase accumulation of the active phosphorylated metabolite of the drug. Recently, a phase I trial was set out to determine the safety, toxicity, and MTD of FDR gemcitabine with bortezomib in advanced solid tumors refractory to standard therapy. One partial response lasting 12 months was observed in breast cancer, and 6 stable disease were observed in 29 patients. The combination of FDR gemcitabine with bortezomib may warrant further studies in advanced breast, lung, ovarianm, and pancreatic cancer patients.41
A marked effect of escalating dose of bortezomib administered twice weekly for 2 weeks every 21 days in combination with a fixed dose of carboplatin (AUC = 5) was assessed in 15 patients with advanced ovarian cancer who had received upfront chemotherapy and up to 2 prior chemotherapy regimens for recurrent disease. The overall response rate to this combination was 47%, with 2 complete responses and 5 partial responses, including 1 complete response in a patient with platinum-resistant disease.42 A Gynecologic Oncology Group phase II trial of single-agent bortezomib in recurrent ovarian cancer is currently ongoing. Kubicek and co-workers have recently presented the preliminary results of a phase I trial of bortezomib, cisplatin, and radiotherapy for advanced head and neck cancer. The combination proved feasible; main toxicity was trombocytopenia; no clinical relevant peripheral neuropathy occurred; and most of the other observed toxicities were clearly attributable to cisplatin and radiation therapy.43 The final paper is awaited with full details on safety and activity.
Phase I combination studies with bortezomib are summarized in Tables 2 and 3.
Table 2.
Phase I combination studies of bortezomib in advanced solid tumors
| Number of patients | 21 | 14 | 25 | 51 | 31 | 29 |
|---|---|---|---|---|---|---|
| Schedule | Starting dose of 0.5 mg/m2 iv twice weekly + 5-FU 500 mg/m2 + leucovorin 20 mg/m2 on day 1 q2 weeks | Starting dose of 0.8 mg/m2 iv on days 2, 5, 9, 12 + docetaxel 25 mg/m2 on days 1, 8 q3 weeks | Starting dose of 0.6 mg/m2 iv on days 2, 5, 9, 12 + paclitaxel 80 mg/m2 on days 1, 8 q3 weeks | Starting dose of 1.3 mg/m2 iv twice a week + irinotecan 125 mg/m2 on days 1, 8 q3 weeks | Starting dose of 1.0 mg/m2 iv twice a week + gemcitabine 1000 mg/m2 on days 1, 8 q3 weeks | Starting dose of 1.0 mg/m2 iv on days 1, 4, 8, 11 + FDr gemcitabine 750, 1000, 1250 mg/m2 on days 1, 8 q3 weeks |
| Best response obtained | 1 PR | 4 SD | 1 PR | 10 SD | 1 PR | 1 PR |
| 8 SD | 7 SD | 6 SD | ||||
| Toxicity observed | Abdominal pain and diarrhea | Hematologic | Neurotoxicity and fatigue | Diarrhea, nausea and vomiting | Abdominal pain and hematologic | Neutropenia and thrombocytopenia |
Abbreviations: FDR, fixed dose rate; PR, partial response; SD, stable disease; 5FU, 5-fluorouracil.
Table 3.
Phase I disease specific combination studies of bortezomib
| Patient population | Advanced NSCLC | Advanced ovarian tumors | Advanced head and neck cancer | Advanced colorectal cancer |
|---|---|---|---|---|
| Number of patients | 16 | 15 | 17 | 13 |
| Schedule | Starting dose of 1.0 mg/m2 iv on days 1, 4, 8, 11 + gemcitabine 800 mg/m2 on days 1, 8 + CBDCA AUC 5 on day 1 q3 weeks | Starting dose of 0.75 mg/m2 iv on days 1, 4, 8, 11 + CBDCA AUC 5 on day 1 q3 weeks | Starting dose of 0.7 mg/m2 iv on days 1, 4, 8, 11 + CDDP 30 mg/m2 on day 1 q3 weeks | Starting dose of 1.03 mg/m2 iv on days 1, 8, 15 + FOLFOX-4 q2 weeks |
| Best response obtained | 4 PR | 2 CR | NR | 5 PR |
| 5 SD | 5 Pr | 5 SD | ||
| Toxicity observed | Myelosuppression | Diarrhea | Thrombocytopenia | Myelosuppression and diarrhea |
Abbreviations: NSLC, nonsmall-cell lung cancer; PR, partial response; SD, stable disease; NR, not reported; CBDCA, carboplatin; CDDP, cisplatin.
Phase II studies
A large number of phase II studies of single-agent bortezomib have been carried out or are currently underway. Phase II trials of bortezomib as single agents in patients with melanoma,44 recurrent or metastatic sarcomas,45 neuroendocrine tumors,46 colorectal cancer,47 advanced renal cancer,48,49 and advanced breast cancer50,51 revealed no significant response rates.
Bortezomib was safe, the most significant clinical adverse event being a peripheral sensory neuropathy. Phase II studies of single agent bortezomib are summarized in Table 4.
Table 4.
Phase II single agent studies of bortezomib
| Patient population | Metastatic melanoma | Recurrent/metastatic soft tissue sarcoma | Metastatic neuroendocrine tumors | Metastatic colorectal cancer | Advanced renal tumors | Metastatic breast cancer |
|---|---|---|---|---|---|---|
| Number of patients | 27 | 25 | 16 | 19 | 60 | 24 |
| Best response obtained | 6 SD | 1 PR | 11 SD | 3 SD | 5 PR 14 SD | 1 SD |
| Toxicity observed | Neurotoxicity, fatigue and thrombocytopenia | Neurotoxicity, myalgia and fatigue | Neurotoxicity, diarrea and vomiting | Neurotoxicity and myalgia | Neurotoxicity | Thrombocytopenia and fatigue |
Abbreviations: PR, partial response; SD, stable disease.
A randomized phase II study was conducted in 87 patients with metastatic pancreatic cancer, who were randomized to receive bortezomib alone (1.5 mg/m2 twice weekly for 2 weeks every 3 weeks) or a combination of bortezomib (1.0 mg/m2 twice weekly for 2 weeks every 3 weeks) plus gemcitabine (1000 mg/m2 on days 1, 8 every 3 weeks). The response rate was 0% in the arm with bortezomib alone, with median survival of 2.5 months and median time to progression of 1.2 months. Four patients achieved a partial response in the combination arm, but the benefit obtained with bortezomib alone or in combination with gemcitabine was low.52
Fanucchi et al investigated the safety and efficacy of bortezomib monotherapy (arm A) compared with the combination of bortezomib and docetaxel (arm B) as second-line therapy in 155 patients with locally advanced and metastatic NSCLC. Overall response rate was 8% in arm A and 9% in arm B. Time to response was 36 to 83 days in arm A, 5 of 6 patients responding within 40 days, and 38 to 99 days in arm B, 2 of 7 patients responding within 41 days.53 Bortezomib plus docetaxel seemed to demonstrate modest benefit compared with bortezomib monotherapy. Phase II combination studies of bortezomib are summarized in Table 5.
Table 5.
Phase II combination studies of bortezomib
| Patient population | Metastatic colorectal cancer | Metastatic pancreatic carcinoma | Pretreated NSCLC |
|---|---|---|---|
| Number of patients | 68 | 87 | 155 |
| Schedule | Arm 1: 1.5 mg/m2 on days 1, 4, 8, 11 q21 days Arm 2: 1.3 mg/m2 on days 1, 4, 8, 11 + CPT 11 125 mg/m2 on day 1 q21 days | Arm 1: 1.5 mg/m2 on days 1, 4, 8, 11 q21 days Arm 2: 1.3 mg/m2 on days 1, 4, 8, 11 + gemcitabine 1000 mg/m2 on day 1 q21 days | Arm 1: 1.5 mg/m2 on days 1, 4, 8, 11 q21 days Arm 2: 1.3 mg/m2 on days 1,4,8,11 + docetaxel 75 mg/m2 on day 1 q21 |
| Response rates % | NA | 10% RR | 42% RR |
| Toxicity observed | Hematologic, neurotoxicity and fatigue | Abdominal pain, fatigue and thrombocytopenia | Neutropenia, neurotoxicity and fatigue |
Abbreviations: RR, response rate; CPT, irinotecan.
Conclusion and future perspectives
The 26S proteasome acts as a housekeeper to eliminate damaged or misfolded proteins. In addition, many regulatory proteins governing the cell cycle, transcription factor activation, apoptosis, and cell trafficking are the substrates for proteasome-mediated degradation. Five years after entering clinical trials, bortezomib has demonstrated efficacy for the treatment of patients with recurrent and refractory multiple myeloma. The clinical results in multiple myeloma provide proof of concept for proteasome inhibition as an anticancer therapy, and the role of bortezomib in other types of cancer therapy is undergoing active investigation. In contrast to the results of myeloma, the treatment of solid tumors with either single-agent bortezomib or bortezomib in combination with conventional chemotherapy agents has not yet yielded significant improvements in treatment response. Probably, a more extensive investigation of the survival signals induced by proteasome inhibition may offer further insight into the poor responses of solid malignancies.
The dysregulation of a variety of pathways, such as NF-κB, epidermal growth factor receptor (EGFR), and Ras/PI3K/Akt, is very common in solid tumors. It is known that bortezomib also interferes with the p44/42 mitogen-activated protein kinase (MAPK), a downstream effector of EGFR pathway that communicates proliferative signals, and induces accumulation of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1.54 Signaling by the EGFR family occurs through several downstream pathways to promote cell proliferation and inhibit apoptosis,55,56 suggesting that EGFR itself might be subjected to ubiquitination and subsequent proteolityc breakdown, and that this process might be affected by proteasome inhibition. On the basis of this assumption, a preclinical study was carried out to evaluate the effect of proteasome inhibitor on EGFR survival signaling in pancreatic cancer cells.57 Intriguingly, the addition of EGFR inhibition only enhanced proteasome inhibitor effects in vivo but not in vitro, suggesting that some other paracrine response may be involved in the effects of EGFR inhibition in vivo. An and Rettig have described the importance of the sequence of drug administration when bortezomib is used in combination with an EGFR tyrosine kinase inhibitor in renal carcinoma cells.58 They concluded that pre-treatment of renal carcinoma cells with the EGFR tyrosine kinase inhibitor prior to bortezomib was cytotoxic, whereas an antagonist interaction resulted with the reverse schedule. The authors also postulated that the diminished AKT and NF-κB, inhibition observed when renal carcinoma cells were pretreated with bortezomib, might result from decreased degradation of signaling proteins that function to converge on the EGFR/PI3K/AKT/NF-κB pathway, although direct evidence to support or refute this hypothesis does not yet exist.
In summary, these findings show that proteasome inhibitor treatment activates several mitogenic signaling pathways that blunt the full potential of the apoptotic response to proteasome inhibitor treatment. As an alternative or addition to EGFR inhibition, selective inhibition of these downstream mitogenic signaling pathways may increase the apoptotic response to proteasome inhibition and further overcome the drug resistance mechanisms in solid tumors. These preclinical studies might identify new drug combinations to enter clinical trials.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 2.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 3.Adams J. The proteasome structure, function, and role in the cell. Cancer Treat Rev. 2003;29:3–9. doi: 10.1016/s0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 4.Ciechanover A, Schwartz AL. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc Natl Acad Sci U S A. 1998;95:2727–2730. doi: 10.1073/pnas.95.6.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or highdose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 8.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 9.Nagata Y, Anan T, Yoshida T, et al. The stabilization mechanism of mutant-type p53 by impaired ubiquitination: The loss of wild-type p53 function and the hsp90 association. Oncogene. 1999;18:6037–6049. doi: 10.1038/sj.onc.1202978. [DOI] [PubMed] [Google Scholar]
- 10.An WG, Hwang SG, Trepel JB, Blagosklonny MV. Protease inhibitor-induced apoptosis: Accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia. 2000;14:1276–1283. doi: 10.1038/sj.leu.2401812. [DOI] [PubMed] [Google Scholar]
- 11.Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 12.Palombella VJ, Conner EM, Fuseler JW, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–1576. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delcros JG, Floc’h MB, Prigent C, Arlot-Bonnemains Y. Proteasome inhibitors as therapeutic agents: current and future strategies. Curr Med Chem. 2003;10:479–503. doi: 10.2174/0929867033368231. [DOI] [PubMed] [Google Scholar]
- 14.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig H, Khayat D, Giaccone G, Facon T. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer. 2005;9:1794–1807. doi: 10.1002/cncr.21414. [DOI] [PubMed] [Google Scholar]
- 16.Almond JB, Cohen GM. The proteasome: structure, function, and role in the cell. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 17.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 18.Olivier S, Robe P, Bours V. Can NF-κB be a target for novel and efficient anti-cancer agents? Biochem Pharmacol. 2006;72:1054–1068. doi: 10.1016/j.bcp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Oikawa T, Sasaki T, Nakamura M, et al. The proteasome is involved in angiogenesis. Biochem Biophys Res Commun. 1998;246:243–248. doi: 10.1006/bbrc.1998.8604. [DOI] [PubMed] [Google Scholar]
- 20.Milano A, Iaffaioli RV, Caponigro F. The proteasome: a worthwhile target for the treatment of solid tumor? Eur J Cancer. 2007;43:1125–1133. doi: 10.1016/j.ejca.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther. 2003;2:835–843. [PubMed] [Google Scholar]
- 22.Shah SA, Potter MW, McDade TP, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem. 2001;82:110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- 23.Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- 24.Russo SM, Tepper JE, Baldwin AS, et al. Enhancement of radiosensitivity by proteasome inhibition; implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NFkappa B. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 26.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 27.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic caner by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 28.Papandreu CN, Daliani VD, Nix D, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observation in androgen-independent prostate cancer. J Clin Oncol. 2004;11:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 29.Dy GK, Thomas JP, Wilding G, et al. A phase I and pharmacologic trial of two schedules of the proteasome inhibitor, PS-341 (Bortezomib, Velcade), in patients with advanced cancer. Clin Cancer Res. 2005;11:3410–3416. doi: 10.1158/1078-0432.CCR-04-2068. [DOI] [PubMed] [Google Scholar]
- 30.Hegewisch-Becker S, Sterneck M, Schubert U, et al. Phase I/II trial of bortezomib in patients with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2004;22(S):4089. [Google Scholar]
- 31.Bardag-Gorce F, Li J, French BA, French SW. Ethanol withdrawal induced CYP2E1 degradation in vivo, blocked by proteasomal inhibitor PS-341. Free Radic Biol Med. 2002;32:17–21. doi: 10.1016/s0891-5849(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 32.Korsmeyer KK, Davoll S, Figueiredo-Pereira ME, Correia MA. Proteolytic degradation of heme-modified hepatic cytochromes P450: A role for phosphorylation, ubiquitination, and the 26S proteasome? Arch Biochem Biophys. 1999;365:31–44. doi: 10.1006/abbi.1999.1138. [DOI] [PubMed] [Google Scholar]
- 33.Messersmith WA, Baker SD, Lassiter L, et al. Phase I trial of bortezomib in combination with docetaxel in patienrts with advanced solid tumors. Clin Cancer Res. 2006;12:1270–1275. doi: 10.1158/1078-0432.CCR-05-1942. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro CL, Ramaswamy B, Young D, et al. Phase I trial of bortezomib (Velcade) in combination with paclitaxel in advanced solid tumor patients (pts) J Clin Oncol 200523S310415860870 [Google Scholar]
- 35.Iqbal S, Cole S, Yang D, et al. Phase I study of PS-341 (bortezomib) with 5-fluorouracil/leucovorin (5-FU/LV) in advanced solid tumors: A California Cancer Consortium study. J Clin Oncol. 2004;22(S):2057. [Google Scholar]
- 36.Ryan DP, O’Neil BH, Supko JG, et al. A phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107:2688–2697. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 37.Caponigro F, Lacombe D, Twelves C, et al. An EORTC phase I study of Bortezomib in combination with oxaliplatin, leucovorin and 5-fluorouracil in patients with advanced colorectal cancer. Eur J Cancer. 2009;45:48–55. doi: 10.1016/j.ejca.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Bergman AM, Pinedo HM, Jongsma AP, et al. Decreased resistance to gemcitabine (2’,2’-difluorodeoxycytidine) of cytosinearabinosideresistant myeloblastic murine and rat leukaemia cell lines: role of altered activity and substrate specificity of deoxycytidine kinase. Biochem Pharmaco. 1999;57:397–406. doi: 10.1016/s0006-2952(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 39.Ryan DP, Appleman LJ, Lynch T, et al. Phase I clinical trial of bortezomib in combination with gemcitabine in patients with advanced solid tumors. Cancer. 2006;107:2482–2489. doi: 10.1002/cncr.22264. [DOI] [PubMed] [Google Scholar]
- 40.Davies M, Lara PN, Lau DH, et al. The proteasome inhibitor, bortezomib, in combination with gemcitabine (Gem) and carboplatin (Carbo) in advanced non-small cell lung cancer (NSCLC): Final results of a phase I California Cancer Consortium study. J Clin Oncol. 2004;22(S):7106. doi: 10.1097/JTO.0b013e31815e8b88. [DOI] [PubMed] [Google Scholar]
- 41.Luu TH, Chow WA, Lim D, et al. Phase I of fixed-dose-rate gemcitabine in combination with bortezomib in patients with advanced solid tumors. J Clin Oncol. 2008;26(S):2563. [Google Scholar]
- 42.Agajanian C, Dizon DS, Sabbatini P, Raizer JJ, Dupont J, Spriggs DR. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2004;23:5943–5949. doi: 10.1200/JCO.2005.16.006. [DOI] [PubMed] [Google Scholar]
- 43.Kubiceck GJ, Machtaym Axelrod RA, et al. Phase I trial of bortezomib (velcade), cisplatin and radiotherapy for advanced head and neck cancer. J Clin Oncol. 2008;26(S):6028. [Google Scholar]
- 44.Markovic S, Geyer SM, Dawkins F, Sharfman W, et al. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 45.Maki RG, Kraft AS, Scheu K, et al. A Multicenter phase II study of bortezomib in recurrent or metastatic sarcomas. Cancer. 2005;103:1431–1438. doi: 10.1002/cncr.20968. [DOI] [PubMed] [Google Scholar]
- 46.Shah MH, Young D, Kindler HL, et al. Phase II Study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 47.Mackay H, Major P, Townsley C, et al. A phase II trial of the proteasome inhibitor PS-341 in patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(S):3109. [Google Scholar]
- 48.Kondagunta GV, Druker B, Schwartz L, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22:3720–3725. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 49.Davis NB, Taber DA, Ansari RH, et al. Phase II trial of PS-341 in patients with renal cell cancer: A University of Chicago phase II Consortium Study. J Clin Oncol. 2004;22:111–119. doi: 10.1200/JCO.2004.07.165. [DOI] [PubMed] [Google Scholar]
- 50.Brown J, Von Roenn J, O’Regan R, et al. A phase II study of the proteasome inhibitor PS-341 in patients (pts) with metastatic breast cancer (MBC) J Clin Oncol. 2004;22(S):546. [Google Scholar]
- 51.Yang CH, Gonzalez-Angulo AM, Reuben JM, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–817. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 52.Alberts SR, Foster NR, Morton RF, et al. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study. Ann Oncol. 2005;16:1654–1661. doi: 10.1093/annonc/mdi324. [DOI] [PubMed] [Google Scholar]
- 53.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of Bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 54.Orlowski RZ. The Ubiquitin Proteasome Pathway from Bench to Bedside. Hematology Am Soc Hematol Educ Program. 2005:220–225. doi: 10.1182/asheducation-2005.1.220. [DOI] [PubMed] [Google Scholar]
- 55.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 56.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 57.Sloss CM, Wang F, Liu R, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14:5116–5123. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An J, Rettig MB. Epidermal growth factor receptor inhibition sensitizes renal cell carcinoma cells to the cytotoxic effects of bortezomib. Mol Cancer Ther. 2007;6:61–69. doi: 10.1158/1535-7163.MCT-06-0255. [DOI] [PubMed] [Google Scholar]