Abstract
Proton pump inhibitors (PPIs) are among the most commonly utilized agents for treatment of symptomatic disorders of the upper gastrointestinal tract, accounting for a significant proportion of sales of both over-the-counter and prescription formulations. A systematic review of the literature was conducted via MEDLINE to evaluate the most rigorous studies linking the potential risk of PPI therapy with adverse events. Emerging data illustrate the potential risks associated with both short-and long-term PPI therapy, including Clostridium difficile–associated diarrhea, community-acquired pneumonia, osteoporotic fracture, vitamin B12 deficiency, and inhibition of antiplatelet therapy. Due to these associations, it is recommended that clinicians assess the continuing need for PPI therapy and use the lowest possible dose to achieve the desired therapeutic goals.
Keywords: Proton pump inhibitors, overutilization, risk
Proton pump inhibitors (PPIs) are among the most commonly used agents for the treatment of upper gastrointestinal (UGI) disorders, accounting for over $11 billion in US sales in 2007. Brand-name PPIs comprise 3 of the top 11 most commonly prescribed medications in the United States and are second only in monetary sales to statins.1 With the advent of over-the-counter omeprazole (Prilosec, AstraZeneca), self-directed PPI therapy for gastroesophageal reflux disease (GERD) is now widely available, though this also increases the potential for inappropriate use.
Recent concerns have arisen regarding the potential for adverse events involving long-term acid suppression.2-4 Currently, there are no guidelines for directing clinicians on how to address potential adverse effects of PPIs in patients with GERD who may use these drugs on a daily basis for many years. The aim of this study was to systematically review the literature and summarize the pertinent adverse risks associated with long-term use of PPIs in the treatment of UGI disorders.
Methods
A computerized literature search was performed using the MEDLINE database from 1966 through November 2008. Criteria for this study selection were determined a priori. The literature search was conducted via components that were each keyed to a specific causal link in a formal problem structure. The search sought to identify all publications using the following terms: proton pump inhibitor, gastroesophageal reflux disease, GERD, overutilization, risk(s), Clostridium difficile–associated diarrhea, community-acquired pneumonia, bone/hip/osteoporotic fracture, vitamin/mineral deficiency, vitamin/mineral malabsorption, clopidogrel, and antiplatelet (therapy). The search was limited to studies involving human subjects published in the English language. Abstracts, book chapters, letters, case reports, and review articles were excluded from the search. Bibliographies from index citations were reviewed for additional relevant studies. After reviewing the abstracts of publications identified through the search algorithm, the authors (J.J.H. and J.M.I.) independently assessed full manuscripts that met inclusion criteria. Study design, methodology, and appropriate and relevant outcomes applicable to the current practice of ambulatory care medicine and gastroenterology were critically reviewed. The adverse events associated with PPIs were specifically identified.
Results
The initial MEDLINE search identified 607 manuscripts using the following search terms: risk(s) and proton pump inhibitor. Review of the abstracts reduced the number of potentially relevant manuscripts to papers identified for inclusion by reviewing the bibliographies of the remaining papers, leaving 47 studies included in the final systematic review. A flowchart highlighting the manuscript selection and review process appears in Figure 1. These studies are summarized in Table 1 according to each category of potential risk.
Figure 1.
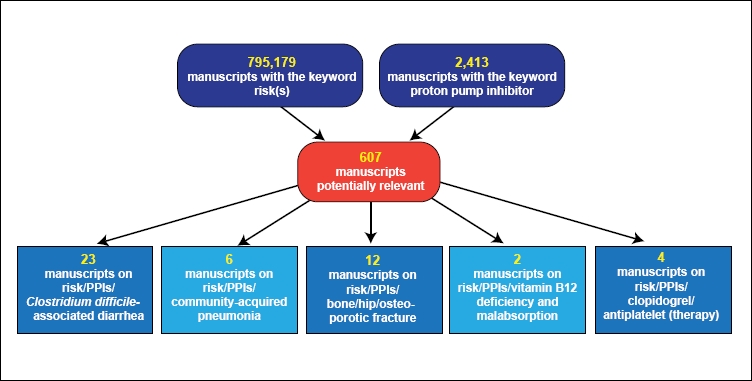
Overview of manuscripts evaluated for the systematic review.
PPI=proton pump inhibitor.
Table 1.
Studies With Validated Outcomes Demonstrating Risk With Use of PPIs
| Study | Study design | Population | Outcomes | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Clostridium difficile–associated diarrhea | ||||
| Cunningham R, et al.5 | Case-control | United Kingdom; hospitalized patients |
|
2.5 (1.5–4.2) 5.4 (2.2–13.2) 43.2 (5.7–330.4) |
| Dial S, et al.6 | Cohort | Montreal, Canada; hospitalized patients who received antibiotics |
|
2.1 (1.2–3.5) 2.1 (1.3–3.4) 4.1 (2.3–7.3) |
| Case-control | Montreal, Canada; hospitalized patients |
|
2.6 (1.3–5.0) 5.1 (1.7–15.2) 2.9 (1.4–6.1) 2.5 (1.2–5.0) 7.8 (2.3–26.0) |
|
| Dial S, et al.7 | 2 population-based case-control studies | United Kingdom; all cases in database and a subgroup of nonhospitalized patients |
|
2.9 (2.4–3.4) 3.6 (2.6–5.1) 3.7 (2.4–5.6) |
| Yearsley KA, et al.8 | Prospective case-control study | United Kingdom; hospitalized patients |
|
13.1 (6.6–26.1) 1.90 (1.10–3.29) |
| Jayatilaka S, et al.9 | Case-control | United States; hospitalized patients |
|
2.75 (1.68–4.52) |
| Aseeri M, et al.10 | Case-control | United States; hospitalized patients |
|
3.6 (1.7–8.3) 5.7 (1.3–39.1) |
| Leonard J, et al.31 | Systematic review | Meta-analysis |
|
1.94 (1.37–2.75) 1.96 (1.28–3.00) 1.40 (0.85–2.29) |
| Community-acquired pneumonia | ||||
| Laheij RJ, et al.11 | Nested case-control | The Netherlands; outpatients |
|
1.27 (1.06–1.54) 1.89 (1.36–2.62) 1.63 (1.07–2.48) |
| Gulmez SE, et al.12 | Case-control | Denmark; hospitalized patients |
|
1.5 (1.3–1.7) 5.0 (2.1–11.7) 1.3 (1.2–1.4) |
| Sarkar M, et al.13 | Nested case-control | United Kingdom; UK GPRD |
|
1.02 (0.97–1.08) 6.53 (3.95–10.80) 3.79 (2.66–5.42) 3.21 (2.46–4.18) |
| Bone fracture | ||||
| Yang YX, et al.14 | Nested case-control | United Kingdom; UK GPRD |
|
1.44 (1.30–1.59) 2.65 (1.80–3.90) |
| Vestergaard P, et al.15 | Case-control | Denmark; community-based |
|
1.18 (1.12–1.43) 1.45 (1.28–1.65) 1.60 (1.25–2.04) |
| Targownik LE, et al.16 | Retrospective matched cohort | Manitoba, Canada; community-based |
|
1.62 (1.02–2.58) 4.55 (1.68–12.29) 1.92 (1.16–3.18) |
| Vitamin B12 deficiency | ||||
| Valuck RJ, Ruscin JM17 | Case-control | United States; university-based |
|
2.01 (0.89–4.35) 1.03 (0.46–2.31) 4.46 (1.49–13.33) |
| Antiplatelet interactions | ||||
| Gilard M, et al.19 | Prospective double-blind randomized placebo-controlled | France; patients with coronary artery disease undergoing coronary stent implantation |
|
|
| Siller-Matula JM, et al.20 | Nonrandomized | Austria; patients with coronary artery disease undergoing percutaneous coronary intervention |
|
|
| Small DS, et al.21 | Single-center open-label randomized 4-period cross-over | United States; healthy subjects with no known coronary heart disease |
|
|
| Ho PM, et al.22 | Retrospective cohort | United States, VA hospitals; veterans discharged with ACS or unstable angina |
|
1.25 (1.11–1.41) 0.91 (0.80–1.05) |
- ACS=
acute coronary syndrome;
- CI=
confidence interval;
- GPRD=
General Practice Research Database;
- H2RA=
histamine-2 receptor antagonist;
- IPA=
inhibition of platelet aggregation;
- MRSA=
methicillin-resistant Staphylococcus aureus;
- OR=
odds ratio;
- PPIs=
proton pump inhibitors;
- SD=
standard deviation;
- VA=
veterans affairs.
Clostridium difficile– associated Diarrhea
Cunningham and colleagues5 conducted a case-control study involving 170 hospitalized patients with diarrhea in the United Kingdom who were positive for C. difficile toxin, with the goal of identifying a potential association between PPI use and C. difficile–associated diarrhea (CDAD). The researchers determined that, compared to matched controls without CDAD, subjects who had taken PPIs within the preceding 2 months were significantly more likely to develop CDAD, with the risk increasing with concomitant antibiotic administration and cytotoxic chemotherapy.
Dial and associates6 examined the risk of CDAD among hospitalized patients in cohort and case-control studies conducted in Montreal, Canada. The cohort study identified all patients in the general and cardiothoracic surgical wards of a teaching hospital who had received antibiotics over a 9-month period. The primary outcome was a new diagnosis of CDAD. To be considered exposed to PPIs, patients had to have taken a PPI for at least 3 days prior to the onset of reported diarrhea. CDAD developed in 6.8% of subjects, with a greater-than-2-fold increase in the risk of CDAD in patients taking PPIs, whereas no increased risk was observed among patients taking histamine-2 receptor antagonists (H2RAs). The risk of CDAD was also significantly greater in subjects taking 3 or more antibiotics and in those in medical versus surgical wards.
The case-control study determined that after adjusting for potential confounding factors, the risk of developing CDAD while taking PPIs was significantly increased in subjects with renal failure, those who had been hospitalized within the previous 3 months, those with methicillin-resistant Staphylococcus aureus (MRSA) colonization, and women.
Dial and coworkers7 also conducted 2 population-based case-control studies in the United Kingdom to determine whether there was an association between acid-suppression therapy (AST) and community-acquired CDAD. When examining time trends of CDAD diagnosis, the investigators determined that the incidence of CDAD increased from less than 1 case per 100,000 in 1994 to 22 per 100,000 in 2004. When examining all 1,672 cases of patients with CDAD in the database, they found that significant predictors of CDAD were age 65 years or older, prior hospitalization, and antibiotic use within 90 days prior to diagnosis. In a subanalysis limited to nonhospitalized patients, factors associated with CDAD included age 71 years or older, female gender, inflammatory bowel disease, malignancy, colonization with MRSA, and PPI exposure.
Yearsley and colleagues8 conducted a case-control study of hospitalized patients in the United Kingdom to determine whether AST was associated with an increased risk of CDAD. The risk of developing CDAD was independently associated with antibiotic use (odds ratio [OR], 13.1; 95% confidence interval [CI], 6.6–26.1) and PPI therapy (OR, 2.03; 95% CI, 1.21–3.41).
Jayatilaka and associates9 conducted a study in a US urban medical center examining a 5-year period of infection rates in association with PPI use. They found an increase of nearly 60% in C. difficile colitis cases during this period. Moreover, PPI usage increased significantly over the period of observation and correlated strongly with the increase in CDAD incidence (rs=1.00). PPI use either prior to or during admission was significantly associated with CDAD, unlike H2RA use; however, there was no observable dose-response relationship with PPIs. Although antibiotic usage correlated with PPI use, PPIs remained a significant independent predictor of CDAD.
Aseeri and coworkers10 performed a case-control study in a US medical center to examine the association between PPI use and CDAD in hospitalized patients. The type and duration of antibiotic use was closely monitored, and exposure to AST was defined as administration prior to admission or at least 3 days prior to development of CDAD as an inpatient. The likelihood of developing CDAD increased more than 3-fold with concomitant PPI use and more than 2-fold with concomitant H2RA use.
Community-acquired Pneumonia
Laheij and colleagues11 examined the association between AST and the development of CAP in a nested case-control trial in the Netherlands. Their study population of 364,683 subjects included 5,551 patients who developed first-time pneumonia. The incidence of community-acquired pneumonia (CAP) was 2.5 per 100 person-years among subjects taking PPIs, 2.3 per 100 person-years among those taking H2RAs, and 0.6 per 100 person-years among those not taking AST. Patients taking AST developed CAP 4.5 times more often compared to those who had never taken AST, and a significant dose response was observed. Conversely, a significant dose response was not observed in subjects taking H2RAs. Among current users of AST, the risk of developing CAP was greatest in those subjects who began AST within 30 days prior to CAP diagnosis.
Gulmez and associates12 conducted a population-based case-control study in Denmark that examined the association between PPIs and the risk of CAP by identifying cases with a hospital discharge diagnosis of a patient’s first CAP episode. Subjects were considered exposed to a PPI if they had fulfilled a prescription for a PPI within 90 days of hospital admission. A total of 7,642 cases were identified, 11% of which were current PPI users. Current PPI users were 50% more likely to develop CAP, and no significant association was noted between H2RA use and CAP. The authors were unable to detect a dose response between either PPIs or H2RAs and CAP. There was a significant temporal relationship between initiation of PPI therapy and diagnosis of CAP, most prominent within the first 7 days of treatment; however, the risk was not increased among subjects who began PPI therapy more than 84 days prior to diagnosis.
Sarkar and coworkers13 investigated the association between PPI use and CAP in adults in general practices in the United Kingdom via a nested case-control study. The researchers examined 80,066 cases of first CAP and identified the use of any PPI within 30 days prior to diagnosis. Although patients who developed CAP were more likely to have been on PPIs, this association lost significance after adjustment for confounding factors. However, the subset of patients who were on PPI therapy for less than 30 days did experience an increased risk that was inversely proportional to the duration of PPI use.
Bone Fracture
Yang and colleagues14 performed a nested case-control study of residents in the United Kingdom to determine the relationship between PPI use and hip fracture. They found a crude incidence rate of hip fracture of 4.0 per 1,000 person-years among patients with more than 1 year of continuous PPI use compared to 1.8 per 1,000 person-years in those subjects not taking AST. After adjusting for potential confounding factors, the relationship between PPI use and hip fracture associated with more than 1 year of PPI therapy remained significant (OR, 1.44; 95% CI, 1.30–1.59). Support for a causal link was increased through demonstration of a dose effect with respect to both the duration and dose of PPI therapy. The association between hip fracture and long-term PPI use was greater in men than women (OR, 1.78 and 1.36, respectively).
Vestergaard and associates15 conducted a case-control study in Denmark examining the risk of PPIs, H2RAs, and antacids on the risk of fracture. They determined that in patients with osteoporotic fractures of the hip and spine who had used AST within the last year, these fractures were more likely to have occurred in patients who took PPIs compared to H2RAs, whereas the risk of forearm fracture did not differ across drug classes. There was an increased risk of fracture associated with AST use within the year prior to fracture, and no significant association was seen among those who had not used AST for over 1 year. No dose-response relationship was identified between fracture and PPI use, yet a trend toward decreasing fracture risk was seen with an increasing dose of H2RAs. Overall, however, the fracture risk was significantly lower among patients taking H2RAs compared to PPIs, and there was no gender difference observed.
Targownik and coworkers16 performed a retrospective matched cohort study in Manitoba, Canada to determine whether osteoporotic fractures correlated with the duration of continuous treatment with PPIs. Cases were defined as individuals 50 years of age and older who had hip, spine, or wrist fracture. Duration of PPI therapy was defined as continuous exposure if a patient was taking a PPI more than 70% of the time prior to fracture, whereas noncontinuous exposure was defined as a patient taking a PPI less than 70% of the time prior to fracture, and no exposure was defined as a patient not taking a PPI prior to the fracture. The researchers found no statistically significant association between the use of PPIs and the occurrence of an osteoporotic fracture within 1–6 years of defined continuous PPI therapy, but they did find that this risk was elevated after 7 years of continuous therapy.
Vitamin B12 Deficiency
Valuck and Ruscin17 conducted a case-control study in a university-based geriatric primary care setting in the United States and reported an association between AST and vitamin B12 (cobalamin) deficiency. They identified 53 vitamin B12-deficient cases and compared them to 212 controls with respect to past or current use of prescription H2RAs or PPIs, with current use further classified as less than 12 months (short-term use) or greater than or equal to 12 months (chronic use) of AST. Vitamin B12 deficiency was found to be significantly associated with chronic use of AST, with the vast majority of patients in this study using H2RAs compared to PPIs; however, vitamin B12 deficiency was not associated with either past or short-term use of AST.
Antiplatelet Interactions
Recent studies have evaluated the potential interaction of the antiplatelet agent clopidogrel in patients concomitantly treated with PPIs, suggesting that PPIs decrease antiplatelet effects due to competitive inhibition of the cytochrome CYP2C19 enzyme, though this may not be a class effect.18,19 Gilard and colleagues19 conducted a double-blind, randomized, placebo-controlled trial in 124 patients with coronary artery disease (CAD) undergoing coronary artery stent implantation in which all patients received aspirin and clopidogrel and were randomized to receive either omeprazole or placebo. They determined that omeprazole significantly decreased clopidogrel’s effects on platelet activation via dephosphorylation of intraplatelet vasodilator-stimulated phosphoprotein and cytochrome P-450 metabolism. No clinical outcomes were examined in this study.
Siller-Matula and associates20 evaluated 300 patients with CAD undergoing percutaneous coronary intervention who were receiving both clopidogrel 75 mg daily and aspirin 100 mg daily and assigned them to treatment with either pantoprazole or esomeprazole versus no PPI therapy. Via examination of platelet reactivity index, there was no statistically significant difference observed between patients who received either pantoprazole or esomeprazole compared to those who received no PPI therapy. This study suggested that PPI-clopidogrel interaction may not be a class effect.
Small and coworkers21 examined 24 healthy subjects without a history of cardiovascular disease. Patients were randomized to receive either prasugrel (Effient, Eli Lilly; a thienopyridine prodrug antiplatelet agent similar to clopidogrel) 60 mg with or without lansoprazole 30 mg, or clopidogrel 300 mg with or without lansoprazole 30 mg. Lansoprazole did not significantly affect the pharmacodynamics of either prasugrel or clopidogrel; however, lansoprazole decreased inhibition of platelet aggregation (IPA) in subjects who had achieved a high IPA after a clopidogrel loading dose, though it did not interfere with IPA in the same subjects after a loading dose of prasugrel. The authors postulated that the dichotomy in these results could be best explained by differences in the pharmacokinetics of prasugrel and clopidogrel, including differences in the pathways uinvolved in the antiplatelet agents and lansoprazole.
Ho and colleagues22 examined a cohort of 8,205 US veterans after hospitalization for acute coronary syndrome (ACS) and found that use of clopidogrel plus PPI was associated with a 25% increased risk of death or rehospitalization for ACS compared to use of clopidogrel without PPI. Considering secondary outcomes, patients taking clopidogrel plus PPI were at increased risk of rehospitalization for recurrent ACS and revascularization procedures; however, no increased risk of all-cause mortality was noted compared to those using clopidogrel without PPI.
Other Risks
Long-term use of PPIs has been associated with benign conditions, including the development of benign gastric and fundic polyps, but data supporting this concept are mixed. The development of fundic polyps is thought to be largely a benign condition that regresses upon cessation of PPI therapy.23 A case-control study determined that long-term PPI therapy conferred no increased risk of development of fundic polyps.24 Case reports suggest the potential for alteration in calcium, aluminum, magnesium, and iron metabolism with the use of PPIs, but to date, no adequate long-term studies exist to prove a cause-and-effect relationship. One study in laboratory rats found that omeprazole inhibited absorption of ferrous iron, but not ferric iron, though there is a lack of adequate human studies to support this relationship.25 Omeprazole has been found to decrease the concentration of vitamin C in the biologically active form of ascorbic acid in both gastric juice and serum concentration.26 Esomeprazole has been reported to compromise upper gastrointestinal barrier function, causing a transmucosal leak of proteins in patients with GERD, the significance of which is currently unclear.27
Discussion
PPIs are associated with several adverse events mechanistically linked to hypochlorhydria. Some studies have suggested a dose-response relationship between PPI therapy and adverse events, though this has not been proven to be ubiquitous. Data on PPI therapy are stronger than data on H2RA therapy, most likely due to the more profound degree of acid suppression with PPIs.
Clostridium difficile– associated Diarrhea
C. difficile is a Gram-positive anaerobic spore-forming bacterium that is the leading cause of diarrhea in hospitalized patients, and is increasing in incidence in developed countries. The clinical spectrum ranges from asymptomatic carrier state to acute diarrhea with pseudomembranous colitis and an increased risk for colonic perforation. The mortality rates quoted in the current literature are estimated to be 1–2%, though these rates may be underestimated. The associated morbidity is even more challenging to predict.28,29 The most common risk factor for acquiring CDAD is the use of antibiotics, particularly broad-spectrum agents, including cephalosporins, clindamycin, and quinolones, in either single- or multiple-treatment courses.10,29,30 The current literature has provided substantial evidence regarding the relationship between PPI therapy and CDAD,5-10 centered on the premise that the pathogenicity of C. difficile is related to the ability of its spores to resist destruction by the normal gastric acid environment, allowing for intestinal colonization.
A systematic review of the risk of enteric infection in patients taking AST by Leonard and associates31 high-lights pooled data demonstrating an increased risk of CDAD with PPI use compared to H2RA use. The studies by Dial and coworkers6,7 postulate that the incidence of CDAD may be due to increased testing and reporting, though AST is more commonly prescribed for patients with UGI disorders, which should not influence testing for CDAD.
These data raise concern for clinicians caring for patients in the hospital setting. Many patients who are admitted may already be on AST for an appropriate condition, though others may be inappropriately placed on a PPI for stress ulcer prophylaxis and may even be discharged on them.32 One expert has suggested that all patients who are admitted on a PPI be discontinued while hospitalized to minimize potential risk of CDAD,33 whereas another expert proposes continuing PPI therapy when appropriately indicated at the lowest effective dose and fostering protective barrier nursing and prudent hand washing.34
Community-acquired Pneumonia
Microorganisms are commonly swallowed, yet few survive the acidic environment in the stomach. Alteration of this important defense mechanism via AST allows for successful gastric colonization of bacteria that are commonly found in the oral cavity and hypopharynx. Typically, the presence of pathogens in the stomach has been associated with nosocomial respiratory infections and ventilator-associated pneumonia.35,36
Attention over recent years has turned to the risk of CAP associated with PPI therapy and has uncovered a dose-dependent relationship. Laheij and colleagues11 observed a significant difference in CAP incidence between subjects currently taking PPIs compared to those who had stopped using PPIs. The researchers admitted that their data may represent a small degree of misclassification of outcomes, as the diagnosis of CAP is often clinical and not radiographic. In addition, pneumonia symptoms may have prompted clinicians to prescribe AST for UGI symptoms related to CAP. Although the overall risk of CAP in the general population without significant comorbidities is low, this study, along with the study by Gulmez and associates,12 provides insight for the care of elderly patients who are more likely to take a PPI and have a higher risk of developing CAP.
Sataloff37 suggested that GERD may contribute to the development of CAP in PPI users, though the evidence to support this hypothesis is conflicting. Gulmez and colleagues12 postulated that the most basic mechanical explanation is that profound inhibition of acid secretion disrupts a barrier for pathogens to ascend the esophagus into the pharynx and become microaspirated into the lungs, as the risk of infection with airborne pathogens has not been shown to be negatively affected by PPI use.
Bone Fracture
Conflicting evidence exists with regard to the role of hydrochloric acid in the absorption of calcium. PPIs inhibit the intragastric secretion of hydrochloric acid that mediates small intestinal absorption of calcium.38 However, in patients with normal gastric acid secretion (not in patients with pernicious anemia), insoluble calcium is absorbed at the same rate as soluble calcium.39 Osteoclasts are known to also possess proton pumps; thus, their activity is potentially thought to be directly affected by PPIs and reduce bone resorption.40
To date, there are no adequate long-term studies that have evaluated the effect of PPIs on calcium absorption. Several short-term studies have found a decrease in calcium absorption in patients taking PPIs, but the confounding element was that these patients had end-stage renal disease and were on hemodialysis.41,42
The findings from the study by Yang and coworkers14 drew similar conclusions to the study by Vestergaard and associates15 with regard to the increased risk of hip fracture with patients taking PPIs. However, the study by Vestergaard and colleagues did not identify either a dose- or duration-response effect, in contrast to the Yang and associates study. Although neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy, the authors postulated that there may be an alternative explanation for various effects with either standard-or high-dose PPIs. Clinically, it was suggested that elderly patients who require long-term and high-dose PPI therapy should consider increased dietary and/or supplementary calcium intake.
In contrast to the study by Yang and coworkers, the study by Targownik and colleagues16 found an increase in the overall risk of fracture among patients with 7 or more years of PPI therapy and an increased risk of hip fracture with 5 or more years of PPI therapy. Short-term risk of fracture was not found to be significant. Their study suggests that the risk of osteoporotic fracture increases with the duration of exposure to PPI therapy, but not in a dose-dependent fashion.4
Vitamin B12 Deficiency
Vitamin B12 deficiency is a common disorder, affecting up to 20% of elderly individuals, and is related to food-cobalamin absorption syndrome, pernicious anemia, and insufficient dietary intake.43 Clinical deficiency is often undetected and sometimes found incidentally; severe cases may present with profound neuropsychiatric and hematologic findings that may herald underlying disease. Malabsorption is thought to result from the development of atrophic gastritis and hypochlorhydria/achlorhydria.44 As patients advance in age and accrue comorbidities, including Helicobacter pylori infection, gastrectomy, vagotomy, HIV infection, and alcoholism, they are at an increased risk of cobalamin deficiency.43,45
A review by Howden46 concluded that inhibition of gastric acid secretion by PPIs may lead to vitamin B12 deficiency, as elevation of intragastric pH may impair the extraction of cobalamin from dietary protein and affect binding to salivary R proteins. The reduction in the amount of gastric acid in the upper small intestine may promote bacterial overgrowth that allows for increased bacterial consumption of cobalamin, as increased bacterial counts in the upper small intestine have been identified in patients on long-term PPI therapy, though the clinical correlation of adverse effects on nutritional status have never been determined.
To date, there is no guideline to support routine screening of serum vitamin B12 levels in patients on either short-or long-term PPI therapy. Valuck and Ruscin17 determined that with an assumed baseline 5% risk of cobalamin deficiency in the elderly, the number needed to harm (NNH) would be 7; with an assumed baseline risk of 10% deficiency, the NNH would be 4. Howden suggests that if a patient with decreased serum cobalamin levels warrants appropriate long-term PPI treatment, the benefit of treatment must be weighed against any potential risks, which in most cases should not outweigh an insignificant reduction in cobalamin levels.46
Antiplatelet Therapy
The currently accepted guidelines for management of patients with ACS consist of combination treatment with antiplatelet agents and antithrombotic medications, which can predispose patients to an increased risk of gastrointestinal bleeding.47 Barada and colleagues48 conducted a retrospective trial of 1,023 patients hospitalized with ACS, with a predominant outcome measurement of incidence of inhospital UGI bleeding and utilization rate of PPIs. Less than 1% of all patients exhibited UGI bleeding in the study. Major predictors of UGI bleeding included prior history of peptic ulcer disease or UGI bleeding and at-home consumption of aspirin, clopidogrel, or nonsteroidal anti-inflammatory drugs. The authors concluded that the risk of UGI bleeding in patients with ACS is low and may not be significantly reduced with the administration of PPIs, despite therapy with antiplatelet and antithrombotic agents.
The practical and clinical implications highlighted in the studies by Gilard and associates,19 Siller-Matula and colleagues,20 Small and coworkers,21 and Ho and associates22 remain a challenge in current practice. Omeprazole metabolism may interfere with conversion of clopidogrel, a prodrug, to its active form. Data on esomeprazole and pantoprazole are less conclusive. There are currently no guidelines to provide an evidence-based recommendation regarding PPI therapy in patients receiving antiplatelet therapy either during ACS or during maintenance therapy after ACS or a cerebrovascular accident. Additional research is needed to further examine this issue in larger cohorts, with head-to-head comparisons across all PPIs.
Conclusion
PPIs have revolutionized therapy for many UGI disorders and command a substantial percentage of the US pharmaceutical market share. Appropriate utilization of these drugs for appropriate diagnoses, periodic reassessment of patient symptoms to determine the lowest effective dosage and duration of therapy, as well as close surveillance for potential adverse risks, will not only minimize cost expenditure but will maximize favorable outcomes. The development of local and national guidelines for appropriate use of AST may also help to minimize adverse risk.
Contributor Information
Joel J. Heidelbaugh, Dr. Heidelbaugh serves as Clinical Associate Professor in the Department of Family Medicine at the University of Michigan in Ann Arbor, Michigan.
Kathleen L. Goldberg, Dr. Goldberg is a Clinical Pharmacist in the Department of Veterans Administration in the VA Ann Arbor Healthcare System in Ann Arbor, Michigan.
John M. Inadomi, Dr. Inadomi serves as Professor of Medicine at the University of California, San Francisco and in the Division of Gastroenterology of San Francisco General Hospital in San Francisco, California.
References
- 1. [December 1]. 2008. Pharmacy Facts & Figures. Drug Topics [Web site]. Available at: http://drug-topics.modernmedicine.com/Pharmacy+Facts+&+Figures.
- 2.Cote GA, Howden CW. Potential adverse effects of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:208–214. doi: 10.1007/s11894-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 3.Inadomi JM, Fendrick AM. PPI use in the OTC era: who to treat, with what, and for how long? Clin Gastroenterol Hepatol. 2005;3:208–215. doi: 10.1016/s1542-3565(04)00717-7. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen BC, Ferris TG, Shea TL, Mahlis EM, Lee TH, Wang TC. Who is using chronic acid suppression therapy and why? Am J Gastroenterol. 2003;98:51–58. doi: 10.1111/j.1572-0241.2003.07186.x. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243–245. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 6.Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 7.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhea. Aliment Pharmacol Ther. 2006;24:613–619. doi: 10.1111/j.1365-2036.2006.03015.x. [DOI] [PubMed] [Google Scholar]
- 9.Jayatilaka S, Shakov R, Eddi R, Bakaj G, Baddoura WJ, DeBari VA. Clostridium difficile infection in an urban medical center: five-year analysis of infection rates among adult admissions and association with the use of proton pump inhibitors. Ann Clin Lab Sci. 2007;37:241–247. [PubMed] [Google Scholar]
- 10.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium-difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 11.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 12.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167:950–955. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 14.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 15.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 16.Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Gilard M, Arnaud B, Le Gal G, Abgrall JF, Boschat J. Influence of ompeprazole on the antiplatelet action of clopidogrel associated to aspirin. J Thromb Haemost. 2006;4:2508–2509. doi: 10.1111/j.1538-7836.2006.02162.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin. The randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) Study. J Am Coll Cardiol. 2008;51:256–260. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J. 2009;157:148.e1–148.e5. doi: 10.1016/j.ahj.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Small DS, Farid NA, Payne CD, Weerakkody GJ, Li YG, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasurgel and clopidogrel. J Clin Pharmacol. 2008;48:475–484. doi: 10.1177/0091270008315310. [DOI] [PubMed] [Google Scholar]
- 22.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 23.Stolte M. Fundic gland polyps: a rare, innocuous, and reversible disturbance. Gastroenterology. 1993;105:1590–1591. doi: 10.1016/0016-5085(93)90187-h. [DOI] [PubMed] [Google Scholar]
- 24.Vieth M, Stolte M. Fundic gland polyps are not induced by proton pump inhibitor therapy. Am J Clin Pathol. 2001;116:716–720. doi: 10.1309/XFWR-LXA7-7TK1-N3Q8. [DOI] [PubMed] [Google Scholar]
- 25.Golubov J, Flanagan P, Adams P. Inhibition of iron absorption by omeprazole in rat model. Dig Dis Sci. 1991;36:405–408. doi: 10.1007/BF01298866. [DOI] [PubMed] [Google Scholar]
- 26.Mowat C, Carswell A, Wirz A, McColl KE. Omeprazole and dietary nitrate independently affect levels of vitamin C and nitrite in gastric juice. Gastroenterology. 1999;116:813–822. doi: 10.1016/s0016-5085(99)70064-8. [DOI] [PubMed] [Google Scholar]
- 27.Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28:1317–1325. doi: 10.1111/j.1365-2036.2008.03824.x. [DOI] [PubMed] [Google Scholar]
- 28.Poutanen SM, Simor AE. Clostridium difficile-associated diarrhoea in adults. Can Med Assoc J. 2004;171:51–58. doi: 10.1503/cmaj.1031189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 30.Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 31.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 32.Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-intensive care unit hospitalized patients. Am J Gastroenterol. 2006;101:2200–2205. doi: 10.1111/j.1572-0241.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 33.Thachil J. Overprescribing PPIs: time for a hospital antacid policy on Clostridium difficile. BMJ. 2008;336:109. doi: 10.1136/bmj.39458.465845.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metz D. Clostridium difficile colitis: wash your hands before stopping the proton pump inhibitor. Am J Gastroenterol. 2008;103:2314–2316. doi: 10.1111/j.1572-0241.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 35.Laheij RJ, Van Ijzendoorn MC, Janssen MJ, Jansen JB. Gastric acid-suppressive therapy and community-acquired respiratory infections. Aliment Pharmacol Ther. 2003;18:847–851. doi: 10.1046/j.1365-2036.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- 36.Inglis TJ, Sherratt MJ, Spoat LJ, Gibson JS, Hawkey PM. Gastroduodenal dysfunction and bacterial colonization of the ventilated lung. Lancet. 1993;341:911–913. doi: 10.1016/0140-6736(93)91208-4. [DOI] [PubMed] [Google Scholar]
- 37.Sataloff RT. Community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2005;293:795–796. doi: 10.1001/jama.293.7.795-c. [DOI] [PubMed] [Google Scholar]
- 38.Bo-Linn GW, Davis GR, Buddrus DJ, Morawski SG, Santa Ana C, Fordtran JS. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640–647. doi: 10.1172/JCI111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med. 1987;317:532–536. doi: 10.1056/NEJM198708273170903. [DOI] [PubMed] [Google Scholar]
- 40.Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033–2048. doi: 10.2174/1381612023393369. [DOI] [PubMed] [Google Scholar]
- 41.Graziani G, Badalamenti S, Como G, Gallieni M, Finazzi S, et al. Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload. Role of gastric acid secretion. Nephron. 2002;91:274–479. doi: 10.1159/000064290. [DOI] [PubMed] [Google Scholar]
- 42.Hardy P, Sechet A, Hottelart C, Oprisiu R, Abighanem O, et al. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artif Organs. 1998;22:569–573. doi: 10.1046/j.1525-1594.1998.06200.x. [DOI] [PubMed] [Google Scholar]
- 43.Andres E, Loukili N, Noel E, Kaltenbach G, Abdelgheni MB, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171:251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Force RW, Meeker AD, Cady PS, Culbertson VL, Force WS, Kelley CM. Increased vitamin B12 requirement associated with chronic acid suppression therapy. Ann Pharmacother. 2003;37:490–493. doi: 10.1345/aph.1C037. [DOI] [PubMed] [Google Scholar]
- 45.Kaptan K, Beyan C, Yalcin A. Helicobacter pylori—is it a novel causative agent in vitamin B12 deficiency? Arch Intern Med. 2000;160:1349–1353. doi: 10.1001/archinte.160.9.1349. [DOI] [PubMed] [Google Scholar]
- 46.Howden C. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. J Clin Gastroenterol. 2000;30:29–33. doi: 10.1097/00004836-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Sinnaeve PR, Huang Y, Bogaerts K, Vahanian A, Adgey J, et al. Age, outcomes and treatment effects of fibrinolytic and antithrombotic combinations: findings from Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT)-3 and ASSENT-3 PLUS. Am Heart J. 2006;152684:e1–684.e9. doi: 10.1016/j.ahj.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Barada K, Karrowni W, Abdallah M, Shamseddeen W, Sharara AI, Dakik HA. Upper gastrointestinal bleeding in patients with acute coronary syndromes. Clinical predictors and prophylactic role of proton pump inhibitors. J Clin Gastroenterol. 2008;42:368–372. doi: 10.1097/MCG.0b013e31802e63ff. [DOI] [PubMed] [Google Scholar]


