Abstract
The risk of lymphoma associated with inflammatory bowel disease is a cause of great anxiety for patients, their families, and their providers. Trepidation regarding the use of immunomodulators and anti-tumor necrosis factor agents, due to their possible association with lymphoma, may influence treatment decisions, compliance with prescribed medications, and, possibly, long-term outcomes if appropriate medical therapy is avoided. Lymphoma is a difficult topic to discuss with patients due to uncertainty regarding the absolute risk. A general message to convey to patients is that there is likely an increased risk of lymphoma associated with the treatment of inflammatory bowel disease but that the substantial benefit of these therapies outweighs the very small risk incurred. This review aims to explain current data regarding the risk of lymphoma associated with inflammatory bowel disease itself and the immune suppressant therapy used for its treatment.
Keywords: Crohn's disease, ulcerative colitis, inflammatory bowel disease, lymphoma, immunomodulator, anti-tumor necrosis factor
The fear of lymphoma influences physicians, patients, and their families when deciding among options for the treatment of inflammatory bowel disease (IBD). Although the absolute risks are uncertain, and at most small, the thought of causing cancer with medication clearly raises concern and becomes a focus of discussion with patients. Conclusions involving the amount of risk attributable to medical therapy versus the disease itself are limited by the available data. As a majority of IBD patients with long-standing disease are at some point prescribed immunomodulators, and nearly all anti-tumor necrosis factor (anti-TNF)–treated patients have been previously or concomitantly exposed to immunomodulators, proper control groups are difficult to compile. Randomized controlled trials (RCTs) are vastly underpowered to detect a difference in lymphoma rates and will likely never be adequate. For example, due to the infrequency of lymphoma, a study designed to detect a difference in lymphoma rates between treated and untreated patients would require over 8,000 patients in each group (assuming no dropouts). Therefore, we need to rely on carefully completed outcomes studies to establish our best estimates of the influence of medical treatment on the occurrence of lymphoma in IBD patients. This is not only important to clarify the safety of the medications, but to clearly relay the information to patients so that they can make the decision themselves as to whether the risk of treatment is worth the potential benefit of therapy. The aim of this review is to discuss what is known regarding the risk of lymphoma associated with IBD itself and the available treatments, including immunomodulators and biologic agents.
Lymphoma Classification and Epidemiology
Referring to lymphoma as a homogeneous group of diseases is a gross oversimplification. Even acknowledging the differences between Hodgkin and non-Hodgkin lymphoma (NHL) still ignores over 20 subtypes of NHLs.1 These NHL subtypes are broadly categorized into B-cell or T- and NK-cell neoplasms. The distinction is important, as the subtypes have a different epidemiology, treatment, and prognosis. A description of overall rates of lymphoma in IBD patients compared to the expected rate in the general population is difficult to interpret due to these differences. Most importantly, Hodgkin disease and NHL should be described separately. In the United States, the median age of diagnosis of Hodgkin disease is 38 years, the overall incidence is approximately 3/100,000 annually, and the 5-year survival rate is 85%. In contrast, the median age of diagnosis of NHL is 67 years, the overall incidence is approximately 20/100,000 annually, and the 5-year survival rate is 67%. Both are male-predominant diseases; however, Hodgkin disease has a fairly stable incidence rate with age, whereas NHL rises dramatically over time (Figure 1).2 The majority of research comparing the rate of lymphoma in disease states to the expected rate in the general population does not account for these differences.
Figure 1.
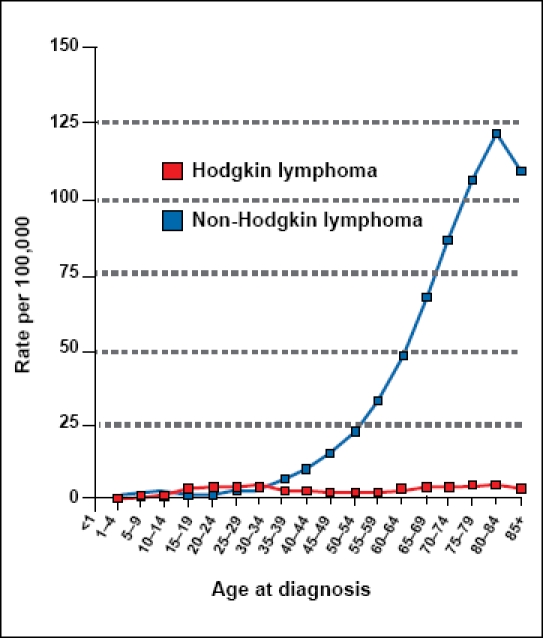
Age-specific (crude) Surveillance Epidemiology End Results (SEER) incidence rates of lymphoma by cancer site (all ages, white, both sexes, 2000–2006).
Cancer sites include invasive cases only unless otherwise noted. Incidence source: SEER 17 areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Alaska Native Registry, rural Georgia, California excluding SF/SJM/LA, Kentucky, Louisiana, and New Jersey).
Datapoints were not shown for rates that were based on fewer than 16 cases.
Figure developed with permission from the National Cancer Institute.2
Lymphoma in Inflammatory Bowel Disease Patients
For many years, there has been concern that IBD itself carries an increased risk of lymphoma. Initial descriptions of lymphosarcoma in a patient with ulcerative colitis in 19283 and reticulum cell sarcoma (now an outdated term for mature B- and T-cell NHL)4 in a patient with Crohn's disease in 19555 have since been followed by numerous case reports, case series, and cohort studies.
Population-based cohort studies may be the least influenced by referral bias, as they include a regional group of patients as opposed to only those referred to specialty centers. The largest study was conducted by Askling and colleagues, who analyzed prospective data from 4 different sources that included nearly 50,000 Swedish patients with IBD. Of these patients, 95% were from a national inpatient registry, with the remaining patients from noninpatient data sources. Standardized incidence ratios (SIRs) were calculated based upon the rates of lymphoma in these populations compared to the expected rates in the general Swedish population. For patients with ulcerative colitis, there was no statistically significant increased risk of lymphoma (SIR 1.0, 95% confidence interval [CI] 0.8–1.3), whether breaking down the analysis by Hodgkin disease or NHL, or by inpatient or outpatient registries. For patients with Crohn's disease, although the overall rate of lymphoma was not significantly higher than that of the comparison group (SIR 1.3, 95% CI 1.0–1.6), the subgroup of those in the inpatient registry had a statistically significant higher rate of NHL (SIR 1.55, 95% CI 1.2–2.0), but not Hodgkin disease (SIR 1.03, 95% CI 0.4–2.2). Adjusting for covariates to control for disease severity did not reveal any differences. Although not significant, the lymphoma risk was higher within the first 5 years of follow-up. In this study, which took place over 3 decades in Sweden, the absolute rate of lymphoma (Hodgkin disease, NHL, and chronic lymphocytic leukemia) was 1.1% for ulcerative colitis and 0.7% for patients with Crohn's disease. The researchers did not have any information on pharmacotherapy. Based upon the time period of the data collection, the cohorts were essentially anti-TNF–naive, but we do not know how many were exposed to immunomodulators. An interpretation of these data is that there may be a slight increased risk of NHL in patients with Crohn's disease, particularly in those who were sick enough to have been hospitalized.
Another large study to address the question of lymphoma risk in IBD utilized the General Practice Research Database.6 This database from the United Kingdom contains computerized records from approximately 2,000 practitioners and 8 million patients. In addition to demographic information and diagnostic codes, prescription medications were also coded within the database. The incidence density ratio (IDR), which is similar to relative risk but allows adjustment for person-time, was also reported in the database. The analysis included nearly 7,000 patients with Crohn's disease, over 10,000 patients with ulcerative colitis, and over 60,000 controls (matched approximately 1:4). The relative risk of lymphoma for patients with Crohn's disease or ulcerative colitis was not statistically elevated in a significant manner compared to the control population (IDR 1.2, 95% CI 0.67–2.06). Multiple analyses were performed and consistent in that there was no increased risk of lymphoma in any of the subgroups. The researchers did not break down lymphoma by subtype (Hodgkin vs non-Hodgkin), as they did not have access to this level of detail in the database [personal communication, James D. Lewis].
In contrast, a population-based study that did show an increased risk of lymphoma in IBD patients was conducted by Bernstein and associates from a patient cohort in Manitoba.7 The incidence of cancer was examined by linking records from the IBD and non-IBD cohorts with the comprehensive Cancer Care Manitoba registry. The researchers reported incidence rate ratios (IRRs) for approximately 6,000 IBD patients compared to matched controls. Results showed an IRR of 3.63 (95% CI 1.53–8.62) for male patients with Crohn's disease, but not for females (IRR 1.09, 95% CI 0.25–4.67) or for ulcerative colitis (IRR 1.03, 95% CI 0.47-2.24). The authors were able to further examine the charts of patients with lymphoma, and none of the Crohn's disease patients with lymphoma were exposed to immunomodulators or anti-TNF agents. Therefore, they concluded that based upon the disease itself, male patients with Crohn's disease are at an increased risk of lymphoma. One criticism is the possibility of finding this result by chance due to the multiple comparisons that were made during this broad study of cancer in IBD; however, this important result needs to be considered when reviewing this topic.
A number of other smaller population-based studies did not show an increased risk of lymphoma in Crohn's disease or ulcerative colitis.8-14 Some hospital-based referral centers did show an increased lymphoma risk in IBD,15,16 but in such studies, issues of referral bias (ie, patients with IBD and lymphoma referred to larger centers) are difficult to ignore. Another explanation for referral centers having higher rates may be that patients with more severe disease are at a higher risk of lymphoma, as has been shown in rheumatoid arthritis.17 Attempts have been made to examine this in IBD within some of the larger studies,6,18 but a relationship between disease severity and lymphoma risk has not been established.
In summary, although some studies examining the risk of lymphoma associated with IBD itself have revealed subgroups that may be at risk, the vast majority of studies, including those from large population-based cohorts, do not confirm these findings. Based upon the available data, it is likely safe to assume that the baseline risk of lymphoma in IBD patients mirrors that of the general population. The next question is whether treatment leads to an increased risk of lymphoma in patients with IBD.
Immunomodulators and Lymphoma Risk
Since at least the 1970s, there has been concern that immune suppression has been associated with an increased risk of cancer.19 Specifically, NHL has been observed to occur in excess of the expected rate within multiple disease states that require immune suppression, such as IBD.20 In the transplant population, NHL associated with immune suppression is called posttransplant lymphoproliferative disorder (PTLD). PTLD occurs in 1–20% of solid organ transplants, and risk factors include younger age, intensity of immune suppression, and Epstein-Barr virus (EBV) serostatus.21 Pediatric patients have a higher likelihood of PTLD than adults (53% vs 15%); this is thought to be, in part, related to EBV status. EBV-naive recipients have a 25–50-fold higher incidence of PTLD when transplanted with EBV-positive donor organs. The connection to pediatric patients is that younger patients are more likely to be EBV-negative than adult patients. Interestingly, in terms of transplanted organs, the intestine is the organ associated with the highest risk. This has been hypothesized to be due to the fact that it holds the highest amount of lymphoid tissue that may be harboring viruses such as EBV and cytomegalovirus (CMV). Although results have not been consistent, Darenkov and colleagues demonstrated, in a cohort of consecutive patients, a decreased incidence of PTLD in those receiving antiviral therapy.22 In this study, patients who received antiviral therapy (ganciclovir if the donor or recipient was CMV-positive and acyclovir if both were CMV-negative) had a PTLD rate of 0.5% compared to 3.9% in those not treated with antiviral agents (P<.05). To decrease the risk of PTLD, transplant physicians extrapolate from these and other data and typically use valganciclovir or valacyclovir as prophylaxis in the posttransplant setting in addition to minimizing immune suppression when possible.
In 1985, Kinlen noted that the almost 50-fold increased rate of NHL in transplant patients was lower (11-fold) in nontransplant immune-suppressed patients.20 One proposed hypothesis involved the lower intensity of immune suppression. This higher rate from baseline still may, in part, be due to EBV. EBV has been considered in association with lymphomas in rheumatoid arthritis patients taking methotrexate23 and also in IBD patients receiving azathioprine (AZA) or 6-mercaptopurine (6-MP).24 Dayharsh and colleagues at the Mayo Clinic reported on 18 patients with lymphoma, 7 of whom developed EBV-positive lymphoma. Five of these 7 patients had been taking AZA or 6-MP compared to only 1 of 11 patients with EBV-negative lymphoma (P=.01).24
Lymphoma was first reported in association with IBD patients treated with immunomodulators over 30 years ago.25 Since then, multiple reports have been published examining the association of immunomodulators and lymphoma in the treatment of Crohn's disease and ulcerative colitis. Across individual reports, results are mixed. A meta-analysis published in 2005 included 6 manuscripts that ranged from 238 to 1,464 IBD patients in each study.6,15,20,26-29 For each of these 6 studies, the SIR ranged from 0 to 37.5 for the rate of lymphoma (Hodgkin and NHL combined) in patients treated with AZA or 6-MP compared to the expected rate (derived from the Surveillance Epidemiology and End Results [SEER] cancer registry). Two of the 6 studies had SIRs that were statistically elevated in a significant manner. When combined as a pooled risk estimate, the relative risk was 4.18 (95% CI 2.07–7.51; 11 observed cases, 2.63 expected). A subanalysis for NHL alone yielded a SIR of 3.92 (95% CI 1.78–7.47), but because of the heterogeneity among studies, the authors warned that these results should be interpreted with caution. A large prospective, population-based study in France also recently examined this question.30 Their cohort included nearly 21,000 patients, with over 50,000 patient-years of follow-up. Approximately 30% of these patients were exposed to AZA, and a total of 19 lymphomas were reported. Of these 19 patients, 13 were exposed to AZA and 8 of these 13 were EBV-positive. The calculated rates of occurrence and relative risks are forthcoming in a full publication; however, these numbers suggest that the rate of lymphoma associated with immunomodulator use may be higher than previously thought.
An aggressive and usually fatal form of lymphoma, hepatosplenic T-cell lymphoma (HSTCL), has recently been of concern in patients treated for IBD. Although the majority of IBD patients who have developed HSTCL have been taking combination immunomodulator and anti-TNF therapy, a number of cases have been reported in young patients on AZA monotherapy.31-33 To date, there have been 18 cases of HSTCL reported worldwide in patients treated with immunomodulators and anti-TNFs. Seventeen of these patients were male, and their ages ranged from 12 to 58 years old (average age, 26). Although over 400,000 patients with IBD have been treated with anti-TNF agents (and over 1 million worldwide for all indications), we do not know how many patients within this age range have been exposed; therefore, we cannot accurately calculate a rate of occurrence. A best guess is that it occurs less frequently than standard NHL in IBD patients treated with immunomodulators. Concerns over HSTCL have significantly impacted attitudes surrounding the use of combination therapy for IBD. Although the risk of HSTCL should be taken seriously, for patients who require the use of 2 drugs to maintain control of their disease, the benefits likely far outweigh the very small risk.
In IBD, there is significantly less written regarding the risk of lymphoma associated with methotrexate than with the thiopurines. In fact, a PubMed search with the search terms “(Crohn's OR ulcerative colitis) AND methotrexate AND lymphoma” does not yield a single study focused on lymphoma in IBD patients treated with methotrexate. In the study by Farrell and associates15 that was included in the meta-analysis by Kandiel and colleagues,28 2 of the 4 cases of NHL occurred in patients treated with methotrexate. Although it is difficult to draw conclusions from such small sample sizes, these 2 patients were out of only 31 patients in the entire 782-person cohort who had any exposure to methotrexate.
In contrast, the same PubMed search switching the IBD terms for rheumatoid arthritis yielded 166 results, a large portion of which were directly related to lymphoproliferative disorders associated with the use of methotrexate in rheumatologic diseases. Patients with rheumatoid arthritis are believed to be at a higher baseline risk of lymphoma than the general population, making it difficult to determine how much treatment contributes to the higher rate.17 EBV may also play a role in these lymphomas. Baecklund and coworkers found that in their large series of 348 lymphomas in patients with rheumatoid arthritis, 37 were EBV-positive.17 Of these patients, 29 had prior exposure to immunomodulators (primarily methotrexate) whereas the other 8 did not. As seen in the IBD literature with AZA and 6-MP, studies examining the relationship between methotrexate and lymphoma show discordant results. In 2008, Buchbinder and colleagues reported on a cohort followed in community practices in Australia that included over 4,000 patient-years of follow-up time.34 They noted that 458 patients had received methotrexate and 8 developed NHL compared to 1.6 expected NHLs, resulting in a SIR of 5.1 (2.2–10). Dr. Frederick Wolfe, who maintains a large prospective registry of rheumatoid arthritis patients in the United States,35 found that, among nearly 20,000 patients (and almost 90,000 patient-years of follow-up), 68% received methotrexate at some point in their disease course. The use of methotrexate alone had an elevated odds ratio of 1.4 for developing lymphoma, but this was not statistically significant (95% CI 0.7–2.9). Interestingly, another recent report found that the use of corticosteroids in rheumatoid arthritis is associated with a decreased risk of lymphoma.36 Perhaps maintaining disease control is a critically important factor.
Anti-tumor Necrosis Factor Agents and Lymphoma Risk
Around the time that infliximab (Remicade, Centocor) was approved for use in Crohn's disease, there was already concern over a possible increased risk of lymphoma associated with this treatment.37 Since then, multiple observational reports have been published on the occurrence of lymphomas in IBD patients treated with anti-TNF agents. In the studies that attempt to quantify an incidence of lymphoma in this clinical setting, the estimates have a broad range. The large Crohn's Therapy Resource, Evaluation, and Assessment Tool (TREAT) registry that includes nearly 25,000 patient-years of follow-up found no increased risk of lymphoma compared to the control population. In contrast, in a Mayo Clinic study of 500 patients, 1 patient developed NHL (for an incidence rate of 0.2%), and a study from Stockholm County, Sweden found an incidence rate of 1.6% (almost 2 per 100). Each of these study designs were accompanied by concerns over the validity of the estimate (eg, the loss to follow-up in the TREAT registry was >15%, and there was a possibility of referral bias at the Mayo Clinic) and add to the uncertainty of the effect of treatment on the occurrence of lymphoma.
A meta-analysis by Peyrin-Biroulet and colleagues analyzed the efficacy and safety of anti-TNF agents for the treatment of Crohn's disease in the setting of RCTs.38 These authors did not find an increased rate of lymphoma associated with treatment; however, they only evaluated RCTs (which include a carefully chosen group of patients), and in the majority of these studies, by design, many of the "control" patients had some exposure to anti-TNF treatment, making interpretation of their results difficult. They did perform a sensitivity analysis of only studies in which control patients were unexposed, but this was not a primary endpoint of their study and it is not clear whether it was powered to provide conclusions on this secondary analysis.
A more recent meta-analysis directly explored the question of NHL in patients with Crohn's disease treated with anti-TNF agents.39 This study analyzed 26 publications, which included 9 RCTs, 3 cohort studies, and 14 case series of consecutive patients. On average, 66% of subjects were concomitantly taking immunomodulators. Thirteen NHLs were identified within the 8,905 patients treated with anti-TNF drugs, with 21,178 patient-years of follow-up. At least 10 of these 13 patients were exposed to immunomodulators as well. The calculated absolute rate of NHL was 6.1 per 10,000 patient-years. This rate was then compared to the expected rate in the SEER registry (Table 1). Overall, the SEER registry reported a NHL rate of 1.9 per 10,000 patient-years. Therefore, the SIR of anti-TNF–treated patients compared to the SEER registry was 3.23 (95% CI 1.5–6.9). The pooled estimate was also compared to the Kandiel and associates' summary estimate from a meta-analysis of IBD patients treated with immunomodulators without anti-TNF exposure.28 The rate from Kandiel was 3.6 per 10,000 patient-years when using only Crohn's patients and only NHL (they combined Crohn's disease and ulcerative colitis, and NHL and Hodgkin disease). Although the rate with anti-TNFs was higher than the rate with immunomodulators, the SIR of 1.7 was not statistically significant (95% CI 0.5–7.1). As NHL appears to be age- and gender-sensitive, further analyses were performed to develop age- and gender-specific rates of NHL. The rate of NHL in anti-TNF exposure patients increased with advancing age; however, as the NHL rate also increases in SEER, the only statistically significant subgroup was men aged 20–54 (SIR 5.4, 95% CI 1.3–18.1). The conclusion of this meta-analysis was that the rate of NHL increases in Crohn's disease patients treated with anti-TNF agents in combination with immunomodulators, but this absolute rate is still very low.
Table 1.
Rate of Non-Hodgkin Lymphoma Associated With Immunomodulator and Anti-tumor Necrosis Factor (anti-TNF) Treatment
| Baseline US population* | Immunomodulator-treated† | Anti-TNF + immunomodulator-treated | |
|---|---|---|---|
| Rate of lymphoma per 10,000 patient-years | 1.9 | 3.6 | 6.1 |
| Relative risk of anti-TNF + immunomodulator therapy | |||
| Compared to baseline: SIR 3.23 (95% CI 1.5–6.9) | |||
| Compared to immunomodulator alone: SIR 1.7 (95% CI 0.5–7.1) | |||
- CI=
confidence interval;
- SIR=
standardized incidence ratio.
Based upon data from the Surveillance Epidemiology End Results registry.
Immunomodulator=azathioprine or 6-mercaptopurine.
As most Crohn's disease patients treated with anti-TNF agents have also been exposed to immunomodulators, there are few anti-TNF–only exposed patients available to study. Therefore, it is currently impossible to know whether anti-TNF agents on their own are associated with an increased rate of NHL or HSTCL. A best guess would be that these lymphomas are related to immune suppression (not necessarily a particular agent) and that, individually, the agents may be associated with higher rates of lymphoma, which is slightly more frequent when using the drugs in combination.
Conclusion
Without appropriate treatment, patients with Crohn's disease and ulcerative colitis can have a greatly diminished quality of life. Immunomodulators and anti-TNF agents have been proven to be effective in the treatment of IBD and can have a significant positive impact on the lives of these patients. Most likely, these medications are associated with a small, but measurable, increased risk of lymphoma. The majority of evidence supports the fact that IBD itself does not increase the risk of lymphoma. Patients, parents, and physicians will have different perceptions of these medications and the disease itself based upon the amount of side effect risk to which they are willing to be exposed.40 It appears that patients with more active disease are willing to accept higher risks of treatment, in most cases substantially higher than the risks described for lymphoma.41 From a population-based standpoint, the benefits of immunomodulator and anti-TNF treatment appear to outweigh the associated risks using the technique of decision analysis.42,43 From an individual patient standpoint, the decision to take these medications will be very personal and should include a thorough conversation between patient and provider to determine whether these treatments are appropriate.
Acknowledgments
Dr. Siegel is supported by a CCFA career development award and grant number K23DK078678 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content of this review is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
References
- 1.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House. Hematol J. 2000;1:53–66. doi: 10.1038/sj.thj.6200013. Virginia, November, 1997. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results Database [database] [[Accessed on July 28, 2009]. Available at: http://seer.cancer.gov.
- 3.Bargen JA. Chronic ulcerative colitis associated with malignant disease. Arch Surg. 1928;17:561–576. doi: 10.1007/BF02054420. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Histiocytic lymphoma [dictionary entry] [Accessed July 28, 2009]. Available at: http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=373019.
- 5.Hughes RK. Reticulum cell sarcoma: a case possibly originating in regional enteritis. Am Surg. 1955;21:770–773. [PubMed] [Google Scholar]
- 6.Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080–1087. doi: 10.1053/gast.2001.28703. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Ekbom A, Helmick C, Zack M, Adami HO. Extracolonic malignancies in inflammatory bowel disease. Cancer. 1991;67:2015–2019. doi: 10.1002/1097-0142(19910401)67:7<2015::aid-cncr2820670731>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Intestinal and extra-intestinal cancer in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther. 2004;19:287–293. doi: 10.1111/j.1365-2036.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 11.Loftus EV, Jr., Tremaine WJ, Habermann TM, Harmsen WS, Zinsmeister AR, Sandborn WJ. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308–2312. doi: 10.1111/j.1572-0241.2000.02316.x. [DOI] [PubMed] [Google Scholar]
- 12.Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, et al. Hodgkin's disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647–653. doi: 10.1053/gast.2000.16487. [DOI] [PubMed] [Google Scholar]
- 13.Persson PG, Karlen P, Bernell O, Leijonmarck CE, Broström O, et al. Crohn's disease and cancer: a population-based cohort study. Gastroenterology. 1994;107:1675–1679. doi: 10.1016/0016-5085(94)90807-9. [DOI] [PubMed] [Google Scholar]
- 14.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 15.Farrell RJ, Ang Y, Kileen P, O'Briain DS, Kelleher D, et al. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514–519. doi: 10.1136/gut.47.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenstein AJ, Mullin GE, Strauchen JA, Heimann T, Janowitz HD, et al. Lymphoma in inflammatory bowel disease. Cancer. 1992;69:1119–1123. doi: 10.1002/cncr.2820690510. [DOI] [PubMed] [Google Scholar]
- 17.Baecklund E, Askling J, Rosenquist R, Ekbom A, Klareskog L. Rheumatoid arthritis and malignant lymphomas. Curr Opin Rheumatol. 2004;16:254–261. doi: 10.1097/00002281-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Askling J, Brandt L, Lapidus A, Karlén P, Björkholm M, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54: 617–622. doi: 10.1136/gut.2004.051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinlen LJ, Sheil AG, Peto J, Doll R. Collaborative United Kingdom-Austral-asian study of cancer in patients treated with immunosuppressive drugs. Br Med J. 1979;2:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinlen LJ. Incidence of cancer in rheumatoid arthritis and other disorders after immunosuppressive treatment. Am J Med. 1985;78:44–49. doi: 10.1016/0002-9343(85)90245-1. [DOI] [PubMed] [Google Scholar]
- 21.Everly MJ, Bloom RD, Tsai DE, Trofe J. Posttransplant lymphoproliferative disorder. Ann Pharmacother. 2007;41:1850–1858. doi: 10.1345/aph.1G706. [DOI] [PubMed] [Google Scholar]
- 22.Darenkov IA, Marcarelli MA, Basadonna GP, Friedman AL, Lorber KM, et al. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation. 1997;64:848–852. doi: 10.1097/00007890-199709270-00010. [DOI] [PubMed] [Google Scholar]
- 23.Dawson TM, Starkebaum G, Wood BL, Willkens RF, Gown AM. Epstein-Barr virus, methotrexate, and lymphoma in patients with rheumatoid arthritis and primary Sjogren's syndrome: case series. J Rheumatol. 2001;28:47–53. [PubMed] [Google Scholar]
- 24.Dayharsh GA, Loftus EV, Jr., Sandborn WJ, Tremaine WJ, Zinsmeister AR, et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 25.Hecker R, Sheers R, Thomas D. Hodgkin's disease as a complication of Crohn's disease. Med J Aust. 1978;2:603. doi: 10.5694/j.1326-5377.1978.tb131771.x. [DOI] [PubMed] [Google Scholar]
- 26.Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–1252. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 27.Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002;16:1225–1232. doi: 10.1046/j.1365-2036.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 28.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korelitz BI, Mirsky FJ, Fleisher MR, Warman JI, Wisch N, Gleim GW. Malignant neoplasms subsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol. 1999;94:3248–3253. doi: 10.1111/j.1572-0241.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 30.Beaugerie L, Carrat F, Bouvier AM, Brousse N, Carbonnel F, et al. Excess risk of lymphoproliferative disorders (LPD) in inflammatory bowel diseases (IBD): Interim results of the CESAME cohort. Gastroenterology. 2008;134:A–116. [Google Scholar]
- 31.Lemann M, de La Valussiere G, Bouhnik Y, Allez M, Touze Y, et al. Intravenous cyclosporine for refractory attacks of Crohn's disease (CD): Long-term follow-up of patients. Gastroenterology. 1998;114:A1020. [Google Scholar]
- 32.Mittal S, Milner BJ, Johnston PW, Culligan DJ. A case of hepatosplenic gamma-delta T-cell lymphoma with a transient response to fludarabine and alemtuzumab. Eur J Haematol. 2006;76:531–534. doi: 10.1111/j.1600-0609.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 33.Navarro JT, Ribera JM, Mate JL, Granada I, Juncá J, et al. Hepatosplenic T-gamma delta lymphoma in a patient with Crohn's disease treated with azathioprine. Leuk Lymphoma. 2003;44:531–533. doi: 10.1080/1042819021000035662. [DOI] [PubMed] [Google Scholar]
- 34.Buchbinder R, Barber M, Heuzenroeder L, Wluka AE, Giles G, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008;59:794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56:1433–1439. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 36.Hellgren K, Iliadou A, Rosenquist R, Feltelius N, Backlin C Rheumatoid arthritis, treatment with corticosteroids, and risk of malignant lymphomas - results from a case-control study. Ann Rheum Dis. 2009. May 12. [Epub ahead of print] [DOI] [PubMed]
- 37.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel CA, Levy LC, Mackenzie TA, Sands BE. Patient perceptions of the risks and benefits of infliximab for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1–6. doi: 10.1002/ibd.20283. [DOI] [PubMed] [Google Scholar]
- 41.Johnson FR, Ozdemir S, Mansfield C, Hass S, Miller DW, et al. Crohn's disease patients' risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133:769–779. doi: 10.1053/j.gastro.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–1024. doi: 10.1016/s0016-5085(00)70353-2. [DOI] [PubMed] [Google Scholar]
- 43.Siegel CA, Hur C, Korzenik JR, Gazelle GS, Sands BE. Risks and benefits of infliximab for the treatment of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1017–1024. doi: 10.1016/j.cgh.2006.05.020. [DOI] [PubMed] [Google Scholar]


