Abstract
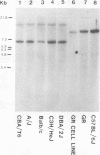
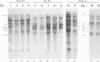
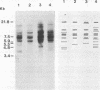
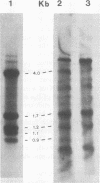
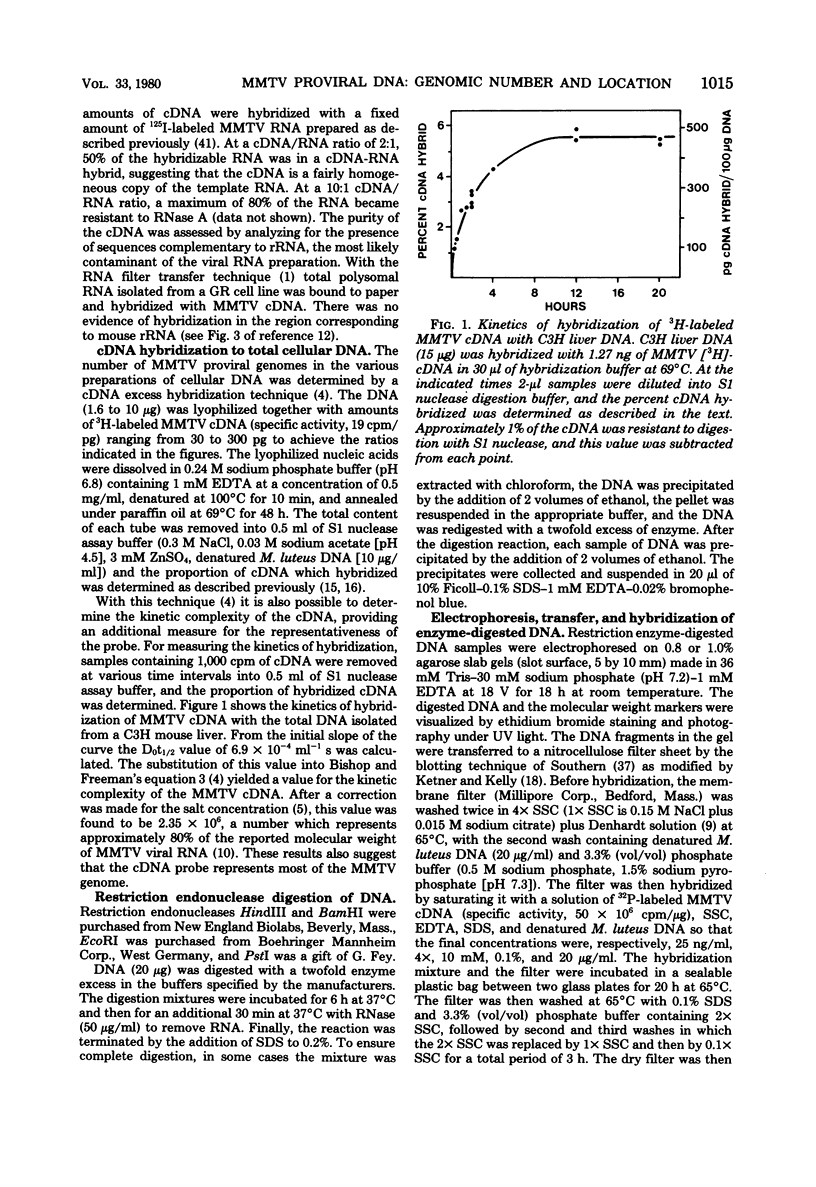
The Southern DNA filter transfer technique was used to characterize the genomic location of the mouse mammary tumor proviral DNA in different inbred strains of mice. Two of the strains (C3H and CBA) arose from a cross of a Bagg albino (BALB/c) mouse and a DBA mouse. The mouse mammary tumor virus-containing restriction enzyme DNA fragments of these strains had similar patterns, suggesting that the proviruses of these mice are in similar genomic locations. Conversely, the pattern arising from the DNA of the GR mouse, a strain genetically unrelated to the others, appeared different, suggesting that its mouse mammary tumor proviruses are located in different genomic sites. The structure of another gene, that coding for beta-globin, was also compared. The mice strains which we studied can be categorized into two classes, expressing either one or two beta-globin proteins. The macroenvironment of the beta-globin gene appeared similar among the mice strains belonging to one genetic class. Female mice of the C3H strain exogenously transmit mouse mammary tumor virus via the milk, and their offspring have a high incidence of mammary tumor occurrence. DNA isolated from individual mammary tumors taken from C3H mice or from BALB/c mice foster nursed on C3H mothers was analyzed by the DNA filter transfer technique. Additional mouse mammary tumor virus-containing fragments were found in the DNA isolated from each mammary tumor. These proviral sequences were integrated into different genomic sites in each tumor.
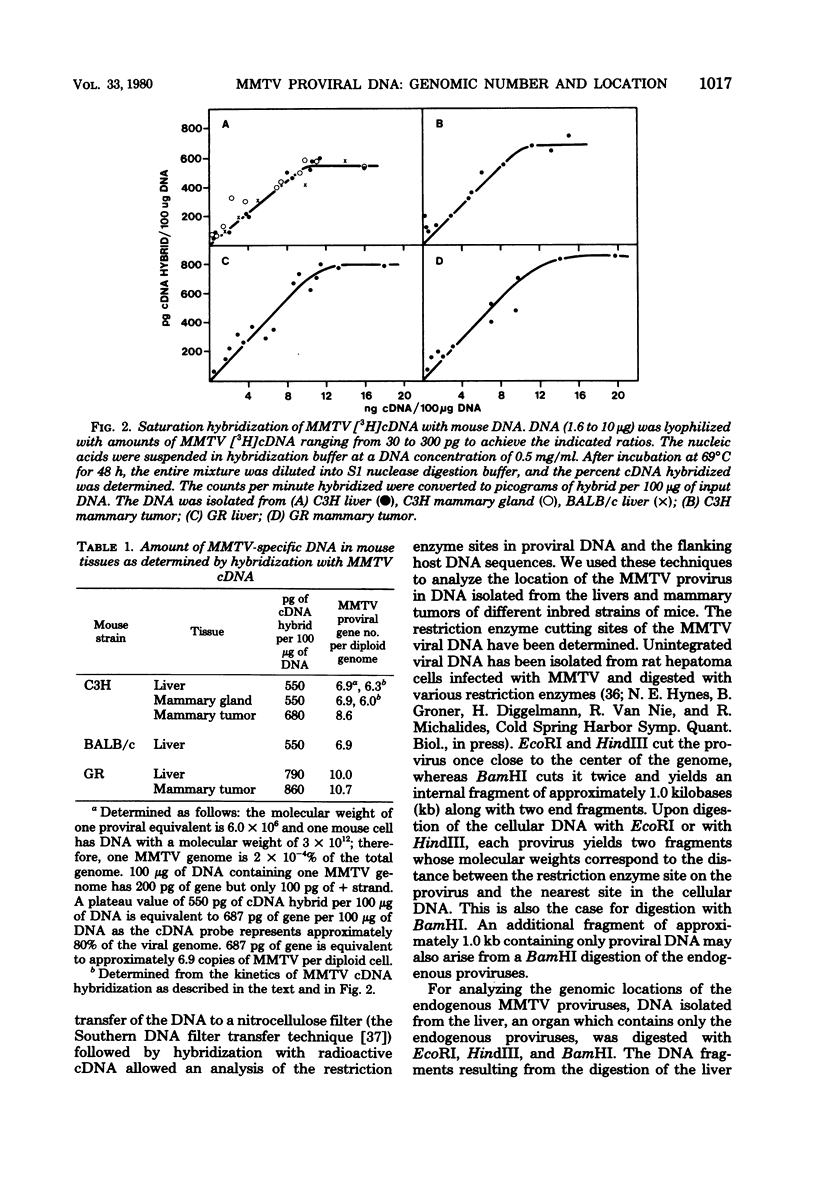
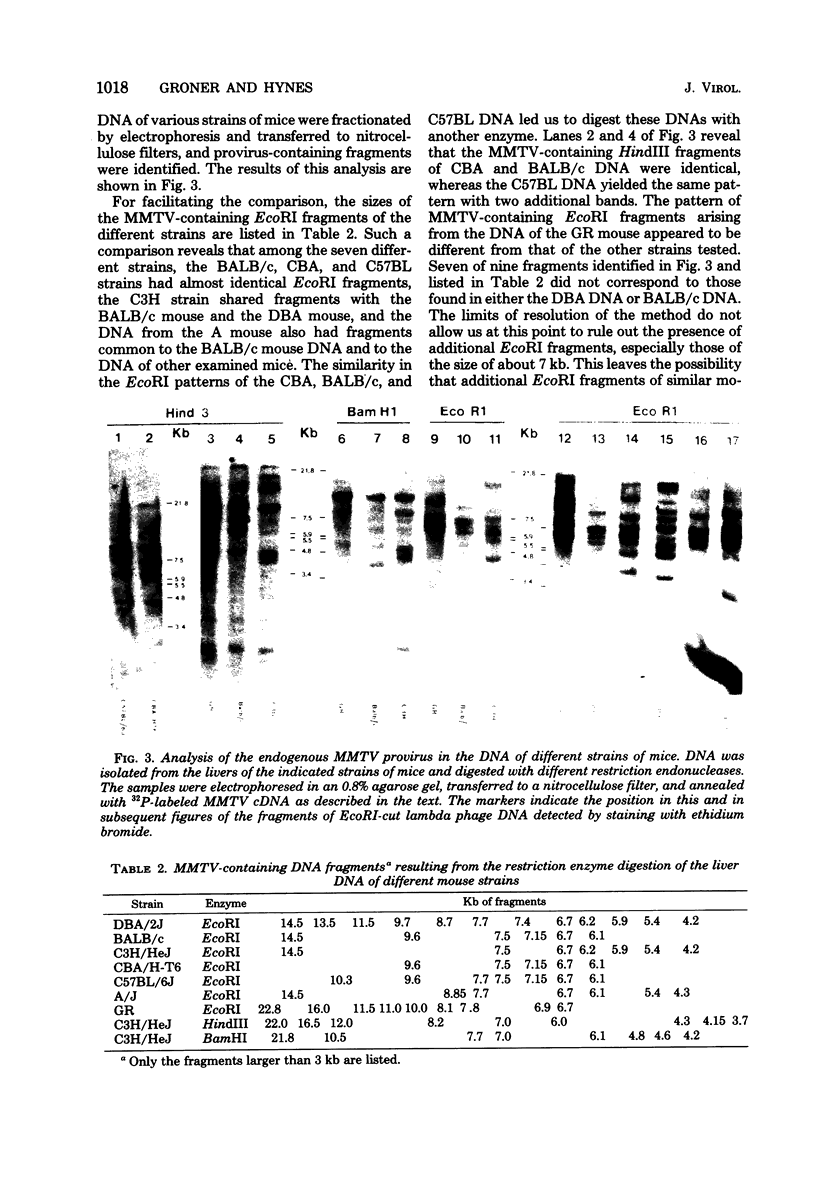
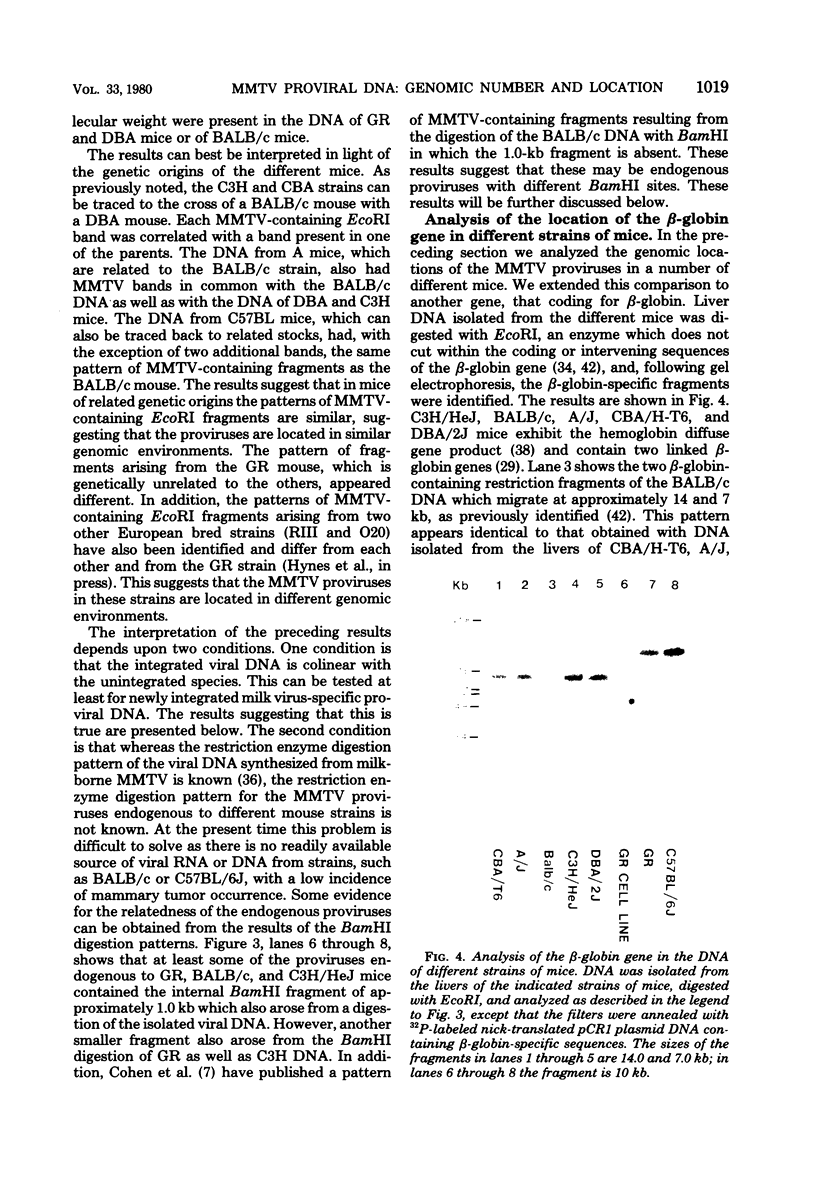
Full text
PDF


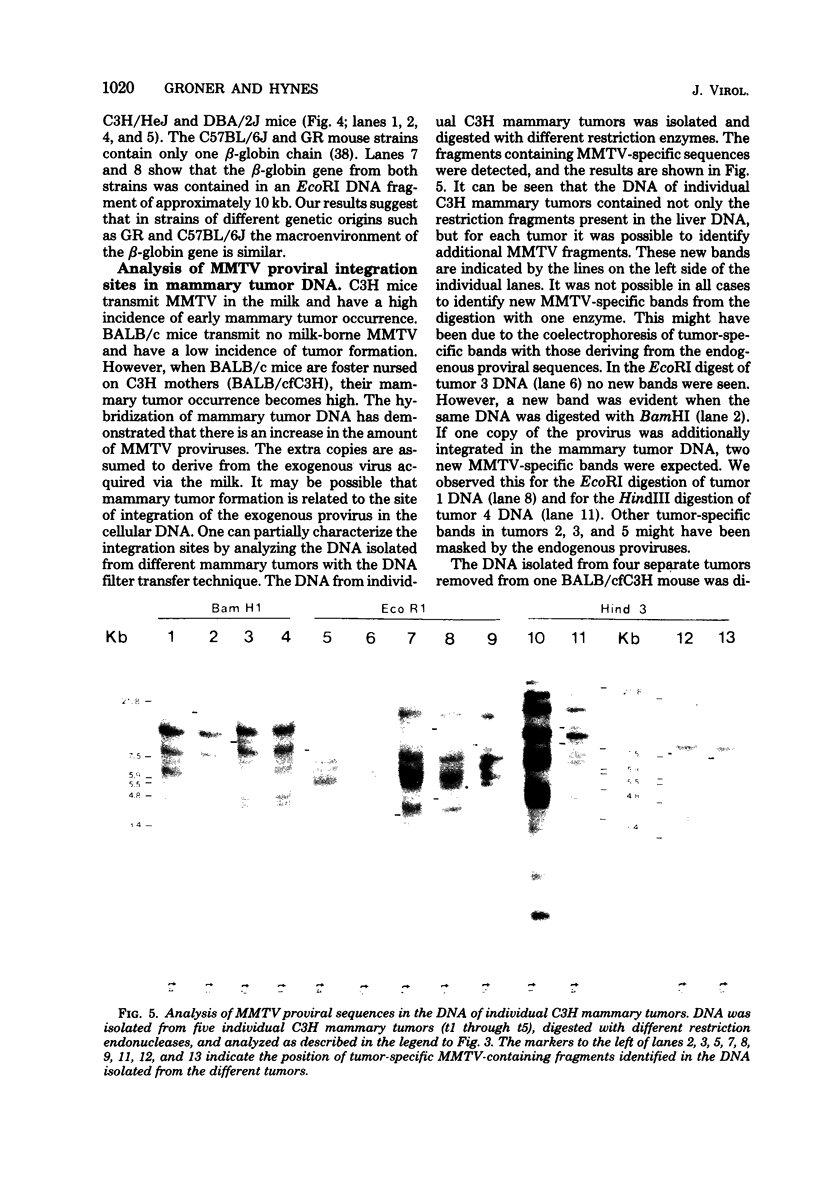
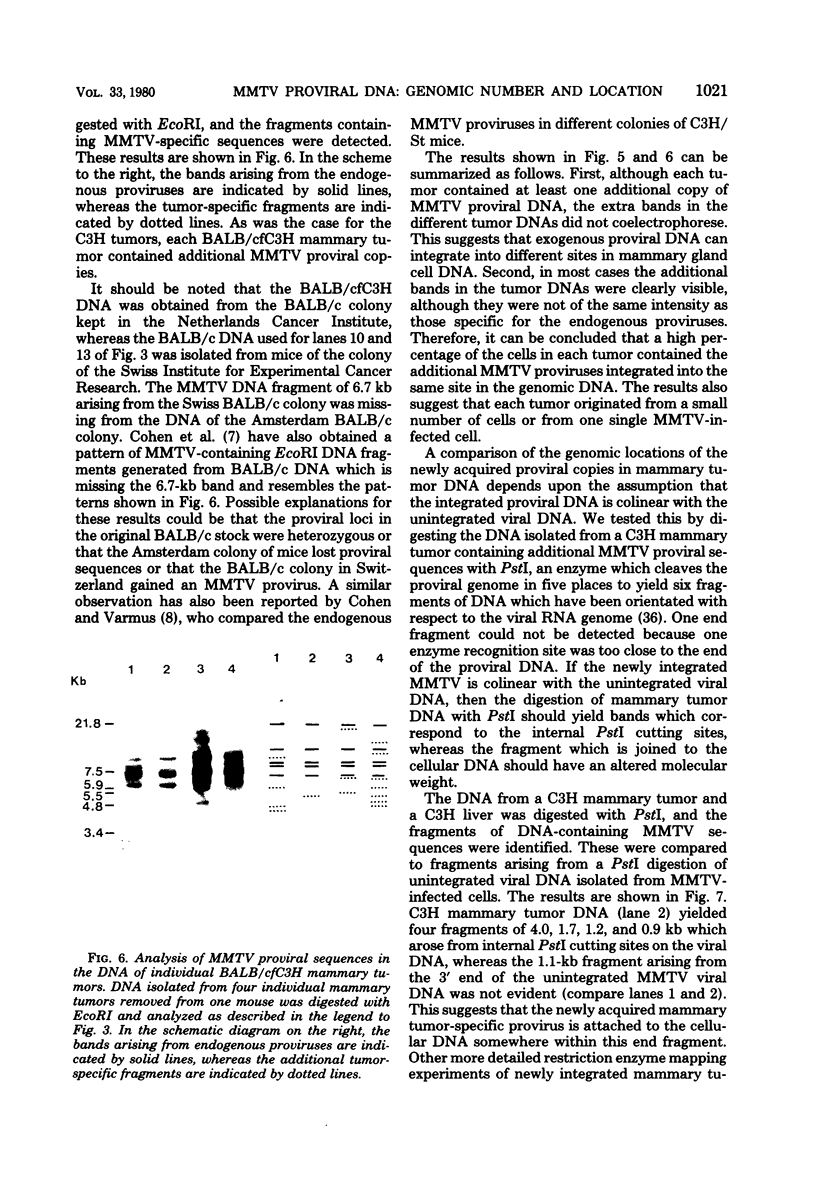




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P., Daams J. H. Hereditary infections with mammary tumor viruses in mice. J Natl Cancer Inst. 1969 Nov;43(5):1025–1035. [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Freeman K. B. DNA sequences neighboring the duck hemoglobin genes. Cold Spring Harb Symp Quant Biol. 1974;38:707–716. doi: 10.1101/sqb.1974.038.01.076. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Heine U. I., Pomenti A. A., Korb J., Weber G. H. Electrophoretic analysis of the molecular weight of murine mammary tumor virus RNA. J Virol. 1977 Jun;22(3):822–825. doi: 10.1128/jvi.22.3.822-825.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Morris V. L., Goodman H. M., Bishop J. M., Varmus H. E. Differences between genomes of two strains of mouse mammary tumor virus as shown by partial RNA sequence analysis. Virology. 1976 Jul 15;72(2):330–340. doi: 10.1016/0042-6822(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J., Bentvelzen P. Interaction between viral and genetic factors in murine mammary cancer. Adv Cancer Res. 1978;26:143–195. doi: 10.1016/s0065-230x(08)60087-1. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Vlahakis G., Schlom J. A biochemical approach to the study of the transmission of mouse mammary tumor viruses in mouse strains RIII and C3H. Int J Cancer. 1976 Jul 15;18(1):105–115. doi: 10.1002/ijc.2910180114. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Röpcke G., Boot L. Involvement of mouse mammary tumor virus in spontaneous and hormone-induced mammary tumors in low-mammary-tumor mouse strains. J Virol. 1978 Sep;27(3):551–559. doi: 10.1128/jvi.27.3.551-559.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Kozak C., Cohen J. C., Shank P. R., Jolicoeur P., Ruddle F., Varmus H. E. Endogenous mouse mammary tumor virus DNA is distributed among multiple mouse chromosomes. Virology. 1979 Jan 15;92(1):46–55. doi: 10.1016/0042-6822(79)90213-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Mühlbock O. Note on a new inbred mouse-strain GR-A. Eur J Cancer. 1965 Oct;1(2):123–124. doi: 10.1016/0014-2964(65)90003-4. [DOI] [PubMed] [Google Scholar]
- Old J., Clegg J. B., Weatherall D. J., Ottolenghi S., Comi P., Giglioni B., Mitchell J., Tolstoshev P., Williamson R. A direct estimate of the number of human gamma-globin genes. Cell. 1976 May;8(1):13–18. doi: 10.1016/0092-8674(76)90180-x. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G. Sequence of amino acids in the major and minor chains of the diffuse hemoglobin from BALB-c mice. Biochim Biophys Acta. 1973 Mar 23;303(1):61–67. doi: 10.1016/0005-2795(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Blair P. B., Bishop J. M., Varmus H. E. Nucleotide sequence homologies among mouse mammary tumor viruses. Virology. 1976 Apr;70(2):550–553. doi: 10.1016/0042-6822(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Shank P. R., Varmus H. E. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977 Jan;10(1):19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of alpha and beta mouse globin gene sequences synthesised in vitro. Gene. 1977 May;1(3-4):229–239. doi: 10.1016/0378-1119(77)90047-6. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W., Kawakami T., Kohne D., Okabe H., Gilden R., Hatanaka M. Primate and murine type-C viral nucleic acid association kinetics: analysis of model systems and natural tissues. J Virol. 1974 Feb;13(2):363–369. doi: 10.1128/jvi.13.2.363-369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: sixth listing. Cancer Res. 1976 Dec;36(12):4333–4377. [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Callahan R., Lieber M. M., Sherr C. J. Endogenous primate and feline type C viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1159–1168. doi: 10.1101/sqb.1974.039.01.133. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Nowinski R. C., Sarker N. H. Mammary tumour virus specific nucleotide sequences in mouse DNA. Nat New Biol. 1972 Aug 9;238(84):189–191. doi: 10.1038/newbio238189a0. [DOI] [PubMed] [Google Scholar]
- Weaver S., Haigwood N. L., Hutchison C. A., 3rd, Edgell M. H. DNA fragments of the Mus musculus beta globin haplotypes Hbbs and Hbbd. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1385–1389. doi: 10.1073/pnas.76.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]