Abstract
Approximately 20% of all inflammatory bowel disease (IBD) first presents in childhood or adolescence, and approximately 10% of the estimated 1.4 million Americans with IBD are under age 17. Diagnosis in pediatric patients may be complicated at presentation due to atypical symptoms and/or extraintestinal manifestations (eg, short stature, chronic anemia, unexplained fever, arthritis, mouth ulcers). Pediatric IBD is traditionally diagnosed using endoscopic evaluations of the upper and lower gastrointestinal tract with mucosal biopsies for histologic confirmation. Less invasive serologic testing for IBD may be particularly valuable in pediatric patients, particularly given the association between serum immune reactivity and severe disease phenotypes that is drawing increasing attention. These serologic markers may help stratify risk and identify appropriate pediatric candidates for early aggressive therapy. Serologic testing in pediatric patients includes traditional IBD serologic markers such as anti–Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibody, as well as newer antimicrobial antibodies, including antibodies to outer membrane porin C; I2, a bacterial sequence derived from Pseudomonas fluorescens; and CBir1 flagellin, a colitogenic antigen of the enteric microbial flora in C3H/HeJBir mice strain. Given recent data associating seropositivity with aggressive clinical phenotypes and rapid disease progression, serologic testing may allow early initiation of therapy, maintenance of remission, reduction of corticosteroid exposure, facilitation of mucosal healing, and restoration of normal growth velocity.
Keywords: Inflammatory bowel disease, diagnosis, serologic markers, pediatric, phenotypes, prognostic, biologic therapy
Although the onset of inflammatory bowel disease (IBD) can occur at any age, approximately 20% of all cases of IBD first present in childhood or adolescence.1 Children and adolescents younger than age 17 constitute approximately 10% of the estimated 1.4 million Americans with IBD.2 Pediatric Crohn's disease is more common than ulcerative colitis. Although true incidence rates are uncertain, Crohn's disease is diagnosed annually in children at a rate of 0.2–8.5 per 100,000 whereas ulcerative colitis is diagnosed at a rate of 0.5–4.3 per 100,000.3,4 This disparity is further demonstrated by results of a large population-based North American study that found the incidence of Crohn's disease to be 4.56 per 100,000, more than double that of ulcerative colitis.5 Moreover, the incidence of pediatric Crohn's disease appears to be increasing, with a recent population-based study in northern France showing a 20% increase in incidence (from 2.1 to 2.6) over 12 years.6 Similarly, pediatric studies from the United States, Scandinavia, and the United Kingdom have all documented increases in overall mean annual Crohn's disease rates over the past 40 years.7
The prevalence of pediatric indeterminate colitis appears to be higher than adult indeterminate colitis,4,8 ranging from 5% to 30% in children8-11 and 10% to 15% in adults.12 Indeterminate colitis is generally considered a temporary diagnosis in children.8 The lower incidence of indeterminate colitis in adults versus children likely arises from the greater amount of time adults have had the disease and the greater likelihood of making a specific diagnosis of Crohn's disease or ulcerative colitis.4 Conversely, other data indicate that pediatric patients with indeterminate colitis retain their diagnoses, suggesting that indeterminate colitis is a distinct clinical phenotype existing along the spectrum of IBD.8,13
Several epidemiologic features of pediatric IBD differ from those of adult-onset IBD. The highest rate of ulcerative colitis and Crohn's disease onset among children was shown to occur at approximate 15 years of age, with 12.5 years being the mean age of diagnosis of IBD and 15 years being the median age for diagnosis of Crohn's disease and ulcerative colitis.5 Indeterminate colitis is prevalent among children with IBD who are younger than age 2.2 Although only slight gender-related differences are observed in adults with IBD,14 the risk of Crohn's disease in children younger than 16 years of age is higher among boys than girls; however, the risk increases among girls in older age groups.2,15
The etiology of IBD in adults and children is believed to have multiple causes, arising from a complex interaction among environmental, genetic, and immune factors.3 Genetically determined defects in the innate immune system lead to overly aggressive mucosal responses to local resident bacterial flora, eventually resulting in the chronic inflammatory lesions typical of IBD.15-17 A strong association is reported between Crohn's disease and variants of the NOD2/CARD15 gene on chromosome 16.15,17 Data from 10 studies involving more than 1,300 children of European descent showed that the risk of Crohn's disease associated with NOD2/CARD15 is similar between pediatric and adult patients.15 Genetics may play a particularly important role in the pediatric population, as children typically lack the environmental exposures associated with IBD in adults (eg, cigarette smoking, use of nonsteroidal anti-inflammatory drugs) and have had less time to be influenced by such immune modifiers. Variants of the IBD5 and tumor necrosis factor-α(TNF-α) genes may also increase IBD susceptibility in children, though these associations have not been consistently demonstrated.15,18
The contribution of the immune system to IBD pathogenesis is highlighted by the abnormal immune responses to various cellular and microbial antigens noted among adult and pediatric patients with IBD.17,19 In their study of the diagnostic accuracy of anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear antineutrophil cytoplasmic antibodies (pANCA) in IBD, Reese and colleagues noted that antibodies directed toward Saccharomyces cerevisiae (ie, ASCA) have been detected in up to 68% of patients with Crohn's disease, whereas pANCA has been detected in 40–80% of patients with ulcerative colitis.19 The presence of ASCA-immunoglobulin A (IgA) and -IgG is highly specific for Crohn's disease in children,20 and a number of newer serologic markers for Crohn's disease have been identified in this population.21,22
An association between serum immune reactivity and severe disease phenotypes in children is drawing increasing attention.16,23,24 Several studies have correlated ASCA positivity with stricturing and penetrating disease, increased risk for surgery and complications, and shorter time to developing complications.23,25,26 The ability to establish aggressive disease phenotypes early in the disease course may be particularly valuable in helping clinicians identify subgroups of children at risk for severe disease who may benefit from the initiation of early aggressive therapy.17,27
Clinical Presentation of Inflammatory Bowel Disease in Pediatric Patients
As in adults, the presentation of IBD in children is assessed in terms of disease location and extent of inflammation.3,4 The most common presenting symptoms of ulcerative colitis include weight loss, rectal bleeding, diarrhea, and abdominal pain, whereas symptoms more characteristic of Crohn's disease include insidious onset of abdominal pain and weight loss.1,3,28 Up to 35% of pediatric patients with IBD present with at least 1 extraintestinal manifestation, arthritis being the most common.3,4,28 Table 1 summarizes extraintestinal manifestations in IBD. Although typically concurrent with exacerbation of disease in adults, extraintestinal symptoms in children with IBD may precede the onset of gastrointestinal symptoms by years.3,28
Table 1.
Extraintestinal Manifestations of Inflammatory Bowel Disease
| Body system | Symptom |
|---|---|
| Blood |
|
| Bone |
|
| Eye |
|
| General |
|
| Growth |
|
| Joints |
|
| Kidney |
|
| Liver |
|
| Lungs |
|
| Mouth |
|
| Pancreas |
|
| Skin |
|
Adapted with permission from Mamula, et al.4
Childhood-onset IBD may have a more aggressive or complicated clinical course than adult disease.16,29 Children typically present with moderate-to-severe disease activity,17 and up to 37% have extensive involvement (ie, pancolitis) at presentation.4,30 An increased risk at younger age of onset and complicated disease behaviors have been correlated with the presence of NOD2/CARD15 variants,31-34 as well as serum immune reactivity.16,23,24 Children with indeterminate colitis also tend to have a particularly aggressive phenotype, characterized by the early age of onset and rapid progression to pancolitis.8 In a retrospective analysis of 250 children registered in The Johns Hopkins Children's IBD Center database from 1996 to 2001, 59 of 74 (79.7%) patients with indeterminate colitis had pancolitis at diagnosis, with the remaining 15 children progressing to pancolitis within a mean of 6.5 years.8
Special Considerations in Pediatric Inflammatory Bowel Disease
Despite obvious similarities between childhood- and adult-onset disease, a number of concerns are unique to the pediatric IBD population. A primary concern and differentiating feature of pediatric IBD is the well-established impairment in growth and skeletal development associated with the disease.2,4,7,35,36 More than 35% of pediatric Crohn's disease patients and up to 10% of pediatric ulcerative colitis patients have impaired linear growth, which can precede the initial diagnosis by years.7 Indeed, growth failure has been documented in up to 85% of children with Crohn's disease and 65% of those with ulcerative colitis at the time of diagnosis.4,37
The pathogenesis of growth impairment in pediatric IBD is multifactorial and may be attributed to the increased nutritional demands that normally occur during childhood in combination with increased metabolic demands and inadequate nutrition resulting from poor dietary intake, altered nutrient use, and increased fecal losses of protein and essential trace elements.2 Growth and sexual maturation also may be delayed by cytokines and other immunologic and endocrine abnormalities.2 As with adults, children and adolescents with IBD may have bone demineralization with chronic corticosteroid use, decreased physical activity, and poor nutritional intake serving as contributing factors.36,38
The serious and potentially irreversible consequences of growth impairment underscore the need to ensure adequate growth and nutritional status in the management of IBD in pediatric patients. Appropriate anti-inflammatory therapy and caloric supplementation, including enteral and parenteral nutrition during acute exacerbations, can improve nutritional status and promote growth.2,4 Iron supplementation is important in preventing iron-deficiency anemia commonly resulting from gastrointestinal blood loss and poor dietary intake.39 Despite these measures, however, some children with IBD may not reach their predicted height potential.2 Strategies for optimizing bone mineral density include periodic radiographic studies (DEXA scans); monitoring serum calcium, phosphorous, and alkaline phosphatase levels; and ensuring adequate calcium and vitamin D intake.7
IBD may present profound psychosocial challenges for pediatric patients. Children and adolescents with IBD face considerable stress associated with a lifelong disease characterized by a relapsing and unpredictable disease course and potentially embarrassing symptoms.40-43 Studies in pediatric IBD patients have revealed various difficulties in psychosocial functioning, feelings of vulnerability and lack of control, lower self-esteem and energy levels, concerns regarding body image, and perceptions of themselves as different from their healthy peers and siblings.4,40,44 Not surprisingly, children and adolescents have demonstrated higher rates of anxiety and depression compared to control populations.40 Although not as well studied in pediatric as in adult populations, quality of life is more frequently compromised in children with IBD, and those with higher disease activity scores have less severe or inactive disease.45,46
Pediatric patients with IBD as well as adults with childhood-onset IBD are at increased risk for other comorbidities and complications as well. The risk of cancer appears to be particularly high in patients diagnosed with Crohn's disease before 25 years of age and in those with a history of pancolitis.3 Crohn's colitis patients have a risk of adenocarcinoma of the colon that occurs at an incidence 4–20 times greater than that of the general population. Those with disease of the small intestine carry a 50–100 times increased likelihood of developing carcinoma of the small intestine.28 The risk of colorectal cancer in ulcerative colitis is directly related to disease duration, such that there is an incidence of 5% at 20 years and 40% at 35 years among children who develop ulcerative colitis before 15 years of age.47 The safety and efficacy of routine childhood immunizations for vaccine-preventable diseases are a clinical issue in pediatric patients, though recommendations for most children with IBD do not differ from schedules recommended for the general population.48 However, a decrease in immune response to influenza vaccination has been documented among children receiving infliximab and immunomodulatory therapy.49 Finally, the transition of IBD patients from pediatric to adult care is a unique situation that requires time and careful planning.7 Successful transition requires a well-designed transition plan, creation of a current and accessible medical summary, access to continuous health-care insurance coverage, and identification of a healthcare professional who understands the needs of adolescents as they reach adulthood.7,50
Is Early Aggressive Therapy Warranted in Pediatric Patients?
Traditionally, the primary goals of medical therapy in children with IBD have been to promote and maintain remission of active disease, promote growth through adequate nutrition and suppression of inflammation, enhance physical and psychosocial functioning, and reduce the risk of surgery.27 Although most medical therapies used to treat IBD in adults are available to treat pediatric patients, few randomized controlled trials have been conducted in children.7,35 Although inducible with mesalamine, corticosteroids, immunomodulators, and infliximab, remission is generally more difficult to maintain. In particular, corticosteroids have not been found to be effective in maintaining remission and may contribute to growth impairment in pediatric patients. In contrast, immunomodulators maintain remission in pediatric Crohn's disease, significantly reduce corticosteroid exposure, and may reduce the number of hospitalizations.51,52 Accordingly, a recent prospective, multicenter, observational study indicates that the overwhelming majority (80%) of patients receive an immunomodulator within the first 12 months of a Crohn's disease diagnosis.52
With the introduction of biologic therapies and widespread use of immunomodulators in pediatric patients, potential treatment goals are expanding to include altering the natural course of disease and slowing the progression of complications.27,35 The TNF-α antagonist infliximab not only induces and prolongs remission in children with IBD,53,54 it also achieves mucosal healing and/or endoscopic improvement in this population.53,55 In addition, infliximab has been found to reverse growth failure in children with severe refractory Crohn's disease,55 reduce corticosteroid exposure,56 and delay or avoid emergency colectomy in children with ulcerative colitis.18
Particular benefit of infliximab has been noted in pediatric patients when initiated early in the course of disease. In a small study of 15 pediatric patients with medically refractory Crohn's disease, infliximab infusion maintained a prolonged clinical response (through 12 months) in 50% of patients with early disease (less than 2 years from the time of diagnosis) compared to no patients with late disease (>2-year history of intestinal inflammation).54 A similarly prolonged response to infliximab was observed among patients with early disease (<1 year's duration) in a retrospective series of pediatric patients with refractory and/or fistulizing Crohn's disease.57
Despite the potential benefits of early aggressive therapy, significant questions regarding this strategy remain unanswered. The benefits of biologic therapy have not been confirmed in prospective controlled trials in large numbers of children, and little data have linked biologic therapy with objective outcomes (ie, reduced hospitalizations, surgeries) in this population. Currently, there is only limited experience with biologic agents other than infliximab (eg, adalimumab, certolizumab pegol, natalizumab), and data on long-term use of any of these agents in pediatric patients are generally not available. Finally, biologic agents are associated with a number of rare but potentially serious toxicities in adults, including serious infections and malignancy, particularly when used in combination with immunosuppressant agents.58,59 Larger numbers of patients treated with these agents are necessary to characterize the safety profile of biologics in children. Thus, the potential benefits of early and aggressive use of biologic agents in children must be weighed against the potential complications of these therapies, as well as the known consequences of inadequately treated Crohn's disease.27
Diagnosis of Pediatric Inflammatory Bowel Disease
Given the serious consequences of IBD on growth and development, early and accurate diagnosis of pediatric patients is essential. Although diagnostic evaluation in children and adolescents is recommended as soon as IBD is suspected,7 delayed diagnosis remains a significant problem in this population.4 The mean delay in diagnosis is 7–11 months for pediatric Crohn's disease and 5–8 months for pediatric ulcerative colitis.4 Not surprisingly, the mean delay in diagnosing indeterminate colitis in pediatric patients exceeds 1 year (14 months). The diagnosis may be particularly difficult in children and adolescents who present with less than typical symptoms and/or extraintestinal manifestations (eg, short stature, chronic anemia, unexplained fever, arthritis, mouth ulcers) rather than with typical gastrointestinal symptoms (eg, diarrhea).4,60 In addition, diagnosis of pediatric IBD may be more challenging in patients with disease isolated to the colon,1 as rectal sparing has been noted in pediatric ulcerative colitis.11,61
As in adults, evaluation of children and adolescents should be directed at determining whether IBD is present and then differentiating between Crohn's disease and ulcerative colitis.1 The initial evaluation includes a thorough clinical history regarding symptom onset, recent travel, food intolerance, contact with enteric illnesses, medications, and family history of IBD.60 Routine laboratory evaluation should include markers of inflammation (eg, C-reactive protein, erythrocyte sedimentation rate, fecal leukocytes, lactoferrin), complete blood count, nutritional laboratory tests, and stool studies to rule out infection.7,60 Normal results do not exclude a diagnosis of IBD but do have a good negative predictive value for ruling out infectious disease.60,62
The gold standard for diagnosing pediatric IBD remains endoscopic evaluation of the upper and lower gastrointestinal tracts, with mucosal biopsies for histopathologic confirmation.7,63 Small-bowel follow-through is the standard for small-bowel investigation, providing information on the extent of disease and possible complications.60 Computed tomography enteroclysis and magnetic resonance enteroclysis may be useful alternatives in children. Video capsule endoscopy allows examination of the small bowel without the need for sedation, but the ability of small children to swallow the capsule may be limited.60,64 Thus, endoscopic placement of the capsule in the duodenum is required when used in most young children.62
Serologic Testing
Noninvasive tests for IBD are particularly valuable in pediatric patients.65 Fecal calprotectin correlates strongly with the severity and extent of mucosal inflammation, but has limited specificity for IBD.60,66,67 Although testing for variations in the NOD2 gene identifies 25% of patients with Crohn's disease, NOD2 genotyping cannot differentiate between ulcerative colitis and Crohn's disease of the colon.11 In contrast, serologic markers such as ASCA and pANCA have a high degree of specificity in IBD, with positivity for both ASCA-IgA and -IgG found to be 100% specific for pediatric Crohn's disease.20 In pediatric ulcerative colitis, a specificity of 92%20 has been found when ANCA is assayed using enzyme-linked immunosorbent assay and immunofluorescence, followed by DNase treatment.
In addition to the traditional serologic markers ASCA and pANCA, new antimicrobial antibodies have been detected in the sera of Crohn's disease patients. These markers include antibodies to outer membrane porin C (anti-OmpC), the outer membrane porin of gram-negative bacteria originally isolated from Escherichia coli; I2, a bacterial sequence derived from Pseudomonas fluorescens; and CBir1 flagellin (anti-CBir1), a colitogenic antigen of the enteric microbial flora in C3H/HeJBir mice strain.21,22 Several US laboratories test sera for IBD markers, and some laboratories use proprietary procedures to test for specific markers.68 One such panel of markers available commercially, the IBD Serology 7 from Prometheus Laboratories, combines a proprietary test for IBD-specific pANCA with tests for anti-OmpC, anti-CBir1, and both ASCA-IgA and -IgG. This test uses a diagnostic algorithm that is based on pattern recognition data rather than a specific cutoff value to assess and differentiate IBD.68 The sensitivity and specificity of utilizing this proprietary diagnostic algorithm in pediatric patients with IBD have been calculated at 79% and 88%, respectively (Data on file, Prometheus Laboratories).
Seroconversion for these biomarkers may be contingent on a mature immune system on exposure to normal commensal intestinal bacteria. Increased small-bowel permeability has been postulated to be a risk factor in presumably healthy first-degree relatives of patients with Crohn's disease.69 This increased permeability may permit the passage of these intestinal bacteria or microbial antigens, thereby activating a dysregulated immune inflammatory response. Because children show an age-specific maturation of their immune system over time, further understanding of the specific physiologic or pathologic function of these circulating antibodies may depend on studies in children.
A recent retrospective analysis of serum biomarkers from a large database found a high percentage of samples from children and adolescents (31.5%) were characterized by anti-CBir1 as the sole elevated marker.70 Prediction of Crohn's disease in adults 17 years of age or older, however, was associated with elevations of both ASCA-IgA and -IgG, illustrating that markers for diagnostic prediction may vary by age (Table 2).70 The recent study by Markowitz and coworkers71 is the first to raise the notion that age at diagnosis within the pediatric age group itself may influence serologic responses in children. Among the 705 children with Crohn's disease in this study, 79 were diagnosed at less than 8 years of age. Of those with isolated colonic disease (n=40), ASCA positivity was uncommon. However, these patients were more likely to express antiCBir1 antibodies, albeit independent of disease location. Although isolated small-bowel disease was higher in the older age group, these differences could not be explained by the frequency of NOD2 variants or the level of CBir1 positivity. The propensity of anti-CBir1 seroconversion in the pediatric age group, whether considering isolated colonic or small-bowel disease, is the most intriguing finding, and may suggest an as yet unrecognized impact of a maturing or dysregulated immune system.71
Table 2.
Distribution of Samples Exhibiting Elevations of a Single Marker
| Age, yr | |||||
|---|---|---|---|---|---|
| Unknown, n (%) | 0–5, n (%) | 6–16, n (%) | 17+, n (%) | Total, n (%) | |
| ANCA | 170 (3.7) | 80 (2.2) | 740 (3.1) | 4,646 (4.1) | 5,636 (3.9) |
| ASCAA | 167 (3.6) | 27 (0.7) | 441 (1.9) | 4,121 (3.6) | 4,756 (3.3) |
| ASCAG | 22 (0.5) | 6 (0.2) | 106 (0.5) | 796 (0.7) | 930 (0.6) |
| Anti-CBir1* | 801 (17.3) | 1,817 (49.5) | 6,751 (28.7) | 16,354 (14.4) | 25,723 (17.7) |
| Anti-OmpC | 203 (4.4) | 49 (1.3) | 450 (1.9) | 5,991 (5.3) | 6,693 (4.6) |
| pANCA | 1 (0.0) | 1 (0.0) | 2 (0.0) | 29 (0.0) | 33 (0.0) |
| Other | 3,256 (70.5) | 1,688 (46.0) | 15,009 (63.9) | 81,903 (71.9) | 101,856 (69.9) |
| Total | 4,620 | 3,668 | 23,499 | 113,840 | 145,627 |
A high percentage of samples had CBir1 as the only identified marker.
Reproduced from Barken, et al.70
Pediatric gastroenterologists have often felt that they deal with more severely ill patients than their colleagues who care for adult patients. The ability to identify children with Crohn's disease who are at the highest risk for rapid progression would be invaluable in guiding therapy. Although the functional significance of having the serologic markers ASCA, anti-OmpC, and anti-CBir1 is not yet known, there is growing evidence that serologic biomarkers may provide clinical insight in predicting aggressive disease behavior, particularly in children with Crohn's disease. In a study of 154 pediatric patients with Crohn's disease, ASCA positivity predicted a more relapsing disease course.23 Although this study was specific to a single serologic marker, a recent study of 796 pediatric patients with Crohn's disease showed that the frequency of internal-penetrating and stricturing small-bowel disease, and the need for early surgical intervention, significantly increased with the number and titer of serologic markers (P trend <.001; Figure 1).24 The odds ratios for the development of these disease complications based on antibody sum and antibody quartile sum score groups are presented in Table 3.24 Patients who were seropositive for all 3 biomarkers (ie, anti-OmpC, anti-CBir1, and ASCA) had odds ratios of 9.5, 6.1, and 10.3 for developing internal-penetrating disease, stricturing disease, or undergoing surgery, respectively. These findings lend credence to the use of serologic markers to delineate phenotypes for purposes of directed treatment.
Figure 1.
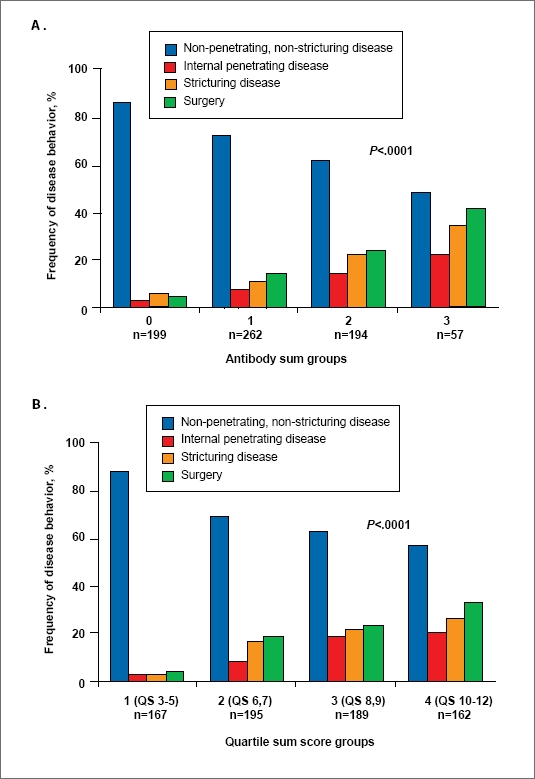
Frequency of nonpenetrating and nonstricturing, internalpenetrating, stricturing, and surgery among (A) antibody sum groups and (B) antibody quartile sum (QS) groups in pediatric patients with Crohn's disease. Antibody sum reflects the number of positive antibodies per individual; antibody QS score is the sum of the quartile scores of 3 antibodies (ASCA-IgA or -IgG, anti-OmpC, and anti-CBir1) where antibody levels <25% = 1; 25–50% = 2; 51−<75% = 3; and >75% = 4.
Reprinted with permission from Dubinsky, et al.24
Table 3.
Odds Ratios for the Development of Specific Disease Behaviors Based on Antibody Sum* and Quartile Sum Scores†: Univariate Analysis
| Complication | Antibody sum 1, OR (95% CI)* | Antibody sum 2, OR (95% CI) | Antibody sum 3, OR (95% CI) | P value | Quartile sum score group 2, OR (95% CI)† | Quartile sum score group 3, OR (95% CI) | Quartile sum score group 4, OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| Non-penetrating, non-stricturing | 0.40 (0.3–0.7) | 0.3 (0.2–0.4) | 0.1 (0.07–0.3) | <.0001 | 0.3 (0.2–0.5) | 0.3 (0.1–0.4) | 0.2 (0.1–0.3) | <.0001 |
| Internal-penetrating | 2.2 (0.9–5.8) | 5.2 (2.1–12.9) | 9.5 (3.4–26.4) | <.0001 | 3.2 (1.0–9.8) | 4.0 (1.3–12.2) | 8.5 (2.9–24.9) | <.0001 |
| Stricturing | 1.7 (0.9–3.4) | 4.2 (2.2–8.1) | 6.1 (2.7–13.5) | <.0001 | 6.8 (2.3–19.9) | 8.6 (2.9–24.9) | 12.5 (4.3–35.9) | <.0001 |
| Surgery | 2.7 (1.4–5.2) | 4.5 (2.4–8.8) | 10.3 (4.8–22.1) | <.0001 | 4.4 (2.0–9.7) | 4.8 (2.1–10.6) | 8.4 (3.8–18.5) | <.0001 |
Baseline antibody sum is 0 (OR=1.0).
Baseline quartile sum (QS) score group is 1 (OR=1).
QS score group 1 = QS of 3–5; QS group 2 = QS of 6,7; QS group 3 = QS of 8,9 and QS group 4 = QS of 10
Reprinted with permission from Dubinsky, et al.24
Conclusion
Although similarities exist, several features of IBD differ in children compared to adults. In addition to specific age- and gender-related patterns, IBD in children may be associated with a more severe and aggressive disease course than in adults. Pediatric patients appear to have a relatively higher prevalence of indeterminate colitis, which may be associated with particularly severe disease. Management considerations for IBD that are unique to the pediatric population include the impact of the disease on growth and nutrition, psychosocial functioning, quality of life, and transition from pediatric to adult care. Although diagnostic approaches and principles are similar in adult and pediatric populations, noninvasive methods may be particularly valuable in pediatric patients when there is diagnostic uncertainty. In such patients, serologic testing may be useful in differentiating Crohn's disease from ulcerative colitis. Moreover, increasing evidence suggests that serologic markers have particular prognostic value in pediatric IBD. Given recent prognostic data associating seropositivity with aggressive clinical phenotypes and rapid disease progression, serologic testing may allow for the selection of patients who may benefit from early aggressive medical therapies with immunomodulators and/or biologic agents. Such therapies may have particular advantages in children, including maintenance of remission, reduced corticosteroid exposure, mucosal healing, and restoration of normal growth velocity. Despite these potential benefits, however, controlled data in large numbers of children are necessary to define the role of this strategy and confirm its safety in the pediatric population.
Acknowledgments
Dr. Cuffari receives research support from and serves as a consultant to Prometheus Laboratories.
Dr. Cuffari thanks John Simmons, MD, for his assistance in the preparation of this manuscript. Dr. Simmons is an employee of Peloton Advantage. Peloton Advantage provides consulting services to Prometheus Laboratories.
References
- 1.Dubinsky M. Special issues in pediatric inflammatory bowel disease. World J Gastroenterol. 2008;14:413–420. doi: 10.3748/wjg.14.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousvaros A, Sylvester F, Kugathasan S, et al. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. doi: 10.1097/01.mib.0000228358.25364.8b. [DOI] [PubMed] [Google Scholar]
- 3.Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol. 2006;12:3204–3212. doi: 10.3748/wjg.v12.i20.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am. 2003;32:967–995. doi: 10.1016/s0889-8553(03)00046-3. viii. [DOI] [PubMed] [Google Scholar]
- 5.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 6.Auvin S, Molinie F, Gower-Rousseau C, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988-1999) J Pediatr Gastroenterol Nutr. 2005;41:49–55. doi: 10.1097/01.mpg.0000162479.74277.86. [DOI] [PubMed] [Google Scholar]
- 7.Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004;126:1550–1560. doi: 10.1053/j.gastro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis. 2006;12:258–262. doi: 10.1097/01.MIB.0000215093.62245.b9. [DOI] [PubMed] [Google Scholar]
- 9.Mamula P, Telega GW, Markowitz JE, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–2010. doi: 10.1111/j.1572-0241.2002.05915.x. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand H, Fredrikzon B, Holmquist L, Kristiansson B, Lindquist B. Chronic inflammatory bowel disease in children and adolescents in Sweden. J Pediatr Gastroenterol Nutr. 1991;13:293–297. doi: 10.1097/00005176-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 12.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Burakoff R. Indeterminate colitis: clinical spectrum of disease. J Clin Gastroenterol. 2004;38:S41–S43. doi: 10.1097/01.mcg.0000123991.13937.7e. [DOI] [PubMed] [Google Scholar]
- 14.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 15.Biank V, Broeckel U, Kugathasan S. Pediatric inflammatory bowel disease: clinical and molecular genetics. Inflamm Bowel Dis. 2007;13:1430–1438. doi: 10.1002/ibd.20213. [DOI] [PubMed] [Google Scholar]
- 16.Dubinsky MC, Lin YC, Dutridge D, et al. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller CA, Markowitz J. IBD in children: lessons for adults. Curr Gastroenterol Rep. 2007;9:528–532. doi: 10.1007/s11894-007-0070-8. [DOI] [PubMed] [Google Scholar]
- 18.Cucchiara S, Latiano A, Palmieri O, et al. Polymorphisms of tumor necrosis factor-alpha but not MDR1 influence response to medical therapy in pediatric-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:171–179. doi: 10.1097/MPG.0b013e31802c41f3. [DOI] [PubMed] [Google Scholar]
- 19.Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–2422. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 21.Peyrin-Biroulet L, Standaert-Vitse A, Branche J, Chamaillard M. IBD serological panels: facts and perspectives. Inflamm Bowel Dis. 2007;13:1561–1566. doi: 10.1002/ibd.20226. [DOI] [PubMed] [Google Scholar]
- 22.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Desir B, Amre DK, Lu SE, et al. Utility of serum antibodies in determining clinical course in pediatric Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:139–146. doi: 10.1016/s1542-3565(03)00321-5. [DOI] [PubMed] [Google Scholar]
- 24.Dubinsky MC, Kugathasan S, Mei L, et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008;6:1105–1111. doi: 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Amre DK, Lu SE, Costea F, Seidman EG. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn's disease patients. Am J Gastroenterol. 2006;101:645–652. doi: 10.1111/j.1572-0241.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 27.Hyams JS, Markowitz JF. Can we alter the natural history of Crohn disease in children? J Pediatr Gastroenterol Nutr. 2005;40:262–272. doi: 10.1097/01.mpg.0000154660.62359.fe. [DOI] [PubMed] [Google Scholar]
- 28.Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. 1999;28:445–458. doi: 10.1016/s0889-8553(05)70064-9. [DOI] [PubMed] [Google Scholar]
- 29.Polito JM, Childs B, Mellits ED, Tokayer AZ, Harris ML, Bayless TM. Crohn's disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;111:580–586. doi: 10.1053/gast.1996.v111.pm8780560. [DOI] [PubMed] [Google Scholar]
- 30.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad T, Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn's disease. Gastroenterology. 2002;122:854–866. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- 32.Abreu MT, Taylor KD, Lin YC, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679–688. doi: 10.1053/gast.2002.35393. [DOI] [PubMed] [Google Scholar]
- 33.Kugathasan S, Collins N, Maresso K, et al. CARD15 gene mutations and risk for early surgery in pediatric-onset Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:1003–1009. doi: 10.1016/s1542-3565(04)00452-5. [DOI] [PubMed] [Google Scholar]
- 34.Russell RK, Drummond HE, Nimmo EE, et al. Genotype-phenotype analysis in childhood-onset Crohn's disease: NOD2/CARD15 variants consistently predict phenotypic characteristics of severe disease. Inflamm Bowel Dis. 2005;11:955–964. doi: 10.1097/01.mib.0000183423.38037.f3. [DOI] [PubMed] [Google Scholar]
- 35.Dubinsky MC. New patients: should children be treated differently? Colorectal Dis. 2006;8(Suppl 1):15–19. doi: 10.1111/j.1463-1318.2006.00987.x. [DOI] [PubMed] [Google Scholar]
- 36.Sentongo TA, Semeao EJ, Piccoli DA, Stallings VA, Zemel BS. Growth, body composition, and nutritional status in children and adolescents with Crohn's disease. J Pediatr Gastroenterol Nutr. 2000;31:33–40. doi: 10.1097/00005176-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Seidman E, Leleiko N, Ament M, et al. Nutritional issues in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1991;12:424–438. doi: 10.1097/00005176-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Semeao EJ, Jawad AF, Stouffer NO, Zemel BS, Piccoli DA, Stallings VA. Risk factors for low bone mineral density in children and young adults with Crohn's disease. J Pediatr. 1999;135:593–600. doi: 10.1016/s0022-3476(99)70058-2. [DOI] [PubMed] [Google Scholar]
- 39.Mamula P, Piccoli DA, Peck SN, Markowitz JE, Baldassano RN. Total dose intravenous infusion of iron dextran for iron-deficiency anemia in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;34:286–290. doi: 10.1097/00005176-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Engstrom I. Inflammatory bowel disease in children and adolescents: mental health and family functioning. J Pediatr Gastroenterol Nutr. 1999;28:S28–S33. doi: 10.1097/00005176-199904001-00004. [DOI] [PubMed] [Google Scholar]
- 41.Mackner LM, Crandall WV, Szigethy EM. Psychosocial functioning in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:239–244. doi: 10.1097/01.MIB.0000217769.83142.c6. [DOI] [PubMed] [Google Scholar]
- 42.Mackner LM, Crandall WV. Psychological factors affecting pediatric inflammatory bowel disease. Curr Opin Pediatr. 2007;19:548–552. doi: 10.1097/MOP.0b013e3282ef4426. [DOI] [PubMed] [Google Scholar]
- 43.van der Zaag-Loonen HJ, Grootenhuis MA, Last BF, Derkx HH. Coping strategies and quality of life of adolescents with inflammatory bowel disease. Qual Life Res. 2004;13:1011–1019. doi: 10.1023/B:QURE.0000025598.89003.0c. [DOI] [PubMed] [Google Scholar]
- 44.Nicholas DB, Otley A, Smith C, Avolio J, Munk M, Griffiths AM. Challenges and strategies of children and adolescents with inflammatory bowel disease: a qualitative examination. Health Qual Life Outcomes. 2007;5:28. doi: 10.1186/1477-7525-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrin JM, Kuhlthau K, Chughtai A, et al. Measuring quality of life in pediatric patients with inflammatory bowel disease: psychometric and clinical characteristics. J Pediatr Gastroenterol Nutr. 2008;46:164–171. doi: 10.1097/MPG.0b013e31812f7f4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35:557–563. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 48.Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677–692. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 49.Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:851–856. doi: 10.1016/j.cgh.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 50.A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- 51.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 52.Punati J, Markowitz J, Lerer T, et al. Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm Bowel Dis. 2008;14:949–954. doi: 10.1002/ibd.20412. [DOI] [PubMed] [Google Scholar]
- 53.Baldassano R, Braegger CP, Escher JC, et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98:833–838. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 54.Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response to infliximab in early but not late pediatric Crohn's disease. Am J Gastroenterol. 2000;95:3189–3194. doi: 10.1111/j.1572-0241.2000.03263.x. [DOI] [PubMed] [Google Scholar]
- 55.Borrelli O, Bascietto C, Viola F, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis. 2004;36:342–347. doi: 10.1016/j.dld.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 56.Stephens MC, Shepanski MA, Mamula P, Markowitz JE, Brown KA, Baldassano RN. Safety and steroid-sparing experience using infliximab for Crohn's disease at a pediatric inflammatory bowel disease center. Am J Gastroenterol. 2003;98:104–111. doi: 10.1111/j.1572-0241.2003.07161.x. [DOI] [PubMed] [Google Scholar]
- 57.Lionetti P, Bronzini F, Salvestrini C, et al. Response to infliximab is related to disease duration in paediatric Crohn's disease. Aliment Pharmacol Ther. 2003;18:425–431. doi: 10.1046/j.1365-2036.2003.01672.x. [DOI] [PubMed] [Google Scholar]
- 58.Toruner M, Loftus EV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 60.Hugot JP, Bellaiche M. Inflammatory bowel diseases: the paediatric gastroen-terologist's perspective. Pediatr Radiol. 2007;37:1065–1070. doi: 10.1007/s00247-007-0573-3. [DOI] [PubMed] [Google Scholar]
- 61.Rajwal SR, Puntis JW, McClean P, et al. Endoscopic rectal sparing in children with untreated ulcerative colitis. J Pediatr Gastroenterol Nutr. 2004;38:66–69. doi: 10.1097/00005176-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Carvalho R, Hyams JS. Diagnosis and management of inflammatory bowel disease in children. Semin Pediatr Surg. 2007;16:164–171. doi: 10.1053/j.sempedsurg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Gupta SK, Fitzgerald JF, Croffie JM, Pfefferkorn MD, Molleston JP, Corkins MR. Comparison of serological markers of inflammatory bowel disease with clinical diagnosis in children. Inflamm Bowel Dis. 2004;10:240–244. doi: 10.1097/00054725-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 64.MacKalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733–741. doi: 10.1136/gut.2005.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubinsky MC, Ofman JJ, Urman M, Targan SR, Seidman EG. Clinical utility of serodiagnostic testing in suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2001;96:758–765. doi: 10.1111/j.1572-0241.2001.03618.x. [DOI] [PubMed] [Google Scholar]
- 66.Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506–513. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177. doi: 10.1097/00005176-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Dubinsky MC. Emerging strategies in the use of IBD-related serologic markers. Gastroenterology & Hepatology. 2008;4:557–559. [PMC free article] [PubMed] [Google Scholar]
- 69.Fries W, Renda MC, Lo Presti MA, et al. Intestinal permeability and genetic determinants in patients, first-degree relatives, and controls in a high-incidence area of Crohn's disease in Southern Italy. Am J Gastroenterol. 2005;100:2730–2736. doi: 10.1111/j.1572-0241.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 70.Barken DM, McGinniss MJ, Nakamura RM, Pan H. Prediction of inflammatory bowel disease (IBD) using serologic testing: a retrospective analysis [abstract 143] Poster presented at the 2008 NASPGHAN annual meeting, San Diego, CA, November 12-15, 2008.
- 71.Markowitz J, Kugathasan S, Dubinsky M, et al. Age of diagnosis influences serologic responses in children with Crohn's disease: a possible clue to etiology? Inflamm Bowel Dis. 2009;15:714–719. doi: 10.1002/ibd.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]


